CIESC Journal ›› 2021, Vol. 72 ›› Issue (3): 1205-1216.DOI: 10.11949/0438-1157.20200921
• Reviews and monographs • Previous Articles Next Articles
WANG Ying1,2( ),LI Qian3,CAO Lixia1,LI Yanxiang1,2(
),LI Qian3,CAO Lixia1,LI Yanxiang1,2( ),LI Wangliang1,2(
),LI Wangliang1,2( )
)
Received:2020-07-09
Revised:2020-08-31
Online:2021-03-05
Published:2021-03-05
Contact:
LI Yanxiang,LI Wangliang
王莹1,2( ),李倩3,曹丽霞1,李艳香1,2(
),李倩3,曹丽霞1,李艳香1,2( ),李望良1,2(
),李望良1,2( )
)
通讯作者:
李艳香,李望良
作者简介:王莹(1994—),女,博士研究生,基金资助:CLC Number:
WANG Ying, LI Qian, CAO Lixia, LI Yanxiang, LI Wangliang. Progress of biomass-based materials for uranium adsorption[J]. CIESC Journal, 2021, 72(3): 1205-1216.
王莹, 李倩, 曹丽霞, 李艳香, 李望良. 生物质基铀吸附材料的研究进展[J]. 化工学报, 2021, 72(3): 1205-1216.
Add to citation manager EndNote|Ris|BibTeX
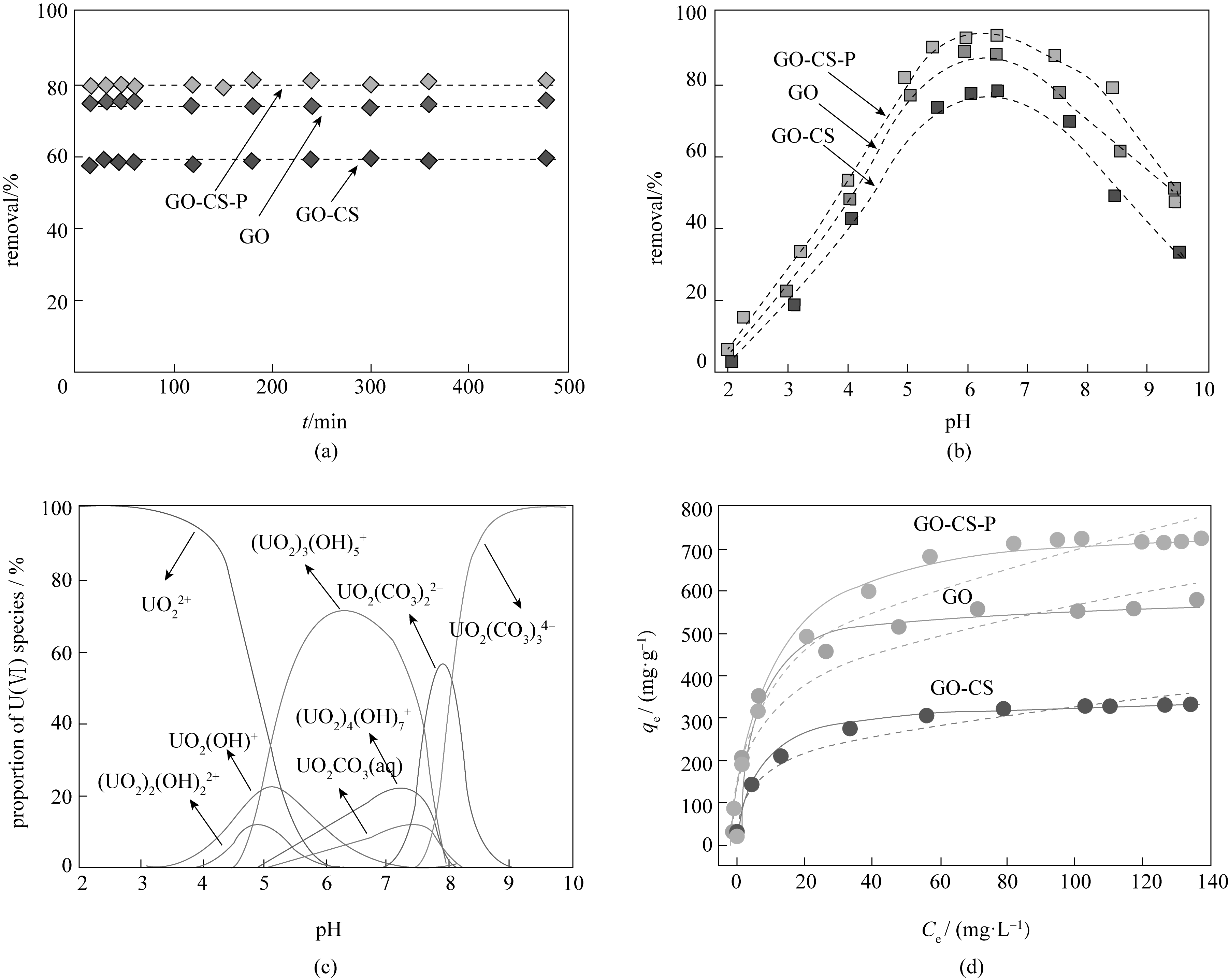
Fig.2 (a) Time-dependent sorption behaviors of U(Ⅵ) on GO, GO-CS and GO-CS-P; (b) Effect of solution pH on the removal of U(Ⅵ) by GO, GO-CS and GO-CS-P; (c) pH-depended speciation of U(Ⅵ) in solution; (d) Sorption isotherm, Langmuir and Freundlich model fits of U(Ⅵ) on GO, GO-CS and GO-CS-P[29]
| 类别 | 吸附剂名称 | 吸附条件 | 最高吸附量/(mg·g-1) | 文献 |
|---|---|---|---|---|
| 壳聚糖颗粒 | 戊二醛和硫脲交联磁性壳聚糖(TTG-MCTS) | C0 = 40 ~ 140 mg·L-1 pH = 5.00,0.60 g·L-1 | 161.3 | [ |
| 二乙烯三胺五乙酸功能化磁性壳聚糖(DTPA-MCS) | C0 = 10 ~ 100 mg·L-1 pH = 5.00,0.40 g·L-1 | 178.20 | [ | |
| 三聚磷酸钠交联磁性壳聚糖树脂(TPP-MCR) | C0 = 40 ~ 150 mg·L-1 pH = 3.00,0.40 g·L-1 | 166.70 | [ | |
| 离子印迹磁性壳聚糖微球(IMCR) | C0 = 15 ~ 420 mg·L-1 pH = 5.00,1.00 g·L-1 | 187.26 | [ | |
| 壳聚糖纤维 | 共混纤维吸附剂(PVP/CS fibers) | C0 = 1~ 270 mg·L-1 pH = 6.00,1.00 g·L-1 | 167±25 | [ |
| 壳聚糖纤维膜(CS-80%) | C0 = 10 ~ 100 mg·L-1 pH = 5.00,0.50 g·L-1 | 196.74 | [ | |
| 壳聚糖复合材料 | 壳聚糖/氧化石墨烯复合材料(CS/GO) | C0 = 2 ~ 100 mg·L-1 pH = 5.00,0.15 g·L-1 | 217.40 | [ |
| 多巴胺交联氧化石墨烯/壳聚糖气凝胶(GO@PDA/CS) | C0 = 10 ~ 220 mg·L-1 pH = 6.00,0.30 g·L-1 | 415.90 | [ | |
| 磷酸化的氧化石墨烯/壳聚糖复合材料(GO-CS-P) | C0 = 2 ~ 140 mg·L-1 pH = 5.00,0.05 g·L-1 | 779.44 | [ | |
| 磷酸化壳聚糖/羧甲基纤维素复合材料(CSP-CMCP) | C0 = 10 ~ 120 mg·L-1 pH = 5.00,0.05 g·L-1 | 977.54 | [ | |
| 壳聚糖-氧化石墨烯/ZIF泡沫吸附剂(GCZ8A) | C0 = 2 ~ 120 mg·L-1 pH = 8.00,0.20 g·L-1 | 361.01 | [ |
Table 1 Summarizes of chitosan-based materials and uranium adsorption abilities
| 类别 | 吸附剂名称 | 吸附条件 | 最高吸附量/(mg·g-1) | 文献 |
|---|---|---|---|---|
| 壳聚糖颗粒 | 戊二醛和硫脲交联磁性壳聚糖(TTG-MCTS) | C0 = 40 ~ 140 mg·L-1 pH = 5.00,0.60 g·L-1 | 161.3 | [ |
| 二乙烯三胺五乙酸功能化磁性壳聚糖(DTPA-MCS) | C0 = 10 ~ 100 mg·L-1 pH = 5.00,0.40 g·L-1 | 178.20 | [ | |
| 三聚磷酸钠交联磁性壳聚糖树脂(TPP-MCR) | C0 = 40 ~ 150 mg·L-1 pH = 3.00,0.40 g·L-1 | 166.70 | [ | |
| 离子印迹磁性壳聚糖微球(IMCR) | C0 = 15 ~ 420 mg·L-1 pH = 5.00,1.00 g·L-1 | 187.26 | [ | |
| 壳聚糖纤维 | 共混纤维吸附剂(PVP/CS fibers) | C0 = 1~ 270 mg·L-1 pH = 6.00,1.00 g·L-1 | 167±25 | [ |
| 壳聚糖纤维膜(CS-80%) | C0 = 10 ~ 100 mg·L-1 pH = 5.00,0.50 g·L-1 | 196.74 | [ | |
| 壳聚糖复合材料 | 壳聚糖/氧化石墨烯复合材料(CS/GO) | C0 = 2 ~ 100 mg·L-1 pH = 5.00,0.15 g·L-1 | 217.40 | [ |
| 多巴胺交联氧化石墨烯/壳聚糖气凝胶(GO@PDA/CS) | C0 = 10 ~ 220 mg·L-1 pH = 6.00,0.30 g·L-1 | 415.90 | [ | |
| 磷酸化的氧化石墨烯/壳聚糖复合材料(GO-CS-P) | C0 = 2 ~ 140 mg·L-1 pH = 5.00,0.05 g·L-1 | 779.44 | [ | |
| 磷酸化壳聚糖/羧甲基纤维素复合材料(CSP-CMCP) | C0 = 10 ~ 120 mg·L-1 pH = 5.00,0.05 g·L-1 | 977.54 | [ | |
| 壳聚糖-氧化石墨烯/ZIF泡沫吸附剂(GCZ8A) | C0 = 2 ~ 120 mg·L-1 pH = 8.00,0.20 g·L-1 | 361.01 | [ |

Fig.3 (a) The uranium adsorption capacity at different pH (C0 = 10 mg·L-1, V = 100 ml, sorbent dose (SD) = 0.03 g, time =8 h); (b) The uranium adsorption capacity at different initial concentration (V = 100 ml, SD = 0.03 g, pH = 5, time =8 h); (c) The experimental data and fitting curves of adsorption isotherms (V = 100 ml, SD = 0.03 g, pH = 5, time =8 h); (d) The experimental data and fitting curves of adsorption kinetics (V = 100 ml, SD = 0.03 g, pH = 5) (the counter ions of uranium were hydroxyl) [50]
| 吸附剂 | 吸附条件 | 最高吸附量/(mg·g-1) | 自然/模拟海水提铀 | 文献 |
|---|---|---|---|---|
丝瓜络 (LC-PAA-PEI) | C0 = 50 ~ 350 mg·L-1 pH = 6.00,0.40 g·L-1 | 444.40 | R>90% | [ |
大麻纤维 (HF-PE1-GDAC) | C0 = 5 ~ 400 mg·L-1 pH = 7.00,0.40 g·L-1 | 414.93 | R=71% | [ |
剑麻纤维 (ATMP-Sisal-150) | C0 = 10 mg·L-1 模拟海水 | 16 | 0.10 mg·g-1 自然海水 | [ |
纤维素纤维 (PAO-CFs) | C0 = 2 ~ 18 mg·L-1 pH = 5.00,0.30 g·L-1 | 52.88 | 1.22 mg U·(g Ads)-1 | [ |
Table 2 Summarizes of cellulose-based materials used for uranium extraction from seawater
| 吸附剂 | 吸附条件 | 最高吸附量/(mg·g-1) | 自然/模拟海水提铀 | 文献 |
|---|---|---|---|---|
丝瓜络 (LC-PAA-PEI) | C0 = 50 ~ 350 mg·L-1 pH = 6.00,0.40 g·L-1 | 444.40 | R>90% | [ |
大麻纤维 (HF-PE1-GDAC) | C0 = 5 ~ 400 mg·L-1 pH = 7.00,0.40 g·L-1 | 414.93 | R=71% | [ |
剑麻纤维 (ATMP-Sisal-150) | C0 = 10 mg·L-1 模拟海水 | 16 | 0.10 mg·g-1 自然海水 | [ |
纤维素纤维 (PAO-CFs) | C0 = 2 ~ 18 mg·L-1 pH = 5.00,0.30 g·L-1 | 52.88 | 1.22 mg U·(g Ads)-1 | [ |
| 1 | Liu C, Hsu P C, Xie J, et al. A half-wave rectified alternating current electrochemical method for uranium extraction from seawater[J]. Nat. Energy, 2017, 2(4): 1-8. |
| 2 | Hoffert M I, Caldeira K, Benford G, et al. Advanced technology paths to global climate stability: energy for a greenhouse planet[J]. Science, 2002, 298(5595): 981-987. |
| 3 | Gloeser S, Espinoza L T, Gandenberger C, et al. Raw material criticality in the context of classical risk assessment[J]. Resour. Policy, 2015, 44: 35-46. |
| 4 | Lindner H, Schneider E. Review of cost estimates for uranium recovery from seawater[J]. Energy Econ., 2015, 49: 9-22. |
| 5 | Kurttio P, Komulainen H, Leino A, et al. Bone as a possible target of chemical toxicity of natural uranium in drinking water[J]. Environ. Health Perspect., 2005, 113(1): 68-72. |
| 6 | Kim J, Tsouris C, Mayes R T, et al. Recovery of uranium from seawater: a review of current status and future research needs[J]. Sep. Sci. Technol., 2013, 48(3): 367-387. |
| 7 | Sholl D S, Lively R P. Seven chemical separations to change the world[J]. Nature, 2016, 532(7600): 435-437. |
| 8 | Abney C W, Mayes R T, Saito T, et al. Materials for the recovery of uranium from seawater[J]. Chem. Rev., 2017, 117(23): 13935-14013. |
| 9 | Bernhard G, Geipel G, Reich T, et al. Uranyl (Ⅵ) carbonate complex formation: validation of the Ca2UO2(CO3)3(aq.) species[J]. Radiochim. Acta, 2001, 89(8): 511-518. |
| 10 | Gill G A, Kuo L J, Janke C J, et al. The uranium from seawater program at the Pacific Northwest National Laboratory: overview of marine testing, adsorbent characterization, adsorbent durability, adsorbent toxicity, and deployment studies[J]. Ind. Eng. Chem. Res., 2016, 55(15): 4264-4277. |
| 11 | Kim J, Tsouris C, Oyola Y, et al. Uptake of uranium from seawater by amidoxime-based polymeric adsorbent: field experiments, modeling, and updated economic assessment[J]. Ind. Eng. Chem. Res., 2014, 53(14): 6076-6083. |
| 12 | Merroun M L, Raff J, Rossberg A, et al. Complexation of uranium by cells and S-layer sheets of Bacillus sphaericus JG-A12[J]. Appl. Environ. Microbiol., 2005, 71(9): 5532-5543. |
| 13 | Yang J, Volesky B. Biosorption of uranium on Sargassum biomass[J]. Water Res., 1999, 33(15): 3357-3363. |
| 14 | 谢志英, 肖化云, 王光辉. 交联壳聚糖对铀的吸附研究[J]. 环境工程学报, 2010, 4(8): 1749-1752. |
| Xie Z Y, Xiao H Y, Wang G H. Study on adsorption of uranium onto cross-linked chitosan[J]. Chin. J. Environ. Eng., 2010, 4(8): 1749-1752. | |
| 15 | 王学刚, 王光辉, 谢志英. 交联壳聚糖吸附处理低浓度含铀废水[J]. 金属矿山, 2010, (9): 133-136. |
| Wang X G, Wang G H, Xie Z Y. Adsorption of uranium in low concentration uranium-bearing wastewater onto cross-linked chitosan[J]. Metal Mine, 2010, (9): 133-136. | |
| 16 | 王凤菊, 丁海云, 宿延涛, 等. 壳聚糖基吸附剂的制备及其对铀的吸附性能研究[J]. 湿法冶金, 2013, 32(4): 243-247. |
| Wang F J, Ding H Y, Su Y T, et al. Study on synthesis and adsorption behavior for uranyl of chitosan-based adsorbents[J]. Hydrometallurgy of China, 2013, 32(4): 243-247. | |
| 17 | 邹晓亮, 王劲松, 莫辉艳, 等. β-环糊精交联磁性壳聚糖对U(Ⅵ)的吸附性能及机制研究[J]. 原子能科学技术, 2013, 47(4): 540-545. |
| Zou X L, Wang J S, Mo H Y, et al. Adsorption properties and mechanism of U (Ⅵ) onto β-cyclodextrin cross-linked magnetic chitosan[J]. Atomic Energy Science and Technology, 2013, 47(4): 540-545. | |
| 18 | 黄国林, 陈中胜, 梁喜珍, 等. 磁性交联壳聚糖对水溶液中铀(Ⅵ)离子的吸附行为[J]. 化工学报, 2012, 63(3): 834-840. |
| Huang G L, Chen Z S, Liang X Z, et al. Adsorption behavior of U(Ⅵ) ions from aqueous solution on novel cross-linked magnetic chitosan beads[J]. CIESC Journal, 2012, 63(3): 834-840. | |
| 19 | Sheng L, Zhou L, Huang Z, et al. Facile synthesis of magnetic chitosan nano-particles functionalized with N/O-containing groups for efficient adsorption of U (Ⅵ) from aqueous solution[J]. J. Radioanal. Nucl. Chem., 2016, 310(3): 1361-1371. |
| 20 | 陈小松, 周利民, 刘峙嵘. 三聚磷酸钠交联磁性壳聚糖树脂对铀酰离子的吸附特性[J]. 原子能科学技术, 2015, 49(6): 972-978. |
| Chen X S, Zhou L M, Liu Z R. Adsorption of UO22+ ion onto tripolyphosphate-crosslinked magnetic chitosan resin[J]. Atomic Energy Science and Technology, 2015, 49(6): 972-978. | |
| 21 | Zhou L, Shang C, Liu Z, et al. Selective adsorption of uranium (Ⅵ) from aqueous solutions using the ion-imprinted magnetic chitosan resins[J]. J. Colloid Interface Sci., 2012, 366(1): 165-172. |
| 22 | Barber P S, Kelley S P, Griggs C S, et al. Surface modification of ionic liquid-spun chitin fibers for the extraction of uranium from seawater: seeking the strength of chitin and the chemical functionality of chitosan[J]. Green Chem., 2014, 16(4): 1828-1836. |
| 23 | Li L, Li Y, Yang C. Chemical filtration of Cr (Ⅵ) with electrospun chitosan nanofiber membranes[J]. Carbohydr. Polym., 2016, 140: 299-307. |
| 24 | Christou C, Philippou K, Krasia-Christoforou T, et al. Uranium adsorption by polyvinylpyrrolidone/chitosan blended nanofibers[J]. Carbohydr. Polym., 2019, 219: 298-305. |
| 25 | Wang Y, Li Y, Li L, et al. Preparation of three-dimensional fiber-network chitosan films for the efficient treatment of uranium-contaminated effluents[J]. Water. Sci. Technol., 2020, 81(1): 52-61. |
| 26 | Zhao G, Wen T, Yang X, et al. Preconcentration of U (Ⅵ) ions on few-layered graphene oxide nanosheets from aqueous solutions[J]. Dalton Trans., 2012, 41(20): 6182-6188. |
| 27 | 李仕友, 史冬峰, 唐振平, 等. 壳聚糖/氧化石墨烯复合材料吸附U(Ⅵ) 的特性与机理[J]. 环境科学学报, 2017, 37(4): 1388-1395. |
| Li S Y, Shi D F, Tang Z P, et al. Characteristics and mechanism of uranium (Ⅵ) adsorption by chitosan/graphene oxide composite[J]. Acta Scientiae Circumstantiae, 2017, 37(4): 1388-1395. | |
| 28 | Liao Y, Wang M, Chen D. Preparation of polydopamine-modified graphene oxide/chitosan aerogel for uranium (Ⅵ) adsorption[J]. Ind. Eng. Chem. Res., 2018, 57(25): 8472-8483. |
| 29 | Cai Y, Wu C, Liu Z, et al. Fabrication of a phosphorylated graphene oxide-chitosan composite for highly effective and selective capture of U (Ⅵ) [J]. Environmental Science: Nano, 2017, 4(9): 1876-1886. |
| 30 | Cai Y, Chen L, Yang S, et al. Rational synthesis of novel phosphorylated chitosan-carboxymethyl cellulose composite for highly effective decontamination of U (Ⅵ)[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(5): 5393-5403. |
| 31 | Guo X, Yang H, Liu Q, et al. A chitosan-graphene oxide/ZIF foam with anti-biofouling ability for uranium recovery from seawater[J]. Chem. Eng. J., 2020, 382: 122850. |
| 32 | 郑伟娜, 夏良树, 王晓, 等. 谷壳对铀(Ⅵ)的吸附性能及机理研究[J]. 原子能科学技术, 2011, 45(5): 534-540. |
| Zheng W N, Xia L S, Wang X, et al. Adsorption behavior and mechanism of uranium by chaff[J]. Atomic Energy Science and Technology, 2011, 45(5): 534-540. | |
| 33 | 冯媛, 易发成. 稻壳对铀吸附性能的研究[J]. 原子能科学技术, 2011, 45(2): 161-167. |
| Feng Y, Yi F C. Adsorptive property of rice husk for uranium[J]. Atomic Energy Science and Technology, 2011, 45(2): 161-167. | |
| 34 | 龙逸云, 李金轩, 李小燕. 改性稻壳对废水中铀的吸附性能[J]. 济南大学学报(自然科学版), 2013, 27(4): 386-389. |
| Long Y Y, Li J X, Li X Y. Adsorptive property of modified chaff for uranium in waste water[J]. Journal of University of Jinan (Sci. & Tech.), 2013, 27(4): 386-389. | |
| 35 | 王晓, 夏良树, 郑伟娜, 等. 改性麦秸对U(Ⅵ)的吸附性能及机理[J]. 过程工程学报, 2010, 10(6): 1084-1090. |
| Wang X, Xia L S, Zheng W N, et al. Adsorption behavior and mechanism of uranium (Ⅵ) on modified wheat straw[J]. The Chinese Journal of Process Engineering, 2010, 10(6): 1084-1090. | |
| 36 | 曹炎森, 王洪江, 喻清. 槟榔渣活性炭对废水中铀(Ⅵ)的吸附实验[J]. 矿业研究与开发, 2019, 39(10): 95-98. |
| Cao Y S, Wang H J,Yu Q. Adsorption experiments of areca residue activated carbon on uranium (Ⅵ) in wastewater[J]. Mining R & D, 2019, 39(10): 95-98. | |
| 37 | 李小燕, 刘义保, 花明, 等. 花生壳吸附溶液中铀的研究[J]. 水处理技术, 2012, 38(3): 38-40+44. |
| Li X Y, Liu Y B, Hua M, et al. Study on adsorption of uranium in aqueous solution by peanut shells[J]. Technology of Water Treatment, 2012, 38(3): 38-40+44. | |
| 38 | 李小燕, 张明, 刘义保, 等. 花生壳活性炭吸附溶液中的铀[J]. 化工环保, 2013, 33(3): 202-205. |
| Li X Y, Zhang M, Liu Y B, et al. Adsorption of uranium from aqueous solution with peanut shell activated carbon[J]. Environmental Protection of Chemical Industry, 2013, 33(3): 202-205. | |
| 39 | Ma D, Hu S, Li Y, et al. Adsorption of uranium on phosphoric acid-activated peanut shells[J]. Sep. Sci. Technol., 2020, 55(9): 1623-1635. |
| 40 | 宋勇, 吕俊文, 张园园, 等. 改性锯末对铀的吸附机理研究[J]. 安全与环境工程, 2018, 25(3): 86-92+129. |
| Song Y, Lyu J W, Zhang Y Y, et al. Study of adsorption mechanism of uranium by modified sawdust[J]. Safety and Environmental Engineering, 2018, 25(3): 86-92+129. | |
| 41 | 王翠苹, 徐伟昌, 庞红顺. 榕树叶对铀吸附的研究[J]. 环境科学与技术, 2004, (2): 19-20+115. |
| Wang C P, Xu W C, Pang H S. Study on uranium biosorption by banyan leaves[J]. Environmental Science & Technology, 2004, (2): 19-20+115. | |
| 42 | 聂小琴, 董发勤, 刘明学, 等. 生物吸附剂梧桐树叶对铀的吸附行为研究[J]. 光谱学与光谱分析, 2013, 33(5): 1290-1294. |
| Nie X Q, Dong F Q, Liu M X, et al. Characteristics of U (Ⅵ) biosorption by biological adsorbent of platanus leaves[J]. Spectroscopy and Spectral Analysis, 2013, 33(5): 1290-1294. | |
| 43 | 吴素强, 刘永, 何鹏. 椰壳活性炭对水溶液中铀(Ⅵ)的吸附研究[J]. 安徽农学通报, 2019, 25(7): 126-129. |
| Wu S Q, Liu Y, He P. Adsorption of uranium (Ⅵ) in aqueous solution by coconut shell activated carbon[J]. Anhui Agri. Sci. Bull., 2019, 25(7): 126-129. | |
| 44 | 夏良树, 谭凯旋, 邓帛辉, 等. 四种生物吸附剂对铀的吸附性能研究[J]. 化学工程, 2008, (2): 9-12. |
| Xia L S, Tan K X, Deng B H, et al. Study on adsorptive capacity of uranium by 4 biosorbents[J]. Chemical Engineering (China), 2008, (2): 9-12. | |
| 45 | Parab H, Joshi S, Shenoy N, et al. Uranium removal from aqueous solution by coir pith: equilibrium and kinetic studies[J]. Bioresour. Technol., 2005, 96(11): 1241-1248. |
| 46 | Su S, Chen R, Liu Q, et al. High efficiency extraction of U (Ⅵ) from seawater by incorporation of polyethyleneimine, polyacrylic acid hydrogel and Luffa cylindrical fibers[J]. Chem. Eng. J., 2018, 345: 526-535. |
| 47 | 王哲, 易发成, 冯媛. 铀在木纤维上的吸附行为及机理分析[J]. 原子能科学技术, 2015, 49(2): 263-272. |
| Wang Z, Yi F C, Feng Y. Adsorption behavior and mechanism of uranium on wood fiber[J]. Atomic Energy Science and Technology, 2015, 49(2): 263-272. | |
| 48 | Bai Z, Liu Q, Zhang H, et al. A novel 3D reticular anti-fouling bio-adsorbent for uranium extraction from seawater: polyethylenimine and guanidyl functionalized hemp fibers[J]. Chem. Eng. J., 2020, 382: 122555. |
| 49 | Tellería-Narvaez A, Talavera-Ramos W, Dos Santos L, et al. Functionalized natural cellulose fibres for the recovery of uranium from seawater[J]. RSC Adv., 2020, 10(11): 6654-6657. |
| 50 | Wang Y, Zhang Y, Li Q, et al. Amidoximated cellulose fiber membrane for uranium extraction from simulated seawater[J]. Carbohydr. Polym., 2020, 245: 116627. |
| 51 | Lovley D R, Phillips E J P, Gorby Y A, et al. Microbial reduction of uranium[J]. Nature, 1991, 350(6317): 413-416. |
| 52 | El-Helow E R, Sabry S A, Amer R M. Cadmium biosorption by a cadmium resistant strain of Bacillus thuringiensis: regulation and optimization of cell surface affinity for metal cations[J]. BioMetals, 2000, 13(4): 273-280. |
| 53 | Liu M, Dong F, Yan X, et al. Biosorption of uranium by Saccharomyces cerevisiae and surface interactions under culture conditions[J]. Bioresour. Technol., 2010, 101(22): 8573-8580. |
| 54 | 胡民火. 生物炭介导微生物还原固定U(Ⅵ) 研究[D]. 赣州: 江西理工大学, 2019. |
| Hu M H. The research on biochar-mediated microbial reduction and immobilization of U (Ⅵ) [D]. Ganzhou: Jiangxi University of Science and Technology, 2019. | |
| 55 | 朱定国, 谢水波, 刘迎九, 等. 植酸改性黑曲霉菌对水中U(Ⅵ)的吸附试验研究[J]. 科学技术与工程, 2019, 19(21): 344-351. |
| Zhu D G, Xie S B, Liu Y J, et al. Experimental study on uranium(Ⅵ) adsorption in water by phytic acid modified Aspergillus niger[J]. Science Technology and Engineering, 2019, 19(21): 344-351. | |
| 56 | 马佳林, 聂小琴, 董发勤, 等. 三种微生物对铀的吸附行为研究[J]. 中国环境科学, 2015, 35(3): 825-832. |
| Ma J L, Nie X Q, Dong F Q, et al. The adsorption behavior on uranium by three kinds of microorganisms[J]. China Environmental Science, 2015, 35(3): 825-832. | |
| 57 | 谭文发, 吕俊文, 唐东山. 生物技术处理含铀废水的研究进展[J]. 生物技术通报, 2015, 31(3): 82-87. |
| Tan W F, Lyu J W, Tang D S. Research advances of biological treatment of uranium-containing wastewater[J]. Biotechnology Bulletin, 2015, 31(3): 82-87. | |
| 58 | 陈灿, 王建龙. 酿酒酵母吸附重金属离子的研究进展[J]. 中国生物工程杂志, 2006, (1): 69-76. |
| Chen C, Wang J L. Review on biosorption of heavy metal by Saccharomyces cerevisiae[J]. China Biotechnology, 2006, (1): 69-76. | |
| 59 | Wang J, Chen C. Biosorption of heavy metals by Saccharomyces cerevisiae: a review[J]. Biotechnol. Adv., 2006, 24(5): 427-451. |
| 60 | Wang J, Chen C. Biosorbents for heavy metals removal and their future[J]. Biotechnol. Adv., 2009, 27(2): 195-226. |
| 61 | Chen C, Hu J, Wang J. Biosorption of uranium by immobilized Saccharomyces cerevisiae[J]. J. Environ. Radioact., 2020, 213: 106158. |
| 62 | 成彬, 李乐, 丁德馨, 等. 黑曲霉磁性生物吸附剂制备及其吸附低浓度铀性能研究[J]. 应用化工, 2018, 47(2): 219-223. |
| Cheng B, Li L, Ding D X, et al. Preparation of nano-Fe3O4 modified Aspergillus niger and its properties for adsorption of low concentration uranium (Ⅵ) [J]. Applied Chemical Industry, 2018, 47(2): 219-223. | |
| 63 | Ding D X, Xin X, Li L, et al. Removal and recovery of U (Ⅵ) from low concentration radioactive wastewater by ethylenediamine-modified biomass of Aspergillus niger[J]. Water, Air, Soil Pollut., 2014, 225(12): 2206. |
| 64 | Li L, Hu N, Ding D, et al. Adsorption and recovery of U (Ⅵ) from low concentration uranium solution by amidoxime modified Aspergillus niger[J]. RSC Adv., 2015, 5(81): 65827-65839. |
| 65 | 庞翠, 刘云海, 李敏, 等. 海藻酸钠固定化桔青霉微球对铀的吸附研究[J]. 化学研究与应用, 2010, 22(11): 1441-1445. |
| Pang C, Liu Y H, Li M, et al. Study on the biosorption property of sodium alginate immobilized penicillium citrinum beads to uranium (Ⅵ) [J]. Chemical Research and Application, 2010, 22(11): 1441-1445. | |
| 66 | 李跃, 谢水波, 林达, 等. 小球藻对U(Ⅵ)的生物吸附特性[J]. 微生物学通报, 2008, (5): 760-764. |
| Li Y, Xie S B, Lin D, et al. The character of U (Ⅵ) biosorption by Chlorella pyrenoidosa[J]. Microbiology China, 2008, (5): 760-764. | |
| 67 | 聂小琴, 董发勤, 刘宁, 等. 少根紫萍对水中U(Ⅵ)的吸附和矿化行为研究[J]. 光谱学与光谱分析, 2015, 35(9): 2613-2619. |
| Nie X Q, Dong F Q, Liu N, et al. Biosorption and biomineralization of uranium (Ⅵ) from aqueous solutions by Landoltia punctata[J]. Spectroscopy and Spectral Analysis, 2015, 35(9): 2613-2619. | |
| 68 | Jiang X, Wang H, Hu E, et al. Efficient adsorption of uranium from aqueous solutions by microalgae based aerogel[J]. Microporous Mesoporous Mater., 2020, 305: 110383. |
| 69 | Zhou L, Bosscher M, Zhang C, et al. A protein engineered to bind uranyl selectively and with femtomolar affinity[J]. Nat. Chem., 2014, 6(3): 236-241. |
| 70 | Kou S, Yang Z, Sun F. Protein hydrogel microbeads for selective uranium mining from seawater[J]. ACS Appl. Mater. Interfaces, 2017, 9(3): 2035-2039. |
| 71 | Yuan Y, Yu Q, Wen J, et al. Ultrafast and highly selective uranium extraction from seawater by hydrogel-like spidroin-based protein fiber[J]. Angew. Chem., Int. Ed. Engl., 2019, 58(34): 11785-11790. |
| 72 | Deng G, Zhang Y, Luo X, et al. Direct extraction of U (Ⅵ) from a simulated saline solution by alkali-activated collagen fiber[J]. J. Radioanal. Nucl. Chem., 2018, 318(2): 1109-1118. |
| 73 | Luo W, Xiao G, Tian F, et al. Engineering robust metal–phenolic network membranes for uranium extraction from seawater[J]. Energy Environ. Sci., 2019, 12(2): 607-614. |
| 74 | Wang D, Song J, Lin S, et al. A marine‐inspired hybrid sponge for highly efficient uranium extraction from seawater[J]. Adv. Funct. Mater., 2019, 29(32):1901009. |
| 75 | Bai Z, Liu Q, Zhang H, et al. Mussel-inspired anti-biofouling and robust hybrid nanocomposite hydrogel for uranium extraction from seawater[J]. J. Hazard. Mater., 2020, 381: 120984. |
| [1] | Minghao SONG, Fei ZHAO, Shuqing LIU, Guoxuan LI, Sheng YANG, Zhigang LEI. Multi-scale simulation and study of volatile phenols removal from simulated oil by ionic liquids [J]. CIESC Journal, 2023, 74(9): 3654-3664. |
| [2] | Bingchun SHENG, Jianguo YU, Sen LIN. Study on lithium resource separation from underground brine with high concentration of sodium by aluminum-based lithium adsorbent [J]. CIESC Journal, 2023, 74(8): 3375-3385. |
| [3] | Ruihang ZHANG, Pan CAO, Feng YANG, Kun LI, Peng XIAO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Analysis of key parameters affecting product purity of natural gas ethane recovery process via ZIF-8 nanofluid [J]. CIESC Journal, 2023, 74(8): 3386-3393. |
| [4] | Yan GAO, Peng WU, Chao SHANG, Zejun HU, Xiaodong CHEN. Preparation of magnetic agarose microspheres based on a two-fluid nozzle and their protein adsorption properties [J]. CIESC Journal, 2023, 74(8): 3457-3471. |
| [5] | Ji CHEN, Ze HONG, Zhao LEI, Qiang LING, Zhigang ZHAO, Chenhui PENG, Ping CUI. Study on coke dissolution loss reaction and its mechanism based on molecular dynamics simulations [J]. CIESC Journal, 2023, 74(7): 2935-2946. |
| [6] | Yuanliang ZHANG, Xinqi LUAN, Weige SU, Changhao LI, Zhongxing ZHAO, Liqin ZHOU, Jianmin CHEN, Yan HUANG, Zhenxia ZHAO. Study on selective extraction of nicotine by ionic liquids composite extractant and DFT calculation [J]. CIESC Journal, 2023, 74(7): 2947-2956. |
| [7] | Jie WANG, Xiaolin QIU, Ye ZHAO, Xinyang LIU, Zhongqiang HAN, Yong XU, Wenhan JIANG. Preparation and properties of polyelectrolyte electrostatic deposition modified PHBV antioxidant films [J]. CIESC Journal, 2023, 74(7): 3068-3078. |
| [8] | Caihong LIN, Li WANG, Yu WU, Peng LIU, Jiangfeng YANG, Jinping LI. Effect of alkali cations in zeolites on adsorption and separation of CO2/N2O [J]. CIESC Journal, 2023, 74(5): 2013-2021. |
| [9] | Zheng ZHANG, Yongping HE, Haidong SUN, Rongzi ZHANG, Zhengping SUN, Jinlan CHEN, Yixuan ZHENG, Xiao DU, Xiaogang HAO. Electrochemically switched ion exchange device with serpentine flow field for selective extraction of lithium [J]. CIESC Journal, 2023, 74(5): 2022-2033. |
| [10] | Chenxin LI, Yanqiu PAN, Liu HE, Yabin NIU, Lu YU. Carbon membrane model based on carbon microcrystal structure and its gas separation simulation [J]. CIESC Journal, 2023, 74(5): 2057-2066. |
| [11] | Shaoyun CHEN, Dong XU, Long CHEN, Yu ZHANG, Yuanfang ZHANG, Qingliang YOU, Chenglong HU, Jian CHEN. Preparation and adsorption properties of monolayer polyaniline microsphere arrays [J]. CIESC Journal, 2023, 74(5): 2228-2238. |
| [12] | Xuanjun WU, Chao WANG, Zijian CAO, Weiquan CAI. Deep learning model of fixed bed adsorption breakthrough curve hybrid-driven by data and physical information [J]. CIESC Journal, 2023, 74(3): 1145-1160. |
| [13] | Xiangshang CHEN, Zhenjie MA, Xihua REN, Yue JIA, Xiaolong LYU, Huayan CHEN. Preparation and mass transfer efficiency of three-dimensional network extraction membrane [J]. CIESC Journal, 2023, 74(3): 1126-1133. |
| [14] | Yu PAN, Zihang WANG, Jiayun WANG, Ruzhu WANG, Hua ZHANG. Heat and moisture performance study of Cur-LiCl coated heat exchanger [J]. CIESC Journal, 2023, 74(3): 1352-1359. |
| [15] | Jiahao JIANG, Xiaole HUANG, Jiyun REN, Zhengrong ZHU, Lei DENG, Defu CHE. Qualitative and quantitative study on Pb2+ adsorption by biochar in solution [J]. CIESC Journal, 2023, 74(2): 830-842. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||