CIESC Journal ›› 2021, Vol. 72 ›› Issue (1): 543-554.DOI: 10.11949/0438-1157.20201045
• Catalysis, kinetics and reactors • Previous Articles Next Articles
GE Bingqing( ),YIN Yixuan,WANG Yaxi,ZHANG Hongwei,YUAN Pei(
),YIN Yixuan,WANG Yaxi,ZHANG Hongwei,YUAN Pei( )
)
Received:2020-07-28
Revised:2020-10-13
Online:2021-01-05
Published:2021-01-05
Contact:
YUAN Pei
通讯作者:
袁珮
作者简介:葛冰青(1995—),女,博士,基金资助:CLC Number:
GE Bingqing, YIN Yixuan, WANG Yaxi, ZHANG Hongwei, YUAN Pei. Study of solvent effect on the dissolution, size, structure and catalytic hydrogenation of nitrile butadiene rubber[J]. CIESC Journal, 2021, 72(1): 543-554.
葛冰青, 阴义轩, 王亚溪, 张宏伟, 袁珮. 溶剂对丁腈橡胶溶解、尺寸、结构和催化加氢的影响研究[J]. 化工学报, 2021, 72(1): 543-554.
Add to citation manager EndNote|Ris|BibTeX
| Structure | Type of H | δ |
|---|---|---|
| 1,4 unit |  CH— CH— | 5.50 |
| —CH2— | 2.15 | |
| —CH(CN)— | 2.65 | |
| —CH2CH(CN)— | 1.70 | |
| 1,2 unit |  CH— and CH— and  CH2 CH2 | 4.90—5.10 |
Table 1 Chemical shifts of H protons in different microstructures of NBR
| Structure | Type of H | δ |
|---|---|---|
| 1,4 unit |  CH— CH— | 5.50 |
| —CH2— | 2.15 | |
| —CH(CN)— | 2.65 | |
| —CH2CH(CN)— | 1.70 | |
| 1,2 unit |  CH— and CH— and  CH2 CH2 | 4.90—5.10 |
Fig.3 Pictures for NBR in weak electrophilic solvents before (a) and after (b) 24 h (Solvents from left to right: TOL,OX,MX,PX,TIPB,MCB,DCM,TCM and EDC)

Fig.4 Pictures for NBR in electron donating solvents before (a) and after (b) 24 h (Solvents from left to right: ACE,MEK,MIBK,CYC,NMP,MAC,EA,IBAC and DMF)
| 编号 | 溶剂 | [η]/(L/g) | ηsp | 编号 | 溶剂 | [η]/(L/g) | ηsp |
|---|---|---|---|---|---|---|---|
| a1 | DCM | 1.84×10-1 | 2.55 | b1 | ACE | 6.88×10-2 | 1.13 |
| a2 | TCM | 1.32×10-1 | 2.17 | b2 | MEK | 6.17×10-2 | 0.92 |
| a3 | EDC | 1.13×10-1 | 2.00 | b3 | MIBK | 5.68×10-2 | 0.97 |
| a4 | MCB | 1.03×10-1 | 1.48 | b4 | CYC | 1.21×10-1 | 1.63 |
| a5 | TOL | 4.90×10-2 | 0.61 | b5 | NMP | 1.35×10-1 | 1.68 |
| a6 | OX | 4.91×10-2 | 0.60 | b6 | MAC | 6.59×10-2 | 1.06 |
| a7 | MX | 2.72×10-2 | 0.38 | b7 | EA | 6.85×10-2 | 1.07 |
| a8 | PX | 3.83×10-2 | 0.41 | b8 | IBAC | 5.13×10-2 | 0.78 |
| a9 | TIPB | 2.31×10-2 | 0.24 | b9 | DMF | 9.56×10-2 | 1.38 |
Table 2 Viscosity of NBR solutions in weak electrophilic solvents (a1—a9) and electron donating solvents (b1—b9)
| 编号 | 溶剂 | [η]/(L/g) | ηsp | 编号 | 溶剂 | [η]/(L/g) | ηsp |
|---|---|---|---|---|---|---|---|
| a1 | DCM | 1.84×10-1 | 2.55 | b1 | ACE | 6.88×10-2 | 1.13 |
| a2 | TCM | 1.32×10-1 | 2.17 | b2 | MEK | 6.17×10-2 | 0.92 |
| a3 | EDC | 1.13×10-1 | 2.00 | b3 | MIBK | 5.68×10-2 | 0.97 |
| a4 | MCB | 1.03×10-1 | 1.48 | b4 | CYC | 1.21×10-1 | 1.63 |
| a5 | TOL | 4.90×10-2 | 0.61 | b5 | NMP | 1.35×10-1 | 1.68 |
| a6 | OX | 4.91×10-2 | 0.60 | b6 | MAC | 6.59×10-2 | 1.06 |
| a7 | MX | 2.72×10-2 | 0.38 | b7 | EA | 6.85×10-2 | 1.07 |
| a8 | PX | 3.83×10-2 | 0.41 | b8 | IBAC | 5.13×10-2 | 0.78 |
| a9 | TIPB | 2.31×10-2 | 0.24 | b9 | DMF | 9.56×10-2 | 1.38 |
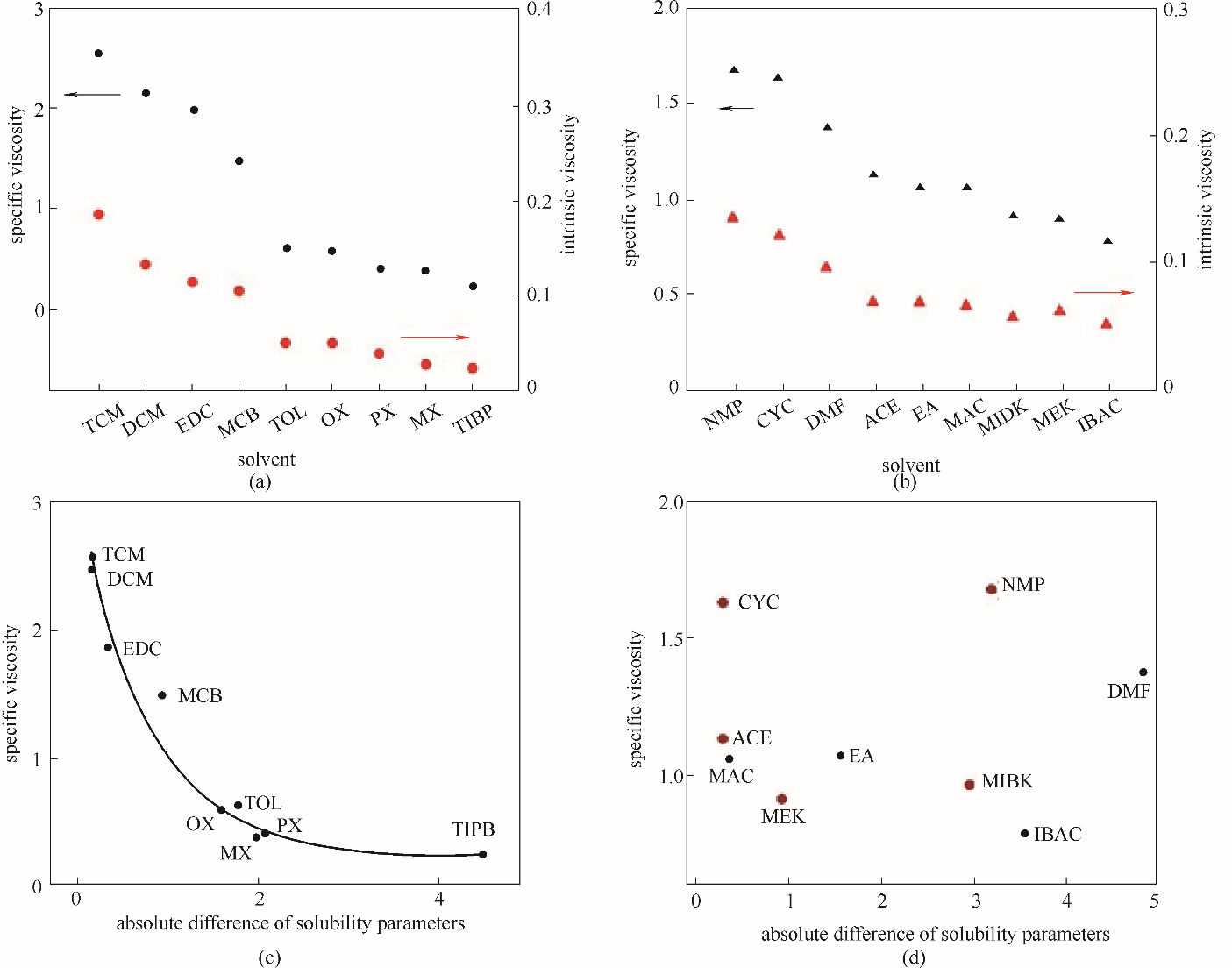
Fig.6 The relationship between the characteristic viscosity and the specific viscosity of NBR solutions in weak electrophilic solvents (a) and electron donating solvents (b); the relationship between specific viscosity of NBR solutions and absolute difference of solubility parameters in weak electrophilic solvents (c) and electron donating solvents (d) (absolute difference of solubility parameters ?δ=|δNBR-δsolvent|)
溶剂 种类 | 溶剂 | Bond length/? | |||||
|---|---|---|---|---|---|---|---|
1,4-C C— C— | 1,2-C C— C— | ||||||
| (1) | (2) | (3) | (4) | (5) | |||
弱亲 电子 溶剂 | TOL | 1.3391 | 1.3355 | 1.3400 | 1.3370 | 1.3371 | 1.3348 |
| OX | 1.3391 | 1.3355 | 1.3401 | 1.3371 | 1.3371 | 1.3348 | |
| TCM | 1.3395 | 1.3359 | 1.3405 | 1.3375 | 1.3376 | 1.3351 | |
| MCB | 1.3397 | 1.3359 | 1.3406 | 1.3376 | 1.3376 | 1.3351 | |
给电子 溶剂 | EA | 1.3396 | 1.3359 | 1.3406 | 1.3376 | 1.3376 | 1.3352 |
| MAC | 1.3397 | 1.3360 | 1.3407 | 1.3376 | 1.3377 | 1.3352 | |
| CYC | 1.3398 | 1.3362 | 1.3409 | 1.3378 | 1.3379 | 1.3354 | |
| MEK | 1.4000 | 1.3364 | 1.3411 | 1.3379 | 1.3381 | 1.3356 | |
| ACE | 1.3399 | 1.3363 | 1.3410 | 1.3378 | 1.3380 | 1.3355 | |
| 气相 | NBR | 1.3384 | 1.3348 | 1.3392 | 1.3365 | 1.3365 | 1.3346 |
Table 3 The length of CC double bonds at different positions of NBR molecule in different solvents
溶剂 种类 | 溶剂 | Bond length/? | |||||
|---|---|---|---|---|---|---|---|
1,4-C C— C— | 1,2-C C— C— | ||||||
| (1) | (2) | (3) | (4) | (5) | |||
弱亲 电子 溶剂 | TOL | 1.3391 | 1.3355 | 1.3400 | 1.3370 | 1.3371 | 1.3348 |
| OX | 1.3391 | 1.3355 | 1.3401 | 1.3371 | 1.3371 | 1.3348 | |
| TCM | 1.3395 | 1.3359 | 1.3405 | 1.3375 | 1.3376 | 1.3351 | |
| MCB | 1.3397 | 1.3359 | 1.3406 | 1.3376 | 1.3376 | 1.3351 | |
给电子 溶剂 | EA | 1.3396 | 1.3359 | 1.3406 | 1.3376 | 1.3376 | 1.3352 |
| MAC | 1.3397 | 1.3360 | 1.3407 | 1.3376 | 1.3377 | 1.3352 | |
| CYC | 1.3398 | 1.3362 | 1.3409 | 1.3378 | 1.3379 | 1.3354 | |
| MEK | 1.4000 | 1.3364 | 1.3411 | 1.3379 | 1.3381 | 1.3356 | |
| ACE | 1.3399 | 1.3363 | 1.3410 | 1.3378 | 1.3380 | 1.3355 | |
| 气相 | NBR | 1.3384 | 1.3348 | 1.3392 | 1.3365 | 1.3365 | 1.3346 |

Fig.9 The bond length of the CC double bonds at different positions of NBR molecule in weak electrophilic solvents (a) and electron donating solvents (b)
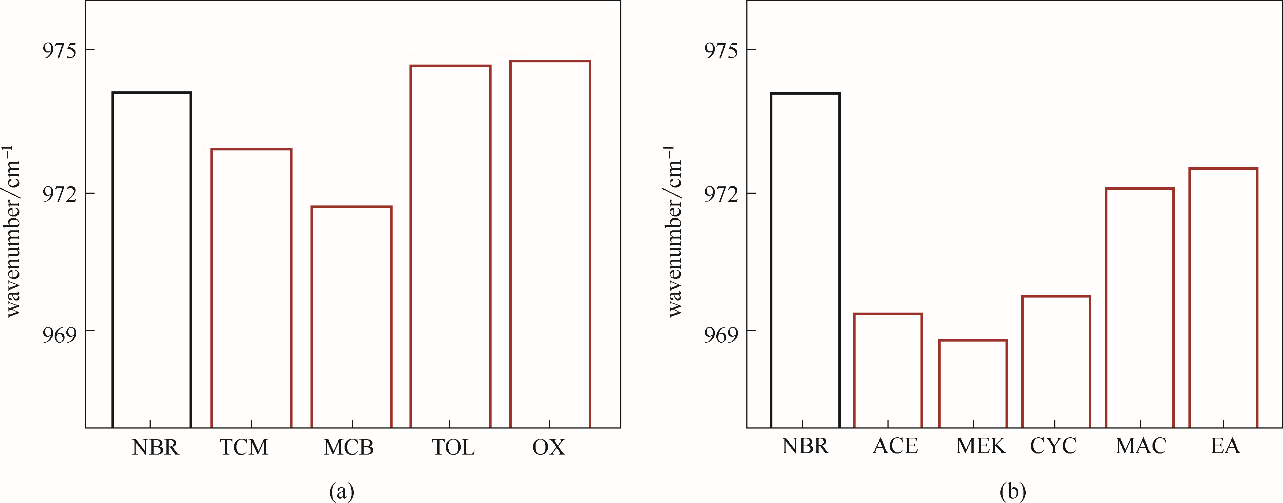
Fig.10 The stretching vibration peak position of the 1, 4-CC— double bond of NBR chain in weak electrophilic solvents (a) and electron donating solvents (b)
溶剂 类型 | 溶剂 | 介电 常数 | ELUMO/ a.u. | EHOMO/a.u. | ?Egap/a.u. | ?G/(kcal/mol) | 偶极矩/D |
|---|---|---|---|---|---|---|---|
弱亲 电子 溶剂 | TOL | 2.24 | 0.01201 | -0.22966 | 0.24167 | -19.14 | 5.99 |
| OX | 2.27 | 0.01264 | -0.23036 | 0.24300 | -18.69 | 6.07 | |
| TCM | 4.90 | 0.01176 | -0.22935 | 0.24111 | -25.87 | 6.62 | |
| MCB | 5.65 | 0.00988 | -0.22837 | 0.23825 | -24.77 | 6.79 | |
给电子 溶剂 | EA | 6.02 | 0.01037 | -0.22847 | 0.23884 | -22.19 | 6.80 |
| MAC | 6.68 | 0.00996 | -0.22838 | 0.23834 | -22.29 | 6.89 | |
| CYC | 18.30 | 0.01206 | -0.22974 | 0.24180 | -22.20 | 7.35 | |
| MEK | 18.51 | 0.01245 | -0.23065 | 0.24310 | -26.31 | 7.50 | |
| ACE | 20.49 | 0.01226 | -0.23006 | 0.24232 | -25.47 | 7.42 | |
| 气相 | NBR | 0 | 0.00420 | -0.22990 | 0.23410 | 0 | 5.01 |
Table 4 Frontier orbit energy, energy gap, solvation energy and dipole moments of NBR in different solvents
溶剂 类型 | 溶剂 | 介电 常数 | ELUMO/ a.u. | EHOMO/a.u. | ?Egap/a.u. | ?G/(kcal/mol) | 偶极矩/D |
|---|---|---|---|---|---|---|---|
弱亲 电子 溶剂 | TOL | 2.24 | 0.01201 | -0.22966 | 0.24167 | -19.14 | 5.99 |
| OX | 2.27 | 0.01264 | -0.23036 | 0.24300 | -18.69 | 6.07 | |
| TCM | 4.90 | 0.01176 | -0.22935 | 0.24111 | -25.87 | 6.62 | |
| MCB | 5.65 | 0.00988 | -0.22837 | 0.23825 | -24.77 | 6.79 | |
给电子 溶剂 | EA | 6.02 | 0.01037 | -0.22847 | 0.23884 | -22.19 | 6.80 |
| MAC | 6.68 | 0.00996 | -0.22838 | 0.23834 | -22.29 | 6.89 | |
| CYC | 18.30 | 0.01206 | -0.22974 | 0.24180 | -22.20 | 7.35 | |
| MEK | 18.51 | 0.01245 | -0.23065 | 0.24310 | -26.31 | 7.50 | |
| ACE | 20.49 | 0.01226 | -0.23006 | 0.24232 | -25.47 | 7.42 | |
| 气相 | NBR | 0 | 0.00420 | -0.22990 | 0.23410 | 0 | 5.01 |
| 1 | 孙黎, 毕忠华, 高梅, 等. 氢化丁腈橡胶的研究进展[J]. 特种橡胶制品, 2020, 41(1): 60-64. |
| Sun L, Bi Z H, Gao M, et al. Research progress of hydrogenated nitrile butadiene rubber[J]. Special Purpose Rubber Products, 2020, 41(1): 60-64. | |
| 2 | Budrugeac P. Thermooxidative degradation of some nitrile-butadiene rubbers[J]. Polymer Degradation and Stability, 1992, 38(2): 165-172. |
| 3 | Zhao J, Yang R, Iervolino R, et al. Investigation of crosslinking in the thermooxidative aging of nitrile–butadiene rubber[J]. Journal of Applied Polymer Science, 2015, 132(3): 41319. |
| 4 | Ciesielski A. An Introduction to Rubber Technology[M]. Smithers Rapra Technology, 1999. |
| 5 | Zhou W, Peng X. Preparation of a novel homogeneous bimetallic Rhodium/Palladium ionic catalyst and its application for the catalytic hydrogenation of nitrile butadiene rubber[J]. Journal of Organometallic Chemistry, 2016, 823: 76-82. |
| 6 | Ning S, Yang S, Wei X, et al. Selective hydrogenation of nitrile-butadiene rubber catalyzed by thermoregulated phase transfer phosphine rhodium complex[J]. Journal of Applied Polymer Science, 2012, 123(2): 1040-1046. |
| 7 | Zhou W, Peng X. Preparation and catalytic application of Rh3+/Ru3+ bimetallic catalyst stabilized by triolefinic macrocycle-terminated poly (propylene imine) dendrimer[J]. Macromolecular Research, 2016, 24(11): 1024-1029. |
| 8 | Zhou W, Zhang D, Wang Y, et al. Preparation of Rh metallic nanoparticle stabilized by 15-membered nitrogen-containing triolefinic macrocycle-ended poly (propylene imine) dendrimer and its catalytic hydrogenation for nitrile-butadiene rubber[J]. Colloid and Polymer Science, 2017, 295(5): 767-772. |
| 9 | Kubo Y, Ohura K. Process for hydrogenation of conjugated diene polymers: US4337329[P]. 1982-06-29. |
| 10 | Kubo Y, Ohura K. Process for hydrogenating conjugated diene polymers: US4452951[P]. 1984-04-09. |
| 11 | Zou R, Li C, Zhang L, et al. Selective hydrogenation of nitrile butadiene rubber (NBR) with rhodium nanoparticles supported on carbon nanotubes at room temperature[J]. Catalysis Communications, 2016, 81: 4-9. |
| 12 | Cao P, Wu M, Zou R, et al. A ternary Rh complex catalyst highly active and stable in the hydrogenation of acrylonitrile–butadiene rubber[J]. New Journal of Chemistry, 2015, 39: 1583-1586. |
| 13 | Cao P, Huang C, Zhang L, et al. One-step fabrication of RGO/HNBR composites via selective hydrogenation of NBR with graphene-based catalyst[J]. Royal Society of Chemistry Advances, 2015, 5(51): 41098-41102. |
| 14 | Chen J, Ma L, Cheng T, et al. [J]. Stable and recyclable Pd catalyst supported on modified silica hollow microspheres with macroporous shells for enhanced catalytic hydrogenation of NBR[J]. Journal of Materials Science, 2018, 53: 15064-15080. |
| 15 | Chen J, Wu Z, Liu H, et al. A surface-cofunctionalized silica supported palladium catalyst for selective hydrogenation of nitrile butadiene rubber with enhanced catalytic activity and recycling performance[J]. Industrial & Engineering Chemistry Research, 2019, 58(27): 11821-11830. |
| 16 | Dyson P J, Jessop P G. Solvent effects in catalysis: rational improvements of catalysts via manipulation of solvent interactions[J]. Catalysis Science & Technology, 2016, 6: 3302-3316. |
| 17 | Li Y, Cheng H, Lin W, et al. Solvent effects on heterogeneous catalysis in the selective hydrogenation of cinnamaldehyde over a conventional Pd/C catalyst[J]. Catalysis Science & Technology, 2018, 8: 3580-3589. |
| 18 | Linda N, Elena S, Alexey B, et al. Selective hydrogenation of 2-methyl-3-butyn-2-ol over Pd-nanoparticles stabilized in hypercrosslinked polystyrene: solvent effect[J]. Catalysis Today, 2015, 241:179-188. |
| 19 | 刘卅, 贾德民. 贮氢合金催化丁腈橡胶溶液加氢反应工艺条件的研究[J]. 弹性体, 2006, 16(2): 19-23. |
| Liu S, Jia D M. Hydrogenation conditions of nitrile rubber solution catalyzed by hydrogen storage alloy[J]. China Elastomerics, 2006, 16(2): 19-23. | |
| 20 | Shirai M, Torii K, Arai M. Hydrogenation of acrylonitrile-butadiene rubbers with palladium loaded mesopore-size controlled clay materials[J]. Studies in Surface Science and Catalysis, 2000, 130: 2105-2110. |
| 21 | Wang S, Ge B, Yin Y, et al. Solvent effect in heterogeneous catalytic selective hydrogenation of nitrile butadiene rubber: relationship between reaction activity and solvents with density functional theory analysis[J]. ChemCatChem, 2020, 12(2): 663-672. |
| 22 | Liu B, Zhang H, Ren J, et al. Effect of solvent aromaticity on poly(9,9-dioctylfluorene)(PFO) chain solution behavior and film condensed state structure[J]. Polymer, 2019, 185: 121986. |
| 23 | Dickson R M, Becke A D. Basis-set-free local density-functional calculations of geometries of polyatomic molecules[J]. Journal of Chemical Physics, 1993, 99(5): 3898-3905. |
| 24 | Fokin A A, Gerbig D, Schreiner P R. σ/σ- and π/π-interactions are equally important: multilayered graphanes[J]. Journal of the American Chemical Society, 2011, 133(50):20036-20039. |
| 25 | Orio M, Pantazis D A, Neese F. Density functional theory[J]. Photosynthesis Research, 2009, 102(2/3): 443-453. |
| 26 | 阴义轩, 成婷婷, 袁珮, 等. 丁腈橡胶非均相加氢催化剂失活原因及再生性能研究[J]. 化工学报, 2019, 70(7): 2528-2539. |
| Yin Y X, Cheng T T, Yuan P, et al. Deactivation and regeneration of heterogeneous hydrogenation catalyst for nitrile butadiene rubber[J]. CISEC Journal, 2019, 70(7): 2528-2539. | |
| 27 | Fukui K,Yonezawa T, Shingu H. A molecular orbital theory of reactivity in aromatic hydrocarbons[J]. Journal of Chemical Physics, 2004, 20(10): 722-725. |
| 28 | Fukui K. Theory of orientation and stereoselection[J]. Reactivity & Structure Concepts in Organic Chemistry, 1975, 15: 1-85. |
| 29 | 周公度, 段连云. 结构化学基础[M]. 北京: 北京大学出版社, 2002: 11-22. |
| Zhou G D, Duan L Y. Fundamentals of Structural Chemistry [M]. Beijing: Peking University Press, 2002: 11-22. | |
| 30 | 付新梅, 李燕, 王景辉. 有机小分子偶极矩影响因素研究[J]. 计算机与应用化学, 2015, (4): 408-412. |
| Fu X M, Li Y, Wang J H. Influencing factors of organic small molecule Dipole moment[J]. Computers and Applied Chemistry, 2015, (4): 408-412. |
| [1] | Zhanyu YE, He SHAN, Zhenyuan XU. Performance simulation of paper folding-like evaporator for solar evaporation systems [J]. CIESC Journal, 2023, 74(S1): 132-140. |
| [2] | Cheng CHENG, Zhongdi DUAN, Haoran SUN, Haitao HU, Hongxiang XUE. Lattice Boltzmann simulation of surface microstructure effect on crystallization fouling [J]. CIESC Journal, 2023, 74(S1): 74-86. |
| [3] | Minghao SONG, Fei ZHAO, Shuqing LIU, Guoxuan LI, Sheng YANG, Zhigang LEI. Multi-scale simulation and study of volatile phenols removal from simulated oil by ionic liquids [J]. CIESC Journal, 2023, 74(9): 3654-3664. |
| [4] | Ruimin CHE, Wenqiu ZHENG, Xiaoyu WANG, Xin LI, Feng XU. Research progress on homogeneous processing of cellulose in ionic liquids [J]. CIESC Journal, 2023, 74(9): 3615-3627. |
| [5] | Xingzhi HU, Haoyan ZHANG, Jingkun ZHUANG, Yuqing FAN, Kaiyin ZHANG, Jun XIANG. Preparation and microwave absorption properties of carbon nanofibers embedded with ultra-small CeO2 nanoparticles [J]. CIESC Journal, 2023, 74(8): 3584-3596. |
| [6] | Tianhua CHEN, Zhaoxuan LIU, Qun HAN, Chengbin ZHANG, Wenming LI. Research progress and influencing factors of the heat transfer enhancement of spray cooling [J]. CIESC Journal, 2023, 74(8): 3149-3170. |
| [7] | Chunyu LIU, Huanyu ZHOU, Yue MA, Changtao YUE. Drying characteristics and mathematical model of CaO-conditioned oil sludge [J]. CIESC Journal, 2023, 74(7): 3018-3027. |
| [8] | Hai WANG, Hong LIN, Chen WANG, Haojie XU, Lei ZUO, Junfeng WANG. Investigation of enhanced boiling heat transfer on porous structural surfaces by high voltage electric field [J]. CIESC Journal, 2023, 74(7): 2869-2879. |
| [9] | Ji CHEN, Ze HONG, Zhao LEI, Qiang LING, Zhigang ZHAO, Chenhui PENG, Ping CUI. Study on coke dissolution loss reaction and its mechanism based on molecular dynamics simulations [J]. CIESC Journal, 2023, 74(7): 2935-2946. |
| [10] | Zhen LONG, Jinhang WANG, Junjie REN, Yong HE, Xuebing ZHOU, Deqing LIANG. Experimental study on inhibition effect of natural gas hydrate formation by mixing ionic liquid with PVCap [J]. CIESC Journal, 2023, 74(6): 2639-2646. |
| [11] | Daoyin LIU, Bingqi CHEN, Zuyang ZHANG, Yan WU. Effect of agglomerate structure on drag force by numerical simulation [J]. CIESC Journal, 2023, 74(6): 2351-2362. |
| [12] | Shaoyun CHEN, Dong XU, Long CHEN, Yu ZHANG, Yuanfang ZHANG, Qingliang YOU, Chenglong HU, Jian CHEN. Preparation and adsorption properties of monolayer polyaniline microsphere arrays [J]. CIESC Journal, 2023, 74(5): 2228-2238. |
| [13] | Chenxin LI, Yanqiu PAN, Liu HE, Yabin NIU, Lu YU. Carbon membrane model based on carbon microcrystal structure and its gas separation simulation [J]. CIESC Journal, 2023, 74(5): 2057-2066. |
| [14] | Jianhua ZHANG, Mengmeng CHEN, Yawen SUN, Yongzhen PENG. Efficient nitrogen and phosphorus removal from domestic wastewater via simultaneous partial nitritation and phosphorus removal combined Anammox [J]. CIESC Journal, 2023, 74(5): 2147-2156. |
| [15] | Jiyuan LI, Jinwang LI, Liuwei ZHOU. Heat transfer performance of cold plates with different turbulence structures [J]. CIESC Journal, 2023, 74(4): 1474-1488. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||