CIESC Journal ›› 2023, Vol. 74 ›› Issue (11): 4475-4486.DOI: 10.11949/0438-1157.20230902
• Thermodynamics • Previous Articles Next Articles
Zongpeng LIU1( ), Shaojian HU1, Yuning ZHANG1, Ling MA2, Lei LI2, Bencheng WU1, Jianhua ZHU1(
), Shaojian HU1, Yuning ZHANG1, Ling MA2, Lei LI2, Bencheng WU1, Jianhua ZHU1( )
)
Received:2023-08-31
Revised:2023-11-01
Online:2024-01-22
Published:2023-11-25
Contact:
Jianhua ZHU
刘宗鹏1( ), 胡少剑1, 张宇宁1, 马玲2, 李磊2, 武本成1, 朱建华1(
), 胡少剑1, 张宇宁1, 马玲2, 李磊2, 武本成1, 朱建华1( )
)
通讯作者:
朱建华
作者简介:刘宗鹏(1994—),男,博士研究生,pzuil5201@163.com
CLC Number:
Zongpeng LIU, Shaojian HU, Yuning ZHANG, Ling MA, Lei LI, Bencheng WU, Jianhua ZHU. Thermodynamic analysis and kinetics study on synthesis reaction of complex polyolester[J]. CIESC Journal, 2023, 74(11): 4475-4486.
刘宗鹏, 胡少剑, 张宇宁, 马玲, 李磊, 武本成, 朱建华. 复合型多元醇酯合成反应的热力学分析及动力学研究[J]. 化工学报, 2023, 74(11): 4475-4486.
Add to citation manager EndNote|Ris|BibTeX
| Group | Benson method | Ducros method | Rozicka-Domalski method | |||
|---|---|---|---|---|---|---|
| ai | bi /K-1 | ci /K-2 | ||||
| C—(C)4 | 2.09 | -146.96 | 0.00 | -2.9353 | 1.4255 | -0.0853 |
| C—(C)(H)3 | -42.20 | 127.32 | 5.65 | 3.8452 | -0.3400 | 0.1949 |
| C—(C)(O)(H)2 | -33.91 | 41.03 | 4.60 | 1.4596 | 1.4657 | -0.2714 |
| O—(C)(H) | -158.68 | 121.71 | 31.80 | 12.9520 | -10.1450 | 2.6261 |
| O—(CO)(H) | -243.25 | 102.66 | 37.87 | -27.5870 | -0.1649 | 2.7483 |
| C—(C)2(H)2 | -20.72 | 39.44 | 4.98 | 2.7972 | -0.0550 | 0.1068 |
| C—(C)(CO)(H)2 | -21.77 | 40.19 | 2.97 | 6.6782 | -2.4473 | 0.4712 |
| CO—(C)(O) | -146.86 | 20.01 | 9.83 | 29.2460 | 3.4261 | -2.8962 |
| C—(C)3(H) | -7.95 | -50.53 | 3.01 | -0.4287 | 0.9381 | 0.0029 |
| O—(C)(CO) | -185.48 | 35.13 | 8.37 | -21.4340 | -4.0164 | 3.0531 |
Table 1 Thermodynamic contribution value of each group in the different methods[14]
| Group | Benson method | Ducros method | Rozicka-Domalski method | |||
|---|---|---|---|---|---|---|
| ai | bi /K-1 | ci /K-2 | ||||
| C—(C)4 | 2.09 | -146.96 | 0.00 | -2.9353 | 1.4255 | -0.0853 |
| C—(C)(H)3 | -42.20 | 127.32 | 5.65 | 3.8452 | -0.3400 | 0.1949 |
| C—(C)(O)(H)2 | -33.91 | 41.03 | 4.60 | 1.4596 | 1.4657 | -0.2714 |
| O—(C)(H) | -158.68 | 121.71 | 31.80 | 12.9520 | -10.1450 | 2.6261 |
| O—(CO)(H) | -243.25 | 102.66 | 37.87 | -27.5870 | -0.1649 | 2.7483 |
| C—(C)2(H)2 | -20.72 | 39.44 | 4.98 | 2.7972 | -0.0550 | 0.1068 |
| C—(C)(CO)(H)2 | -21.77 | 40.19 | 2.97 | 6.6782 | -2.4473 | 0.4712 |
| CO—(C)(O) | -146.86 | 20.01 | 9.83 | 29.2460 | 3.4261 | -2.8962 |
| C—(C)3(H) | -7.95 | -50.53 | 3.01 | -0.4287 | 0.9381 | 0.0029 |
| O—(C)(CO) | -185.48 | 35.13 | 8.37 | -21.4340 | -4.0164 | 3.0531 |
| Component | Tb/K | ||||
|---|---|---|---|---|---|
| AA | -865.20 | 398.84 | 111.30 | 215.28 | 615.27 |
| EH | -367.82 | 512.11 | 67.99 | 176.09 | 488.39 |
| TMP | -638.60 | 489.75 | 119.83 | 203.31 | 574.37 |
| EHA | -1016.57 | 721.71 | 120.63 | 131.05 | 656.25 |
| DEHA | -1167.94 | 1044.57 | 129.96 | 133.81 | 690.37 |
| TMPME | -1438.72 | 1056.15 | 179.16 | 136.44 | 726.72 |
| TMPDE | -2238.84 | 1575.34 | 238.49 | 142.55 | 813.02 |
| TMPTE | -3038.96 | 2104.43 | 297.82 | 146.46 | 873.51 |
| H2O(g)[ | -241.826 | 188.82 | 40.64 | 108.90 | 373.15 |
Table 2 Thermodynamic data of each component in the esterification system
| Component | Tb/K | ||||
|---|---|---|---|---|---|
| AA | -865.20 | 398.84 | 111.30 | 215.28 | 615.27 |
| EH | -367.82 | 512.11 | 67.99 | 176.09 | 488.39 |
| TMP | -638.60 | 489.75 | 119.83 | 203.31 | 574.37 |
| EHA | -1016.57 | 721.71 | 120.63 | 131.05 | 656.25 |
| DEHA | -1167.94 | 1044.57 | 129.96 | 133.81 | 690.37 |
| TMPME | -1438.72 | 1056.15 | 179.16 | 136.44 | 726.72 |
| TMPDE | -2238.84 | 1575.34 | 238.49 | 142.55 | 813.02 |
| TMPTE | -3038.96 | 2104.43 | 297.82 | 146.46 | 873.51 |
| H2O(g)[ | -241.826 | 188.82 | 40.64 | 108.90 | 373.15 |
| Group | ΔTbi /K |
|---|---|
 | 0.2878 |
| —CH2O— | 1.6249 |
| —OH | 3.2152 |
| —COOH | 5.8337 |
| —COO— | 2.6446 |
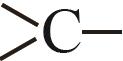 | 0.6033 |
| —CH2— | 0.9225 |
| —CH3 | 0.8894 |
| —CH2COO— | 3.3950 |
Table 3 Thermodynamic contribution value of each group in the Constantinous-Gani method[15]
| Group | ΔTbi /K |
|---|---|
 | 0.2878 |
| —CH2O— | 1.6249 |
| —OH | 3.2152 |
| —COOH | 5.8337 |
| —COO— | 2.6446 |
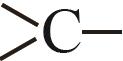 | 0.6033 |
| —CH2— | 0.9225 |
| —CH3 | 0.8894 |
| —CH2COO— | 3.3950 |
| Component | |||
|---|---|---|---|
| a | b×102 | c×104 | |
| AA | 185.14 | 12.62 | 7.15 |
| EH | 273.22 | -71.84 | 26.39 |
| TMP | 390.28 | -207.91 | 60.53 |
| EHA | 401.83 | -6.90 | 14.25 |
| DEHA | 618.52 | -26.42 | 21.34 |
| TMPME | 735.58 | -162.49 | 55.48 |
| TMPDE | 1080.88 | -117.06 | 50.42 |
| TMPTE | 1426.19 | -71.63 | 45.37 |
| H2O(g)[ | 36.54 | -3.48 | 1.17 |
Table 4 Correlation of specific heat capacity and temperature of each component in the esterification system
| Component | |||
|---|---|---|---|
| a | b×102 | c×104 | |
| AA | 185.14 | 12.62 | 7.15 |
| EH | 273.22 | -71.84 | 26.39 |
| TMP | 390.28 | -207.91 | 60.53 |
| EHA | 401.83 | -6.90 | 14.25 |
| DEHA | 618.52 | -26.42 | 21.34 |
| TMPME | 735.58 | -162.49 | 55.48 |
| TMPDE | 1080.88 | -117.06 | 50.42 |
| TMPTE | 1426.19 | -71.63 | 45.37 |
| H2O(g)[ | 36.54 | -3.48 | 1.17 |
| First esterification step (XA≈0—90%) | Second esterification step (XC≈0—80%) | ||||
|---|---|---|---|---|---|
| T/K | Fitting equation | R2 | T/K | Fitting equation | R2 |
| 413.15 | y=0.0088x-0.0165 | 0.9923 | 423.15 | y=0.0113x+0.0034 | 0.9941 |
| 423.15 | y=0.0198x-0.0337 | 0.9936 | 433.15 | y=0.0163x+0.1692 | 0.9923 |
| 433.15 | y=0.0325x-0.0086 | 0.9915 | 443.15 | y=0.0220x+0.2462 | 0.9850 |
| 443.15 | y=0.0582x+0.1234 | 0.9885 | 453.15 | y=0.0339x+0.3084 | 0.9708 |
Table 5 Fitting results of reaction kinetic equations at different temperatures
| First esterification step (XA≈0—90%) | Second esterification step (XC≈0—80%) | ||||
|---|---|---|---|---|---|
| T/K | Fitting equation | R2 | T/K | Fitting equation | R2 |
| 413.15 | y=0.0088x-0.0165 | 0.9923 | 423.15 | y=0.0113x+0.0034 | 0.9941 |
| 423.15 | y=0.0198x-0.0337 | 0.9936 | 433.15 | y=0.0163x+0.1692 | 0.9923 |
| 433.15 | y=0.0325x-0.0086 | 0.9915 | 443.15 | y=0.0220x+0.2462 | 0.9850 |
| 443.15 | y=0.0582x+0.1234 | 0.9885 | 453.15 | y=0.0339x+0.3084 | 0.9708 |
| First esterification step(XA≈0—90%) | Second esterification step(XC≈0—80%) | ||
|---|---|---|---|
| A1/(L·mol-1·min-1) | 7.153×109 | A2/(L·mol-1·min-1) | 1.869×105 |
| Ea1/(kJ·mol-1) | 93.99 | Ea2/(kJ·mol-1) | 58.59 |
Table 6 Kinetic parameters of the two esterification steps
| First esterification step(XA≈0—90%) | Second esterification step(XC≈0—80%) | ||
|---|---|---|---|
| A1/(L·mol-1·min-1) | 7.153×109 | A2/(L·mol-1·min-1) | 1.869×105 |
| Ea1/(kJ·mol-1) | 93.99 | Ea2/(kJ·mol-1) | 58.59 |
| 1 | Nagendramma P, Kaul S. Development of ecofriendly/biodegradable lubricants: an overview[J]. Renewable and Sustainable Energy Reviews, 2012, 16(1): 764-774. |
| 2 | Pichler J, Eder R M, Besser C, et al. A comprehensive review of sustainable approaches for synthetic lubricant components[J]. Green Chemistry Letters and Reviews, 2023, 16(1): 2185547. |
| 3 | Pettersson A. High-performance base fluids for environmentally adapted lubricants[J]. Tribology International, 2007, 40(4): 638-645. |
| 4 | Raghunanan L, Narine S S. Engineering green lubricants (Ⅰ): Optimizing thermal and flow properties of linear diesters derived from vegetable oils[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(3): 686-692. |
| 5 | Eychenne V, Mouloungui Z. Relationships between structure and lubricating properties of neopentylpolyol esters[J]. Industrial & Engineering Chemistry Research, 1998, 37(12): 4835-4843. |
| 6 | Nagendramma P, Khatri P K, Thakre G D, et al. Lubrication capabilities of amino acid based ionic liquids as green bio-lubricant additives[J]. Journal of Molecular Liquids, 2017, 244: 219-225. |
| 7 | Kamalakar K, Mahesh G, Prasad R B N, et al. A novel methodology for the synthesis of acyloxy castor polyol esters: low pour point lubricant base stocks[J]. Journal of Oleo Science, 2015, 64(12): 1283-1295. |
| 8 | 赵东江. 热力学过程的性质、方向和限度判据的研究[J]. 化工高等教育, 2007, 24(1): 47-49, 63. |
| Zhao D J. Study on the nature, direction and limit criterion of thermodynamic process[J]. Higher Education in Chemical Engineering, 2007, 24(1): 47-49, 63. | |
| 9 | Zanjani N G, Pirzaman A K, Yazdanian E. Biodiesel production in the presence of heterogeneous catalyst of alumina: study of kinetics and thermodynamics[J]. International Journal of Chemical Kinetics, 2020, 52(7): 472-484. |
| 10 | Elmelawy M S, El-Meligy A, Mawgoud H A, et al. Synthesis and kinetics study of trimethylolpropane fatty acid triester from oleic acid methyl ester as potential biolubricant[J]. Biomass Conversion and Biorefinery, 2023, 13(3): 1645-1657. |
| 11 | Altuntepe E, Greinert T, Hartmann F, et al. Thermodynamics of enzyme-catalyzed esterifications(Ⅰ): Succinic acid esterification with ethanol[J]. Applied Microbiology and Biotechnology, 2017, 101(15): 5973-5984. |
| 12 | Aziz N A M, Hamid H A, Yunus R, et al. Kinetics and thermodynamics of synthesis of palm oil-based trimethylolpropane triester using microwave irradiation[J]. Journal of Saudi Chemical Society, 2020, 24(8): 552-566. |
| 13 | Ma L L, Han Y, Sun K A, et al. Kinetic and thermodynamic studies of the esterification of acidified oil catalyzed by sulfonated cation exchange resin[J]. Journal of Energy Chemistry, 2015, 24(4): 456-462. |
| 14 | 董新法, 方利国, 陈砺. 物性估算原理及计算机计算[M]. 北京: 化学工业出版社, 2006: 171-180. |
| Dong X F, Fang L G, Chen L. Principle of Physical Property Estimation and Computer Calculation[M]. Beijing: Chemical Industry Press, 2006: 171-180. | |
| 15 | 马沛生. 化工数据[M]. 北京: 中国石化出版社, 2003: 258-276. |
| Ma P S. Chemical Engineering Data[M]. Beijing: China Petrochemical Press, 2003: 258-276. | |
| 16 | 赵国良, 靳长德. 有机物热力学数据的估算[M]. 北京: 高等教育出版社, 1983: 140-156. |
| Zhao G L, Jin C D. Estimation of Thermodynamic Data of Organic Matter[M]. Beijing: Higher Education Press, 1983: 140-156. | |
| 17 | 马沛生, 夏淑倩, 夏清. 化工物性数据简明手册[M]. 北京: 化学工业出版社, 2013: 258-276. |
| Ma P S, Xia S Q, Xia Q. Concise Handbook of Chemical Physical Property Data[M]. Beijing: Chemical Industry Press, 2013: 258-276. | |
| 18 | 张继龙, 赵志仝, 乔燕, 等. 酯交换制油酸甲酯的基团贡献法热力学分析[J]. 化工学报, 2012, 63(6): 1684-1690. |
| Zhang J L, Zhao Z T, Qiao Y, et al. Thermodynamic analysis on preparation of methyl oleate via transesterification by group-contribution method[J]. CIESC Journal, 2012, 63(6): 1684-1690. | |
| 19 | 刘宏晓, 孙伟振, 赵玲. TOME环化反应热力学分析及反应动力学研究[J]. 化工学报, 2020, 71(2): 500-506. |
| Liu H X, Sun W Z, Zhao L. Thermodynamic analysis and kinetics of cyclization of TOME[J]. CIESC Journal, 2020, 71(2): 500-506. | |
| 20 | 吕全明, 孙伟振, 赵玲. 连四甲苯液相氧化过程热力学分析及动力学模拟[J]. 化工学报, 2021, 72(2): 1009-1017. |
| Lyu Q M, Sun W Z, Zhao L. Thermodynamic analysis and kinetic simulation of liquid phase oxidation of prehnitene to mellophanic acid[J]. CIESC Journal, 2021, 72(2): 1009-1017. | |
| 21 | Forsythe C J. Influence of inert gas sparging on fatty acid lactylate esterification kinetics[J]. Reaction Kinetics, Mechanisms and Catalysis, 2013, 108(2): 263-284. |
| 22 | Tesser R, Casale L, Verde D, et al. Kinetics of free fatty acids esterification: batch and loop reactor modeling[J]. Chemical Engineering Journal, 2009, 154(1/2/3): 25-33. |
| 23 | 付丽丽, 蒋登高. 棕榈酸异丙酯合成反应的热力学分析[J]. 高校化学工程学报, 2016, 30(2): 398-403. |
| Fu L L, Jiang D G. Thermodynamic analysis of isopropyl palmitate synthesis[J]. Journal of Chemical Engineering of Chinese Universities, 2016, 30(2): 398-403. | |
| 24 | 王洪海, 李旭, 李春利, 等. 固定化酶催化制备乙酸正丁酯及动力学[J]. 化工学报, 2017, 68(12): 4685-4690. |
| Wang H H, Li X, Li C L, et al. Kinetics of n-butyl acetate prepared by immobilized enzyme[J]. CIESC Journal, 2017, 68(12): 4685-4690. | |
| 25 | Zhou D, Wang L L, Chen X P, et al. Reaction mechanism investigation on the esterification of rosin with glycerol over annealed Fe3O4/MOF-5 via kinetics and TGA-FTIR analysis[J]. Chemical Engineering Journal, 2020, 401: 126024. |
| 26 | Russo V, Taddeo F, Cogliano T, et al. Investigation of the intrinsic reaction kinetics and the mass transfer phenomena of nonanoic acid esterification with 2-ethylhexanol promoted by sulfuric acid or Amberlite IR120[J]. Chemical Engineering Journal, 2021, 408: 127236. |
| 27 | Wang Y Z, Liu Y P, Liu C G. Kinetics of the esterification of low-concentration naphthenic acids and methanol in oils with or without SnO as a catalyst[J]. Energy & Fuels, 2008, 22(4): 2203-2206. |
| 28 | Kamaruzaman M R, Chin S Y, Pui E C L, et al. Synthesis of biobased polyester polyol through esterification of sorbitol with azelaic acid catalyzed by tin(Ⅱ) oxide: a kinetic modeling study[J]. Industrial & Engineering Chemistry Research, 2019, 58(2): 510-516. |
| 29 | Sánchez-Correa C A, Gil-Chaves I D, Rodríguez-Niño G. Kinetics of acetic acid and isoamyl alcohol liquid esterification over Amberlyst-70[J]. Chemical Engineering Research and Design, 2023, 196: 642-655. |
| 30 | 刘新鹏, 吴天祥. 非酸催化酯化合成对苯二甲酸二异辛酯的反应动力学[J]. 高校化学工程学报, 1994, 8(2): 195-200. |
| Liu X P, Wu T X. Reaction kinetics of esterification of synthetic dioctyl terephthalate with nonacid catalyst[J]. Journal of Chemical Engineering of Chinese Universities, 1994, 8(2): 195-200. | |
| 31 | Salmi T, Paatero E, Nyholm P. Kinetic model for the increase of reaction order during polyesterification[J]. Chemical Engineering and Processing: Process Intensification, 2004, 43(12): 1487-1493. |
| 32 | Tian W Y, Zeng Z X, Xue W L, et al. Kinetics of the mono-esterification between terephthalic acid and 1,4-butanediol[J]. Chinese Journal of Chemical Engineering, 2010, 18(3): 391-396. |
| 33 | Sun L, Zhu L, Xue W L, et al. Kinetics of p-toluene-sulfonic acid catalyzed direct esterification of pentaerythritol with acrylic acid for pentaerythritol diacrylate production[J]. Chemical Engineering Communications, 2020, 207(3): 331-338. |
| 34 | Zhou F, Cai J J, Mao X N, et al. Pseudo-homogeneous kinetic modeling of dioctyl terephthalate (DOTP) production by esterification of terephthalic acid and 2-ethylhexanol over tetrabutyl titanate catalyst[J]. Korean Journal of Chemical Engineering, 2022, 39(9): 2324-2333. |
| 35 | Narayan R C, Madras G. Esterification of sebacic acid in near-critical and supercritical methanol[J]. Industrial & Engineering Chemistry Research, 2017, 56(10): 2641-2649. |
| [1] | Cheng CHENG, Zhongdi DUAN, Haoran SUN, Haitao HU, Hongxiang XUE. Lattice Boltzmann simulation of surface microstructure effect on crystallization fouling [J]. CIESC Journal, 2023, 74(S1): 74-86. |
| [2] | Jie CHEN, Yongsheng LIN, Kai XIAO, Chen YANG, Ting QIU. Study on catalytic synthesis of sec-butanol by tunable choline-based basic ionic liquids [J]. CIESC Journal, 2023, 74(9): 3716-3730. |
| [3] | Linzheng WANG, Yubing LU, Ruizhi ZHANG, Yonghao LUO. Analysis on thermal oxidation characteristics of VOCs based on molecular dynamics simulation [J]. CIESC Journal, 2023, 74(8): 3242-3255. |
| [4] | Mengmeng ZHANG, Dong YAN, Yongfeng SHEN, Wencui LI. Effect of electrolyte types on the storage behaviors of anions and cations for dual-ion batteries [J]. CIESC Journal, 2023, 74(7): 3116-3126. |
| [5] | Xiaoyu YAO, Jun SHEN, Jian LI, Zhenxing LI, Huifang KANG, Bo TANG, Xueqiang DONG, Maoqiong GONG. Research progress in measurement methods in vapor-liquid critical properties of mixtures [J]. CIESC Journal, 2023, 74(5): 1847-1861. |
| [6] | Ke CHEN, Li DU, Ying ZENG, Siying REN, Xudong YU. Phase equilibria and calculation of quaternary system LiCl+MgCl2+CaCl2+H2O at 323.2 K [J]. CIESC Journal, 2023, 74(5): 1896-1903. |
| [7] | Yuanjing MAO, Zhi YANG, Songping MO, Hao GUO, Ying CHEN, Xianglong LUO, Jianyong CHEN, Yingzong LIANG. Estimation of SAFT-VR Mie equation of state parameters and thermodynamic properties of C6—C10 alcohols [J]. CIESC Journal, 2023, 74(3): 1033-1041. |
| [8] | Jin YU, Binbin YU, Xinsheng JIANG. Study on quantification methodology and analysis of chemical effects of combustion control based on fictitious species [J]. CIESC Journal, 2023, 74(3): 1303-1312. |
| [9] | Wenting CHENG, Jie LI, Li XU, Fangqin CHENG, Guoji LIU. Experiment and prediction for the solubility of AlCl3·6H2O in FeCl3, CaCl2, KCl and KCl-FeCl3 solutions [J]. CIESC Journal, 2023, 74(2): 642-652. |
| [10] | Xiaodong HONG, Xuan DONG, Meijin LIN, Zuwei LIAO, Congjing REN, Yao YANG, Binbo JIANG, Jingdai WANG, Yongrong YANG. Prediction of thermodynamic properties of hydrocarbon working fluids by graph neural network models [J]. CIESC Journal, 2023, 74(11): 4466-4474. |
| [11] | Lei ZHANG, Xiaohui SONG, Jianting ZHANG, Meiling TU, Asan YANG. Reaction kinetics study of tranexamic acid isomerization process [J]. CIESC Journal, 2023, 74(10): 4173-4181. |
| [12] | Ruizhe CHEN, Yongfeng LIU, Chenyang YIN, Long WANG, Lu ZHANG, Jin’ou SONG. Study of the mechanism of pyrolysis of n-hexane initiated by 1-nitropropane [J]. CIESC Journal, 2023, 74(10): 4319-4329. |
| [13] | Zhi ZHENG, Naisheng GUO, Zhanping YOU, Jiawei WANG. Research on compatibility mechanisms between waste wood oil and petroleum asphalt through molecular dynamics [J]. CIESC Journal, 2023, 74(10): 4037-4050. |
| [14] | Songtao YANG, Dongyang LI, Yuqing NIU, Xingang LI, Shaohui KANG, Hong LI, Kaikai YE, Zhiquan ZHOU, Xin GAO. Molecular simulation progress in studying thermodynamic properties and potential functions of fluorides [J]. CIESC Journal, 2022, 73(9): 3828-3840. |
| [15] | Chen CHEN, Qian YANG, Yun CHEN, Rui ZHANG, Dong LIU. Chemical kinetic study on coal volatiles combustion for various oxygen concentrations [J]. CIESC Journal, 2022, 73(9): 4133-4146. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||