CIESC Journal ›› 2024, Vol. 75 ›› Issue (5): 2001-2016.DOI: 10.11949/0438-1157.20231201
• Energy and environmental engineering • Previous Articles Next Articles
Xu MA1,2( ), Yadong TENG3,4, Jie LIU3,4, Yulu WANG1,2, Peng ZHANG1(
), Yadong TENG3,4, Jie LIU3,4, Yulu WANG1,2, Peng ZHANG1( ), Lianhai ZHANG1, Wanlong YAO5, Jing ZHAN1, Qingbai WU1
), Lianhai ZHANG1, Wanlong YAO5, Jing ZHAN1, Qingbai WU1
Received:2023-11-21
Revised:2024-03-27
Online:2024-06-25
Published:2024-05-25
Contact:
Peng ZHANG
马旭1,2( ), 滕亚栋3,4, 刘杰3,4, 王宇璐1,2, 张鹏1(
), 滕亚栋3,4, 刘杰3,4, 王宇璐1,2, 张鹏1( ), 张莲海1, 姚万龙5, 展静1, 吴青柏1
), 张莲海1, 姚万龙5, 展静1, 吴青柏1
通讯作者:
张鹏
作者简介:马旭(1996—),女,硕士研究生,maxu@nieer.ac.cn
基金资助:CLC Number:
Xu MA, Yadong TENG, Jie LIU, Yulu WANG, Peng ZHANG, Lianhai ZHANG, Wanlong YAO, Jing ZHAN, Qingbai WU. CO2 capture and separation from flue gas by spraying hydrate method[J]. CIESC Journal, 2024, 75(5): 2001-2016.
马旭, 滕亚栋, 刘杰, 王宇璐, 张鹏, 张莲海, 姚万龙, 展静, 吴青柏. 喷雾法水合物法捕集分离烟道气中CO2[J]. 化工学报, 2024, 75(5): 2001-2016.
Add to citation manager EndNote|Ris|BibTeX
| 体 系 | 编号 | 促进剂浓度/% (质量分数) | 雾化喷嘴孔径/mm | 原料气中CO2/%(摩尔分数) | 最终消耗的气体量/(mol/mol H2O) | 水转化为水合物比率/% |
|---|---|---|---|---|---|---|
| CO2/N2/H2O | 1 | 纯水 | 0.8 | 14.35 | 0.0053 | 3.0 |
| 2 | 纯水 | 0.1 | 14.46 | 0.0061 | 3.5 | |
| CO2/N2/L-Met/H2O | 3 | 0.1 | 0.8 | 15.55 | 0.0587 | 33.5 |
| 4 | 1.0 | 0.8 | 16.87 | 0.0537 | 30.7 | |
| 5 | 0.1 | 0.1 | 19.08 | 0.0749 | 42.7 | |
| 6 | 1.0 | 0.1 | 13.19 | 0.0654 | 37.3 | |
| CO2/N2/SDS/H2O | 7 | 0.1 | 0.8 | 18.15 | 0.0848 | 48.4 |
| 8 | 1.0 | 0.8 | 13.67 | 0.0733 | 41.9 | |
| 9 | 0.1 | 0.1 | 15.34 | 0.0407 | 23.3 |
Table 1 Experimental results in different 640 ml systems of SDS and L-Met promoters (7.71 MPa,269.15 K)
| 体 系 | 编号 | 促进剂浓度/% (质量分数) | 雾化喷嘴孔径/mm | 原料气中CO2/%(摩尔分数) | 最终消耗的气体量/(mol/mol H2O) | 水转化为水合物比率/% |
|---|---|---|---|---|---|---|
| CO2/N2/H2O | 1 | 纯水 | 0.8 | 14.35 | 0.0053 | 3.0 |
| 2 | 纯水 | 0.1 | 14.46 | 0.0061 | 3.5 | |
| CO2/N2/L-Met/H2O | 3 | 0.1 | 0.8 | 15.55 | 0.0587 | 33.5 |
| 4 | 1.0 | 0.8 | 16.87 | 0.0537 | 30.7 | |
| 5 | 0.1 | 0.1 | 19.08 | 0.0749 | 42.7 | |
| 6 | 1.0 | 0.1 | 13.19 | 0.0654 | 37.3 | |
| CO2/N2/SDS/H2O | 7 | 0.1 | 0.8 | 18.15 | 0.0848 | 48.4 |
| 8 | 1.0 | 0.8 | 13.67 | 0.0733 | 41.9 | |
| 9 | 0.1 | 0.1 | 15.34 | 0.0407 | 23.3 |
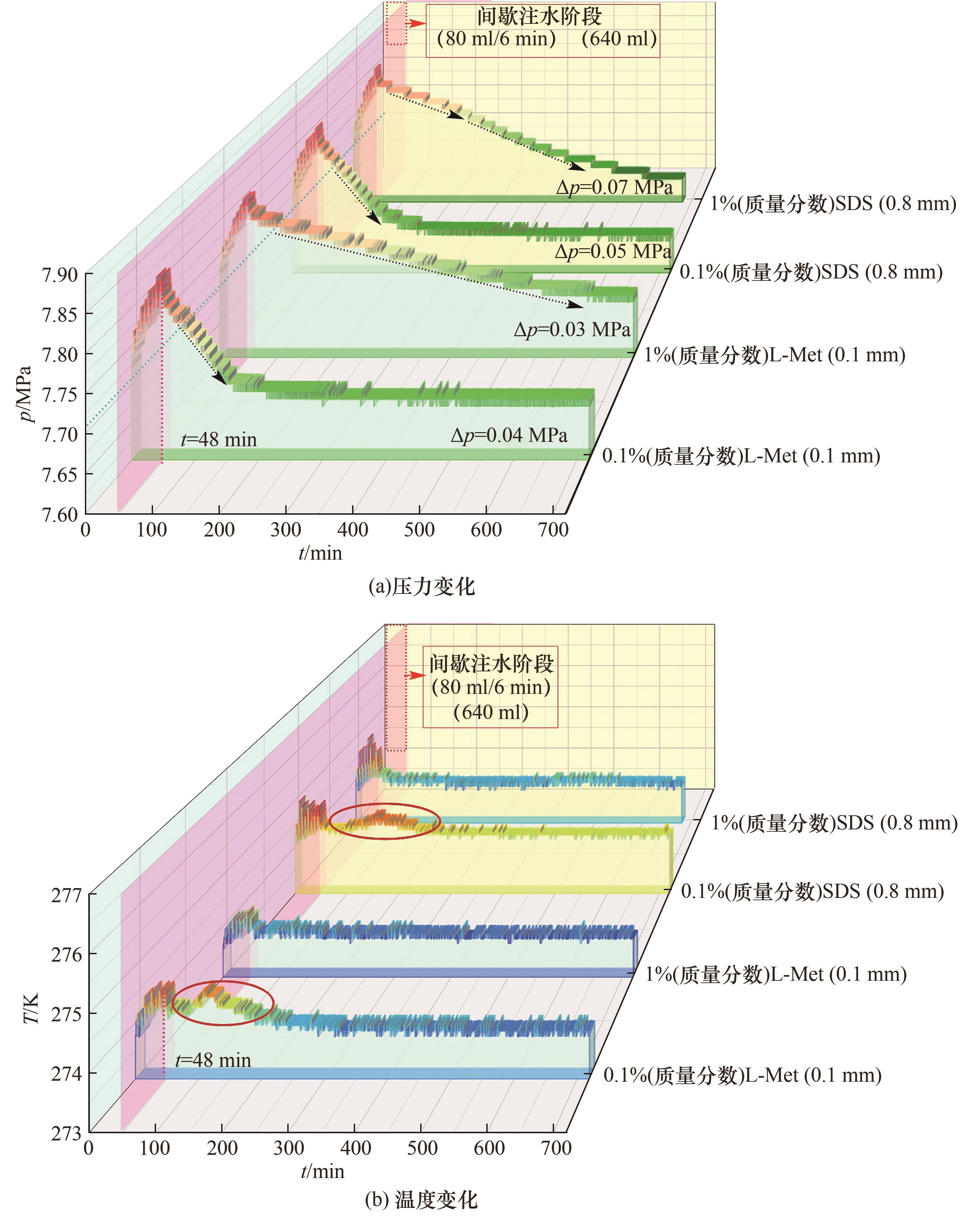
Fig.4 Temperature and pressure as functions of time during hydrate formation from solutions of CO2/H2O /SDS and CO2/H2O /L-Met respectively with concentrations of 0.1% and 1% (mass fraction)
| 体 系 | 编号 | 促进剂浓度/% (质量分数) | 雾化喷嘴孔径/mm | 原料气中CO2/%(摩尔分数) | 最终消耗的气体量/(mol/mol H2O) | 水转化为水合物比率/% |
|---|---|---|---|---|---|---|
| CO2/N2/H2O | 1 | 纯水 | 0.8 | 14.38 | 0.0075 | 4.3 |
| 2 | 纯水 | 0.1 | 14.53 | 0.0111 | 6.3 | |
| CO2/N2/L-Met/H2O | 3 | 0.1 | 0.8 | 16.64 | 0.1220 | 69.6 |
| 4 | 1.0 | 0.8 | 16.84 | 0.1306 | 74.5 | |
| 5 | 0.1 | 0.1 | 19.66 | 0.1599 | 91.2 | |
| 6 | 1.0 | 0.1 | 13.63 | 0.1319 | 75.2 | |
| CO2/N2/SDS/H2O | 7 | 0.1 | 0.8 | 18.41 | 0.1187 | 67.7 |
| 8 | 1.0 | 0.8 | 13.95 | 0.0574 | 30.2 | |
| 9 | 0.1 | 0.1 | 15.56 | 0.0670 | 38.2 |
Table 2 Experimental results in different 160 ml systems of SDS and L-Met promoters (7.71 MPa,269.15 K)
| 体 系 | 编号 | 促进剂浓度/% (质量分数) | 雾化喷嘴孔径/mm | 原料气中CO2/%(摩尔分数) | 最终消耗的气体量/(mol/mol H2O) | 水转化为水合物比率/% |
|---|---|---|---|---|---|---|
| CO2/N2/H2O | 1 | 纯水 | 0.8 | 14.38 | 0.0075 | 4.3 |
| 2 | 纯水 | 0.1 | 14.53 | 0.0111 | 6.3 | |
| CO2/N2/L-Met/H2O | 3 | 0.1 | 0.8 | 16.64 | 0.1220 | 69.6 |
| 4 | 1.0 | 0.8 | 16.84 | 0.1306 | 74.5 | |
| 5 | 0.1 | 0.1 | 19.66 | 0.1599 | 91.2 | |
| 6 | 1.0 | 0.1 | 13.63 | 0.1319 | 75.2 | |
| CO2/N2/SDS/H2O | 7 | 0.1 | 0.8 | 18.41 | 0.1187 | 67.7 |
| 8 | 1.0 | 0.8 | 13.95 | 0.0574 | 30.2 | |
| 9 | 0.1 | 0.1 | 15.56 | 0.0670 | 38.2 |
| 1 | Lee H, Romero J. A report of the intergovernmental panel on climate change. contribution of working groups Ⅰ, Ⅱ and Ⅲ to the sixth assessment report of the intergovernmental panel on climate change[R]. Geneva: IPCC, 2023. |
| 2 | Liu H J, Were P, Li Q, et al. Worldwide status of CCUS technologies and their development and challenges in China[J]. Geofluids, 2017, 2017: 6126505. |
| 3 | Gupta M, Coyle I, Thambimuthu K. CO2 capture technologies and opportunities in Canada[C]//1st Canadian CC&S Technology Roadmap Workshop. Canada: CANMET Energy Technology Centre Natural Resources Canada, 2003: 18-19. |
| 4 | Pellegrini G, Strube R, Manfrida G. Comparative study of chemical absorbents in postcombustion CO2 capture[J]. Energy, 2010, 35(2): 851-857. |
| 5 | Martunus, Helwani Z, Wiheeb A D, et al. Improved carbon dioxide capture using metal reinforced hydrotalcite under wet conditions[J]. International Journal of Greenhouse Gas Control, 2012, 7: 127-136. |
| 6 | Dou B L, Song Y C, Liu Y G, et al. High temperature CO2 capture using calcium oxide sorbent in a fixed-bed reactor[J]. Journal of Hazardous Materials, 2010, 183(1/2/3): 759-765. |
| 7 | Sevilla M, Fuertes A B. CO2 adsorption by activated templated carbons[J]. Journal of Colloid and Interface Science, 2012, 366(1): 147-154. |
| 8 | Zanganeh K E, Shafeen A, Salvador C. CO2 capture and development of an advanced pilot-scale cryogenic separation and compression unit[J]. Energy Procedia, 2009, 1(1): 247-252. |
| 9 | Sloan E D, Koh C A, Koh C A. Clathrate Hydrates of Natural Gases[M]. New York: CRC Press, 2007: 685-692. |
| 10 | Park S, Lee S, Lee Y, et al. CO2 capture from simulated fuel gas mixtures using semiclathrate hydrates formed by quaternary ammonium salts[J]. Environmental Science & Technology, 2013, 47(13): 7571-7577. |
| 11 | Zhang P, Wu Q B, Mu C C. Influence of temperature on methane hydrate formation[J]. Scientific Reports, 2017, 7: 7904. |
| 12 | Kang S P, Lee H, Lee C S, et al. Hydrate phase equilibria of the guest mixtures containing CO2, N2 and tetrahydrofuran[J]. Fluid Phase Equilibria, 2001, 185(1/2): 101-109. |
| 13 | Linga P, Kumar R, Englezos P. Gas hydrate formation from hydrogen/carbon dioxide and nitrogen/carbon dioxide gas mixtures[J]. Chemical Engineering Science, 2007, 62(16): 4268-4276. |
| 14 | Seo Y T, Kang S P, Lee H E, et al. Hydrate phase equilibria for gas mixtures containing carbon dioxide: a proof-of-concept to carbon dioxide recovery from multicomponent gas stream[J]. Korean Journal of Chemical Engineering, 2000, 17(6): 659-667. |
| 15 | Kutergin O B, Melnikov V P, Nesterov A N. Influence of surfactants on the mechanism and kinetics of the formation of gas hydrates[J]. Doklady Akademii Nauk, 1992, 323(3): 549-553. |
| 16 | 郎雪梅, 樊栓狮, 王燕鸿, 等. 笼型水合物为能源化工带来新机遇[J]. 化工进展, 2021, 40(9): 4703-4710. |
| Lang X M, Fan S S, Wang Y H, et al. Opportunities for energy and chemical engineering through clathrate hydrates[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 4703-4710. | |
| 17 | Molokitina N S, Nesterov A N, Podenko L S, et al. Carbon dioxide hydrate formation with SDS: further insights into mechanism of gas hydrate growth in the presence of surfactant[J]. Fuel, 2019, 235: 1400-1411. |
| 18 | Linga P, Kumar R, Lee J D, et al. A new apparatus to enhance the rate of gas hydrate formation: application to capture of carbon dioxide[J]. International Journal of Greenhouse Gas Control, 2010, 4(4): 630-637. |
| 19 | Li G, Liu D P, Xie Y M, et al. Study on effect factors for CO2 hydrate rapid formation in a water-spraying apparatus[J]. Energy & Fuels, 2010, 24(8): 4590-4597. |
| 20 | 石定贤, 赵建忠, 赵阳升. 水合物合成喷雾强化机理研究[J]. 辽宁工程技术大学学报, 2006, 25(1): 131-133. |
| Shi D X, Zhao J Z, Zhao Y S. Research on atomization strengthening mechanics for hydrate formation[J]. Journal of Liaoning Technical University, 2006, 25(1): 131-133. | |
| 21 | Sloan E D. Fundamental principles and applications of natural gas hydrates[J]. Nature, 2003, 426: 353-359. |
| 22 | Hassanpouryouzband A, Joonaki E, Farahani M V, et al. Gas hydrates in sustainable chemistry[J]. Chemical Society Reviews, 2020, 49(15): 5225-5309. |
| 23 | Prasad P S R, Sai Kiran B. Clathrate hydrates of greenhouse gases in the presence of natural amino acids: storage, transportation and separation applications[J]. Scientific Reports, 2018, 8: 8560. |
| 24 | Prasad P S, Kiran B S. Are the amino acids thermodynamic inhibitors or kinetic promoters for carbon dioxide hydrates?[J]. Journal of Natural Gas Science and Engineering, 2018, 52: 461-466. |
| 25 | Bavoh C B, Nashed O, Khan M S, et al. The impact of amino acids on methane hydrate phase boundary and formation kinetics[J]. Journal of Chemical Thermodynamics, 2018, 117: 48-53. |
| 26 | Cai Y H, Chen Y L, Li Q J, et al. CO2 hydrate formation promoted by a natural amino acid l-methionine for possible application to CO2 capture and storage[J]. Energy Technology, 2017, 5(8): 1195-1199. |
| 27 | Liu X J, Ren J J, Chen D Y, et al. Comparison of SDS and L-methionine in promoting CO2 hydrate kinetics: implication for hydrate-based CO2 storage[J]. Chemical Engineering Journal, 2022, 438: 135504. |
| 28 | Pandey J S, Daas Y, Sieverts M, et al. Insights into CO2 capture by flue gas hydrate formation using selected amino acids and surfactant[C]// IUPAC 50th General Assembly. Paris, France, 2019: 1. |
| 29 | Jarrahian A, Nakhaee A. Hydrate-liquid-vapor equilibrium condition of N2+CO2+H2O system: measurement and modeling[J]. Fuel, 2019, 237: 769-774. |
| 30 | Sun S C, Liu C L, Meng Q G. Hydrate phase equilibrium of binary guest-mixtures containing CO2 and N2 in various systems[J]. Journal of Chemical Thermodynamics, 2015, 84: 1-6. |
| 31 | Ballard A L. A non-ideal hydrate solid solution model for a multi-phase equilibria program[D]. Golden: Colorado School of Mines, 2002. |
| 32 | Kumar R, Englezos P, Moudrakovski I, et al. Structural and compositional characterization of hydrates formed from CO2/H2 and CO2/H2/C3H8 gas mixtures in relation to simultaneous CO2 capture and H2 production[J]. AIChE Journal, 2009, 55(6): 1584-94. |
| 33 | Davidson D W, Leaist D G, Hesse R. Oxygen-18 enrichment in the water of a clathrate hydrate[J]. Geochimica et Cosmochimica Acta, 1983, 47(12): 2293-2295. |
| 34 | Ripmeester J A, Ratcliffe C I. Low-temperature cross-polarization/magic angle spinning carbon-13 NMR of solid methane hydrates: structure, cage occupancy, and hydration number[J]. Journal of Physical Chemistry, 1988, 92(2): 337-339. |
| 35 | Kang S P, Lee H E. Recovery of CO2 from flue gas using gas hydrate: thermodynamic verification through phase equilibrium measurements[J]. Environmental Science & Technology, 2000, 34(20): 4397-4400. |
| 36 | Seo Y T, Moudrakovski I L, Ripmeester J A, et al. Efficient recovery of CO2 from flue gas by clathrate hydrate formation in porous silica gels[J]. Environmental Science & Technology, 2005, 39(7): 2315-2319. |
| 37 | Li Y, Maria Gambelli A, Chen J Z, et al. Experimental study on the competition between carbon dioxide hydrate and ice below the freezing point[J]. Chemical Engineering Science, 2023, 268: 118426. |
| 38 | Linga P. Separation of carbon dioxide from flue gas (post-combustion capture) via gas hydrate crystallization[D]. Vancouver: University of British Columbia, 2009. |
| 39 | Burla S K, Pinnelli S R P. Enrichment of gas storage in clathrate hydrates by optimizing the molar liquid water-gas ratio[J]. RSC Advances, 2022, 12(4): 2074-2082. |
| 40 | Zhang X, Huang Y L, Ma Z S, et al. Hydrogen-bond memory and water-skin supersolidity resolving the Mpemba paradox[J]. Physical Chemistry Chemical Physics: PCCP, 2014, 16(42): 22995-23002. |
| 41 | 张学民, 李洋, 姚泽, 等. 表面活性剂对气体水合物生成过程的定量影响[J]. 过程工程学报, 2018, 18(2): 356-360. |
| Zhang X M, Li Y, Yao Z, et al. Quantitative influence of surfactant on the formation process for gas hydrate[J]. Chinese Journal of Process Engineering, 2018, 18(2): 356-360. | |
| 42 | Colbeck S C. Capillary bonding of wet surfaces—the effects of contact angle and surface roughness[J]. Journal of Adhesion Science and Technology, 1997, 11(3): 359-371. |
| [1] | Lihao LIU, Ting HUANG, Yu YONG, Xinhao LUO, Zeming ZHAO, Shangfei SONG, Bohui SHI, Guangjin CHEN, Jing GONG. CH4-hydrate formation and solid-phase deposition in salt-sand coexisting flow systems [J]. CIESC Journal, 2024, 75(5): 1987-2000. |
| [2] | Yifei LI, Xinyu DONG, Weishu WANG, Lu LIU, Yifan ZHAO. Numerical study on heat transfer of dry ice sublimation spray cooling on the surface of micro-ribbed plate [J]. CIESC Journal, 2024, 75(5): 1830-1842. |
| [3] | Yaqing ZANG, Yijun ZHANG, Jinzhao WANG, Qian WANG, Dianqing LI, Junting FENG, Xue DUAN. Low energy consumption preparation of anhydrous calcium chloride from hydrated calcium chloride based on reaction coupling [J]. CIESC Journal, 2024, 75(4): 1508-1518. |
| [4] | Jiaqi WANG, Haoqi WEI, Ajing GOU, Jiaxing LIU, Xinlin ZHOU, Kun GE. Study on the formation mechanism of CO2 hydrate under the action of nanoparticles [J]. CIESC Journal, 2024, 75(3): 956-966. |
| [5] | Baofeng WANG, Shugao WANG, Fangqin CHENG. Progress in preparation and CO2 adsorption properties of solid waste-based sulfur-doped porous carbon materials [J]. CIESC Journal, 2024, 75(2): 395-411. |
| [6] | Mingqing TAO, Minghao MU, Teng CHENG, Bo WANG. Research on spray coupled cooling to enhance the removal of fine particles by cyclone separator [J]. CIESC Journal, 2024, 75(2): 584-592. |
| [7] | Xiaoyang LI, Dong LI, Minglei TAO, Zhifu ZHOU, Lingyi ZHANG, Lizheng SU, Tianning ZHANG, Zhi LI, Bin CHEN. Experimental study on heat transfer characteristics of multi nozzle spray cooling surface [J]. CIESC Journal, 2024, 75(1): 231-241. |
| [8] | Ruohan ZHAO, Mengmeng HUANG, Chunying ZHU, Taotao FU, Xiqun GAO, Youguang MA. Flow and mass transfer study of CO2 absorption by nanofluid in T-shaped microchannels [J]. CIESC Journal, 2024, 75(1): 221-230. |
| [9] | Yue YANG, Dan ZHANG, Jugan ZHENG, Maoping TU, Qingzhong YANG. Experimental study on flash and mixing evaporation of aqueous NaCl solution [J]. CIESC Journal, 2023, 74(8): 3279-3291. |
| [10] | Xudong YU, Qi LI, Niancu CHEN, Li DU, Siying REN, Ying ZENG. Phase equilibria and calculation of aqueous ternary system KCl + CaCl2 + H2O at 298.2, 323.2, and 348.2 K [J]. CIESC Journal, 2023, 74(8): 3256-3265. |
| [11] | Tianhua CHEN, Zhaoxuan LIU, Qun HAN, Chengbin ZHANG, Wenming LI. Research progress and influencing factors of the heat transfer enhancement of spray cooling [J]. CIESC Journal, 2023, 74(8): 3149-3170. |
| [12] | Haopeng SHI, Dawen ZHONG, Xuexin LIAN, Junfeng ZHANG. Experimental study on the downward-facing surface enhanced boiling heat transfer of multiscale groove-fin structures [J]. CIESC Journal, 2023, 74(7): 2880-2888. |
| [13] | Zhen LONG, Jinhang WANG, Junjie REN, Yong HE, Xuebing ZHOU, Deqing LIANG. Experimental study on inhibition effect of natural gas hydrate formation by mixing ionic liquid with PVCap [J]. CIESC Journal, 2023, 74(6): 2639-2646. |
| [14] | Lei MAO, Guanzhang LIU, Hang YUAN, Guangya ZHANG. Efficient preparation of carbon anhydrase nanoparticles capable of capturing CO2 and their characteristics [J]. CIESC Journal, 2023, 74(6): 2589-2598. |
| [15] | Wenchao XU, Zhigao SUN, Cuimin LI, Juan LI, Haifeng HUANG. Effect of surfactant E-1310 on the formation of HCFC-141b hydrate under static conditions [J]. CIESC Journal, 2023, 74(5): 2179-2185. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||