CIESC Journal ›› 2024, Vol. 75 ›› Issue (11): 3857-3869.DOI: 10.11949/0438-1157.20240639
• Reviews and monographs • Previous Articles Next Articles
Jing ZHAO1( ), Gongping LIU1, Wanqin JIN1(
), Gongping LIU1, Wanqin JIN1( ), Nanping XU2
), Nanping XU2
Received:2024-06-07
Revised:2024-07-07
Online:2024-12-26
Published:2024-11-25
Contact:
Wanqin JIN
通讯作者:
金万勤
作者简介:赵静(1989—),女,博士,教授, zhaojingmem@njtech.edu.cn
基金资助:CLC Number:
Jing ZHAO, Gongping LIU, Wanqin JIN, Nanping XU. Precision construction and application of separation membranes based on confined mass transfer mechanism[J]. CIESC Journal, 2024, 75(11): 3857-3869.
赵静, 刘公平, 金万勤, 徐南平. 限域传质分离膜的精密构筑与应用[J]. 化工学报, 2024, 75(11): 3857-3869.
Add to citation manager EndNote|Ris|BibTeX
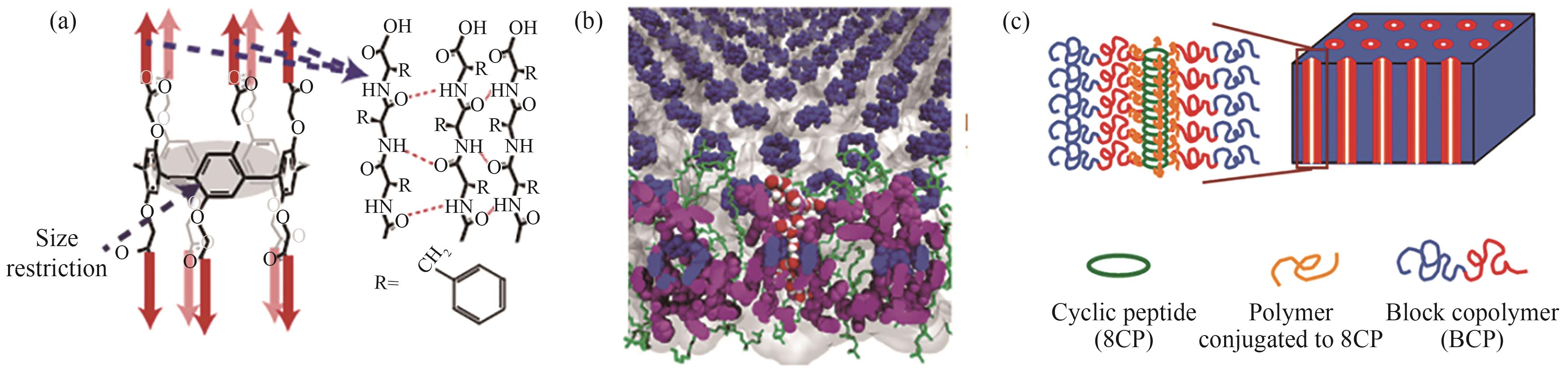
Fig.1 Schematic diagrams of formation of nanotube through assembly of pillar[5]arene[8] (a); embedding of nanotubes in bilayers[8] (b), assembly of cyclic peptide with the assistance of block copolymer[17](c)
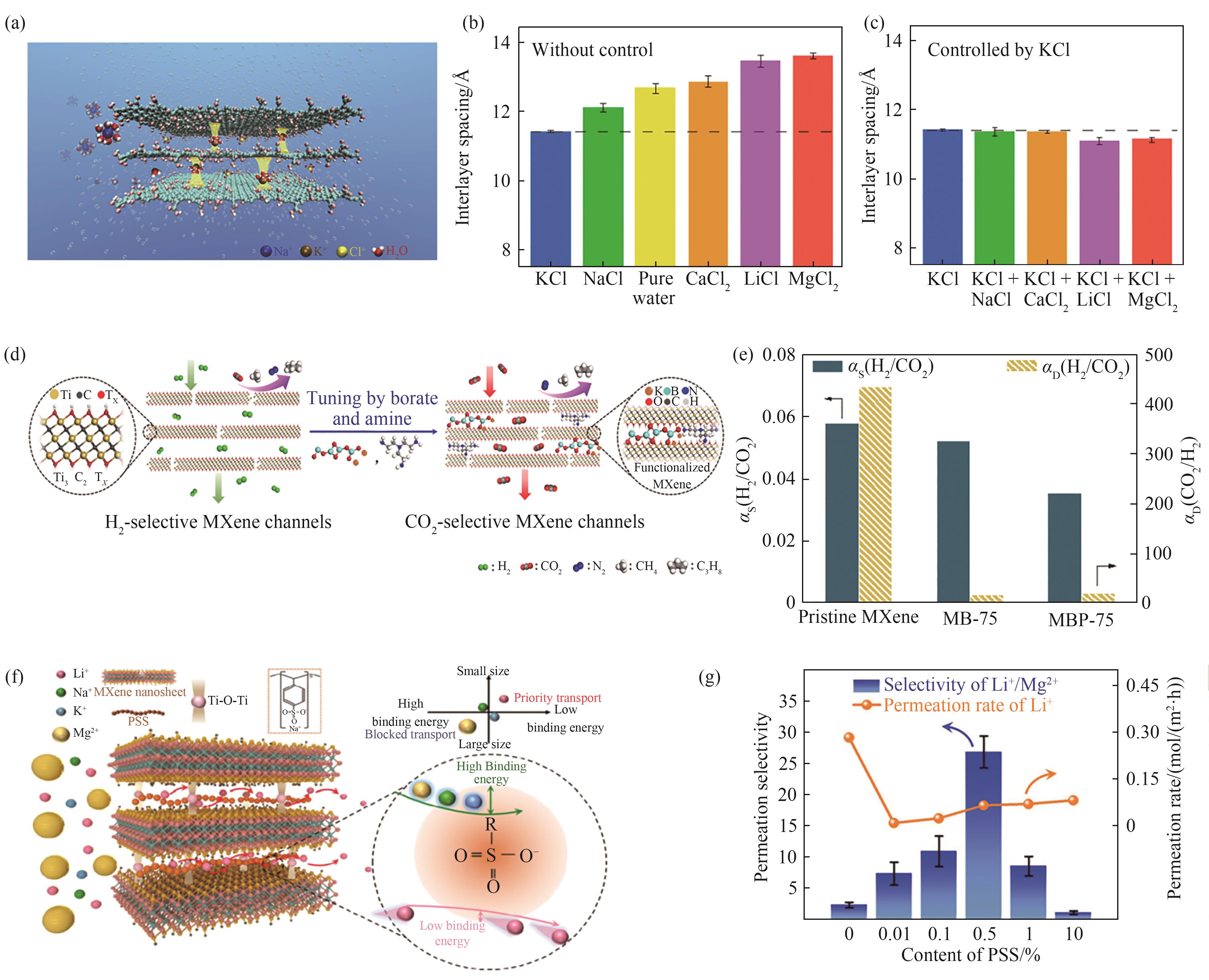
Fig.2 (a) A schematic of how K+ ions fix the interlayer spacing of GO membrane such that other cations are rejected[29]; (b) Interlayer spacings for GO membranes immersed in pure water or in various salt solutions[29]; (c) Interlayer spacings of GO membranes that were soaked in KCl solution, followed by being immersed in various salt solutions[29]; (d) Schematic of the transformation of MXene channels from “diffusion-controlled” to “solution-controlled”[20]; (e) Change of sorption selectivity and diffusion selectivity after chemical tuning of MXene membrane (MB: MXene+borate; MBP: MXene+borate+PEI)[20]; (f) Schematic of the fast transport subnanochannels for Li+ in MXene/PSS composite membranes[21]; (g) The PSS content dependent ion permeation rates and permselectivity of MXene/PSS composite membrane[21]
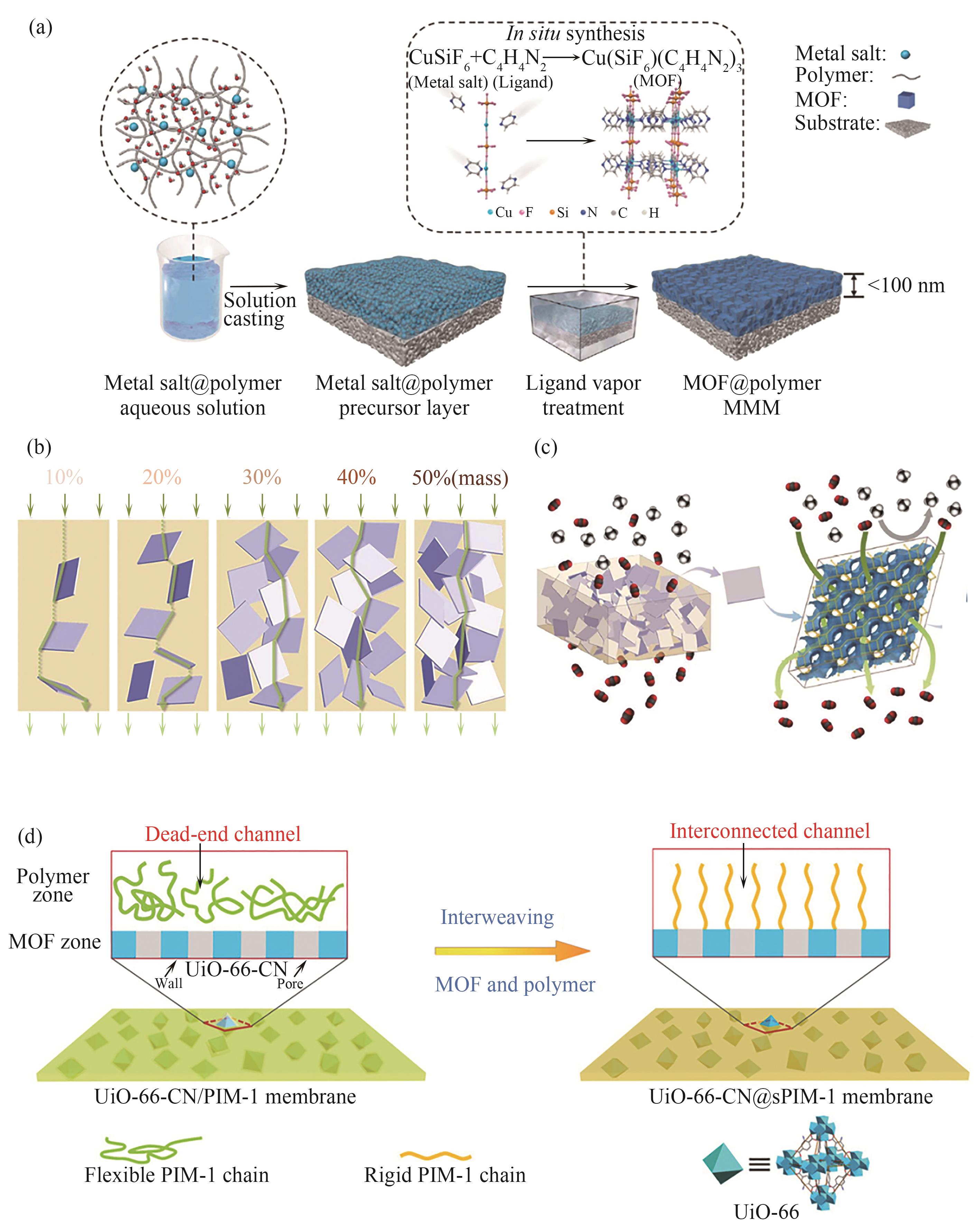
Fig.3 Schematic diagram of fabricating ultrathin and high-loading MOFs mixed matrix membranes via a solid-solvent processing strategy[53]; (b) Illustration of the nonaligned zeolite platelet distribution in the polymer matrix with different zeolite loadings[54]; (c) Illustration of the mixed matrix membrane with quasi-continuous zeolite phase and the unhindered CO2 permeation (indicated with green arrows) through the 3D-channel system of platelet-shaped Na-SSZ-39 filler[54]; (d) Schematic diagram of building CO2 transport freeways through interweaving UiO-66 and PIM-1[43]
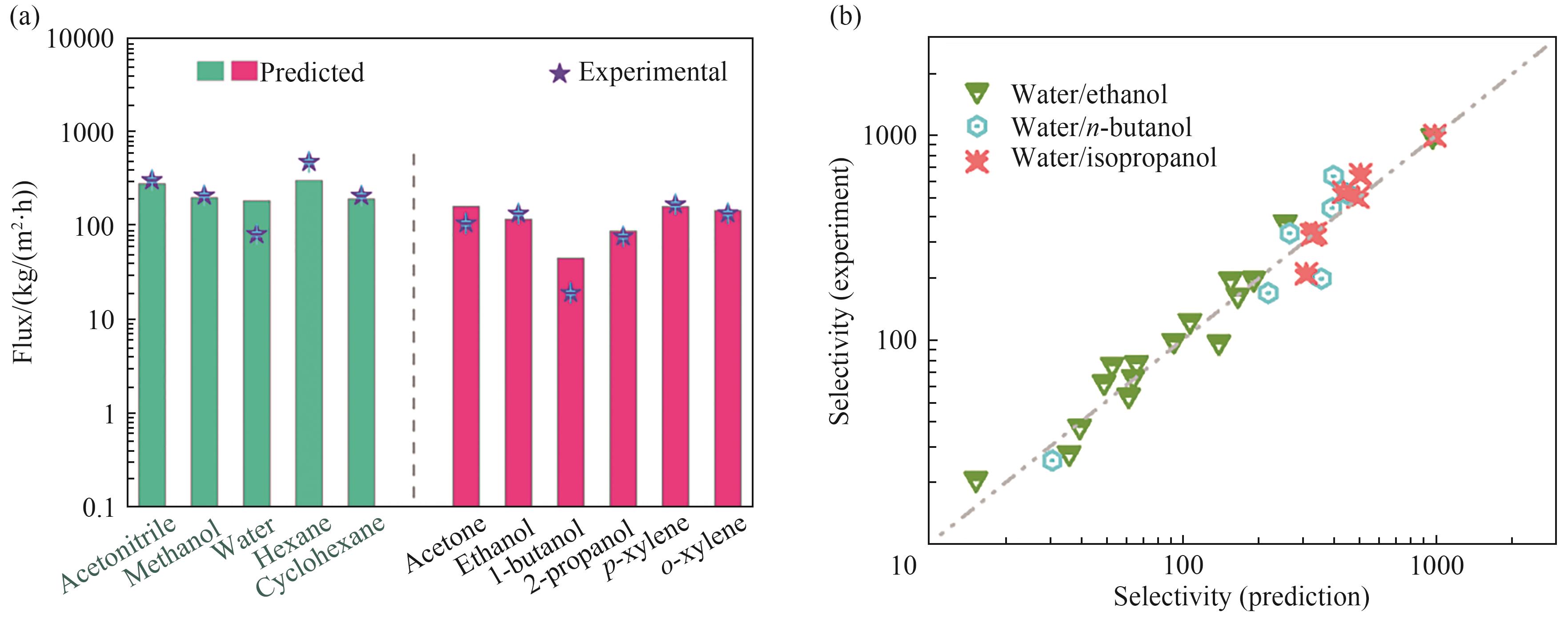
Fig.4 (a) The experimental fluxes (purple stars) and predicted fluxes (bars) of different solvents through Ti3C2/graphene channels[60]; (b) The experimental and predicted selectivities of different water/alcohol systems (water/ethanol, water/n-butanol, and water/isopropanol) in graphene-based membranes[61]
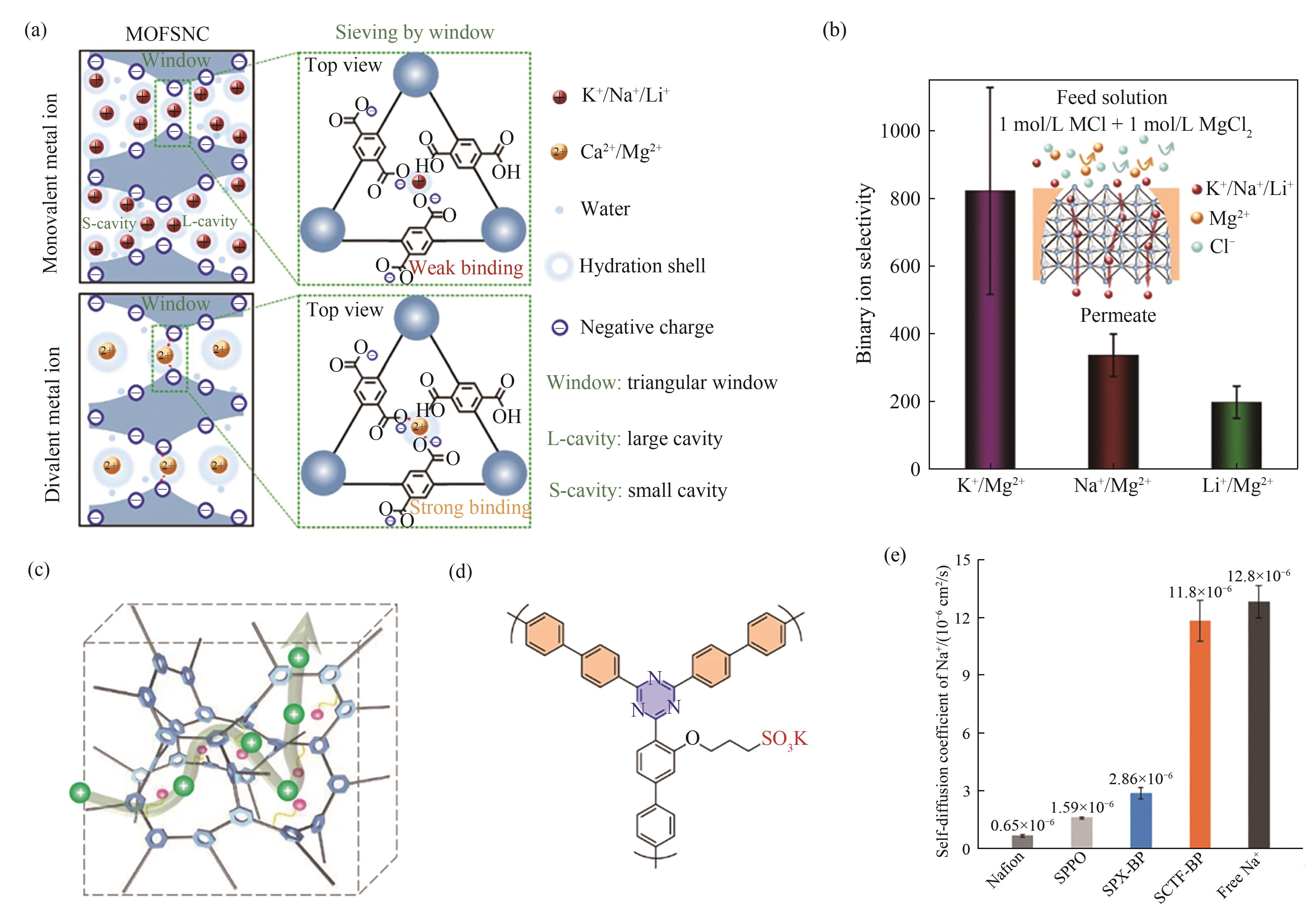
Fig.5 (a) Ion sieving mechanism and (b) binary K+/Mg2+, Na+/Mg2+ and Li+/Mg2+ selectivities in the UiO-66-(COOH)2 subnanochannels (MOFSNC)[63]; (c) Schematic diagram of the rigid ion channels in sulfonated SCTF (SCTF) membrane[65]; (d) Molecular structure of SCTF- biphenyl (SCTF-BP)[65]; (e) Diffusion coefficients of Na+ in water and different membrane samples[65]

Fig.6 (a) Schematic illustration of the COF membrane with hydrophilicity gradient for membrane distillation[66]; (b) Water evaporation flux as a function of pore diameter (molecular dynamics simulations)[66]; (c) Schematic for the channel structures and interactions in the COF membranes formed through binding of COF nanosheets and nanoribbons[67]; (d) Temperature-dependent permeation flux of COF membrane[67]; (e) Schematic illustration of the Ti3C2-graphene membrane structure[60]; (f) Water flux of the Ti3C2-graphene hetero-channel membranes under different feed temperatures[60]
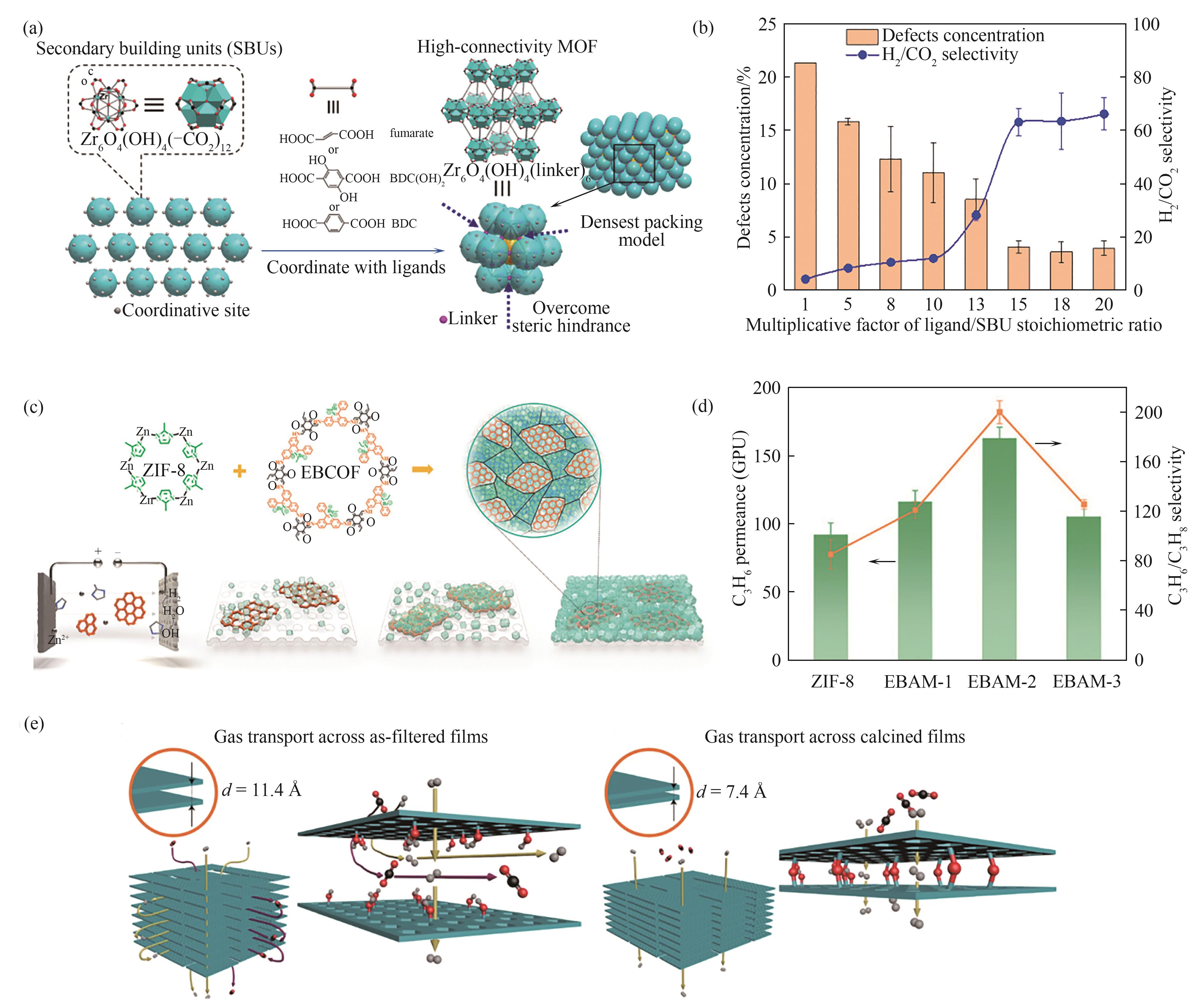
Fig.7 (a) Schematic diagram of the formation of MOF membranes with perfect lattices[71]; (b) The defect concentration and H2/CO2 selectivity of Zr-MOF (fumarate) membranes prepared using various multiplicative factors of the ligand/SBU stoichiometric ratio[71]; (c) Schematic diagram of the construction of MOF-COF alloy membranes[72]; (d) Mixed C3H6/C3H8 separation performance of the MOF-COF alloy membrane[72]; (e) Comparison of the gas transport channel strctures in RUB zeolite membranes before and after calcination[36]
| 1 | Sholl D S, Lively R P. Seven chemical separations to change the world[J]. Nature, 2016, 532(7600): 435-437. |
| 2 | 金万勤, 徐南平. 限域传质分离膜[J]. 化工学报, 2018, 69(1): 50-56. |
| Jin W Q, Xu N P. Membrane separation based on mechanism of confined mass transfer[J]. CIESC Journal, 2018, 69(1): 50-56. | |
| 3 | 朱育丹, 陆小华, 谢文龙, 等. 基于限域传质机制的膜过程定量描述的研究进展[J]. 科学通报, 2017, 62(S1): 223-232. |
| Zhu Y D, Lu X H, Xie W L, et al. The progress of quantitatively description of membrane process based on the mechanism of nanoconfined mass transfer[J]. Chinese Science Bulletin, 2017, 62(S1): 223-232. | |
| 4 | 刘壮, 汪伟, 巨晓洁, 等. 具有限域传质效应的碳基分离膜: 从碳纳米管膜到石墨烯膜[J]. 化工学报, 2018, 69(1): 166-174. |
| Liu Z, Wang W, Ju X J, et al. Carbon-based membranes with confinement effect for mass transport: from carbon nano-tube membranes to graphene membranes[J]. CIESC Journal, 2018, 69(1): 166-174. | |
| 5 | Robeson L M. Correlation of separation factor versus permeability for polymeric membranes[J]. Journal of Membrane Science, 1991, 62(2): 165-185. |
| 6 | Freeman B D. Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes[J]. Macromolecules, 1999, 32(2): 375-380. |
| 7 | Shen J, Liu G P, Han Y, et al. Artificial channels for confined mass transport at the sub-nanometre scale[J]. Nature Reviews Materials, 2021, 6: 294-312. |
| 8 | Shen Y X, Song W, Barden D R, et al. Achieving high permeability and enhanced selectivity for angstrom-scale separations using artificial water channel membranes[J]. Nature Communications, 2018, 9(1): 2294. |
| 9 | Song W, Joshi H, Chowdhury R, et al. Artificial water channels enable fast and selective water permeation through water-wire networks[J]. Nature Nanotechnology, 2020, 15(1): 73-79. |
| 10 | Kocsis I, Sorci M, Vanselous H, et al. Oriented chiral water wires in artificial transmembrane channels[J]. Science Advances, 2018, 4(3): eaao5603. |
| 11 | Hinds B J, Chopra N, Rantell T, et al. Aligned multiwalled carbon nanotube membranes[J]. Science, 2004, 303(5654): 62-65. |
| 12 | Secchi E, Marbach S, Niguès A, et al. Massive radius-dependent flow slippage in carbon nanotubes[J]. Nature, 2016, 537(7619): 210-213. |
| 13 | Vatanpour V, Ali Naziri Mehrabani S, Keskin B, et al. A comprehensive review on the applications of boron nitride nanomaterials in membrane fabrication and modification[J]. Industrial & Engineering Chemistry Research, 2021, 60(37): 13391-13424. |
| 14 | Kim W G, Nair S. Membranes from nanoporous 1D and 2D materials: a review of opportunities, developments, and challenges[J]. Chemical Engineering Science, 2013, 104: 908-924. |
| 15 | Hummer G, Rasaiah J C, Noworyta J P. Water conduction through the hydrophobic channel of a carbon nanotube[J]. Nature, 2001, 414: 188-190. |
| 16 | Huang L B, Hardiagon A, Kocsis I, et al. Hydroxy channels-adaptive pathways for selective water cluster permeation[J]. Journal of the American Chemical Society, 2021, 143(11): 4224-4233. |
| 17 | Xu T, Zhao N N, Ren F, et al. Subnanometer porous thin films by the co-assembly of nanotube subunits and block copolymers[J]. ACS Nano, 2011, 5(2): 1376-1384. |
| 18 | Shen J, Liu G P, Huang K, et al. Subnanometer two-dimensional graphene oxide channels for ultrafast gas sieving[J]. ACS Nano, 2016, 10(3): 3398-3409. |
| 19 | Wang S F, Wu Y Z, Zhang N, et al. A highly permeable graphene oxide membrane with fast and selective transport nanochannels for efficient carbon capture[J]. Energy & Environmental Science, 2016, 9(10): 3107-3112. |
| 20 | Shen J, Liu G Z, Ji Y F, et al. 2D MXene nanofilms with tunable gas transport channels[J]. Advanced Functional Materials, 2018, 28(31): 1801511. |
| 21 | Lu Z, Wu Y, Ding L, et al. A lamellar MXene (Ti3C2T x )/PSS composite membrane for fast and selective lithium-ion separation[J]. Angewandte Chemie International Edition, 2021, 60(41): 22265-22269. |
| 22 | Mei L, Cao Z L, Ying T, et al. Simultaneous electrochemical exfoliation and covalent functionalization of MoS2 membrane for ion sieving[J]. Advanced Materials, 2022, 34(26): 2201416. |
| 23 | Fan Z W, Zhang J, Zuo B Y, et al. Biomimetic guttation feature in 2D hydrotalcite membranes for self-sustaining water purification[J]. Advanced Functional Materials, 2024, 34(26): 2316247. |
| 24 | Liu G P, Jin W Q, Xu N P. Two-dimensional-material membranes: a new family of high-performance separation membranes[J]. Angewandte Chemie International Edition, 2016, 55(43): 13384-13397. |
| 25 | Liu G P, Jin W Q, Xu N P. Graphene-based membranes[J]. Chemical Society Reviews, 2015, 44(15): 5016-5030. |
| 26 | Deng J J, Lu Z, Ding L, et al. Fast electrophoretic preparation of large-area two-dimensional titanium carbide membranes for ion sieving[J]. Chemical Engineering Journal, 2021, 408: 127806. |
| 27 | Nair R R, Wu H A, Jayaram P N, et al. Unimpeded permeation of water through helium-leak-tight graphene-based membranes[J]. Science, 2012, 335(6067): 442-444. |
| 28 | Zheng S X, Tu Q S, Urban J J, et al. Swelling of graphene oxide membranes in aqueous solution: characterization of interlayer spacing and insight into water transport mechanisms[J]. ACS Nano, 2017, 11(6): 6440-6450. |
| 29 | Chen L, Shi G S, Shen J, et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing[J]. Nature, 2017, 550(7676): 380-383. |
| 30 | Zhang M C, Mao Y Y, Liu G Z, et al. Molecular bridges stabilize graphene oxide membranes in water[J]. Angewandte Chemie International Edition, 2020, 59(4): 1689-1695. |
| 31 | Zhang M C, Zhao P X, Li P S, et al. Designing biomimic two-dimensional ionic transport channels for efficient ion sieving[J]. ACS Nano, 2021, 15(3): 5209-5220. |
| 32 | Cheng L, Guo Y N, Liu Q, et al. Metal confined in 2D membranes for molecular recognition and sieving towards ethylene/ethane separation[J]. Advanced Materials, 2022, 34(44): 2206349. |
| 33 | Akbari A, Sheath P, Martin S T, et al. Large-area graphene-based nanofiltration membranes by shear alignment of discotic nematic liquid crystals of graphene oxide[J]. Nature Communications, 2016, 7: 10891. |
| 34 | Liu S, Liu G Z, Chen G N, et al. Scale-up fabrication of two-dimensional material membranes: challenges and opportunities[J]. Current Opinion in Chemical Engineering, 2023, 39: 100892. |
| 35 | Liu Z Y, Ma Z, Qian B T, et al. A facile and scalable method of fabrication of large-area ultrathin graphene oxide nanofiltration membrane[J]. ACS Nano, 2021, 15(9): 15294-15305. |
| 36 | Dakhchoune M, Villalobos L F, Semino R, et al. Gas-sieving zeolitic membranes fabricated by condensation of precursor nanosheets[J]. Nature Materials, 2021, 20(3): 362-369. |
| 37 | Du P, Zhang Y T, Wang X R, et al. Control of zeolite framework flexibility for ultra-selective carbon dioxide separation[J]. Nature Communications, 2022, 13(1): 1427. |
| 38 | Li L, Xu R S, Song C W, et al. A review on the progress in nanoparticle/C hybrid CMS membranes for gas separation[J]. Membranes, 2018, 8(4): 134. |
| 39 | Koh D Y, McCool B A, Deckman H W, et al. Reverse osmosis molecular differentiation of organic liquids using carbon molecular sieve membranes[J]. Science, 2016, 353(6301): 804-807. |
| 40 | Wang H J, Zhai Y M, Li Y, et al. Covalent organic framework membranes for efficient separation of monovalent cations[J]. Nature Communications, 2022, 13(1): 7123. |
| 41 | Hasell T, Cooper A I. Porous organic cages: soluble, modular and molecular pores[J]. Nature Reviews Materials, 2016, 1(9): 16053. |
| 42 | Zhao S, Zhao Z Y, Zha Z Y, et al. Amine-rich molecular nodule-assembled membrane having 5 angstrom channels for CO2/N2 separation[J]. Advanced Functional Materials, 2024, 34(27): 2314469. |
| 43 | Yu G L, Zou X Q, Sun L, et al. Constructing connected paths between UiO-66 and PIM-1 to improve membrane CO2 separation with crystal-like gas selectivity[J]. Advanced Materials, 2019, 31(15): 1806853. |
| 44 | Cooper A I. Conjugated microporous polymers[J]. Advanced Materials, 2009, 21(12): 1291-1295. |
| 45 | Ma Y, Cui F C, Rong H Z, et al. Continuous porous aromatic framework membranes with modifiable sites for optimized gas separation[J]. Angewandte Chemie International Edition, 2022, 61(1): e202113682. |
| 46 | Du W G, Liu L, Yin L Y, et al. Ultrathin free-standing porous aromatic framework membranes for efficient anion transport[J]. Angewandte Chemie International Edition, 2024, 63(22): e202402943. |
| 47 | Wang H J, Wang M D, Liang X, et al. Organic molecular sieve membranes for chemical separations[J]. Chemical Society Reviews, 2021, 50(9): 5468-5516. |
| 48 | Yuan D Q, Lu W G, Zhao D, et al. Highly stable porous polymer networks with exceptionally high gas-uptake capacities[J]. Advanced Materials, 2011, 23(32): 3723-3725. |
| 49 | Chen G N, Liu G Z, Pan Y, et al. Zeolites and metal-organic frameworks for gas separation: the possibility of translating adsorbents into membranes[J]. Chemical Society Reviews, 2023, 52(14): 4586-4602. |
| 50 | Peng Y, Li Y S, Ban Y J, et al. Metal-organic framework nanosheets as building blocks for molecular sieving membranes[J]. Science, 2014, 346(6215): 1356-1359. |
| 51 | Li J R, Zhou H C. Bridging-ligand-substitution strategy for the preparation of metal-organic polyhedra[J]. Nature Chemistry, 2010, 2(10): 893-898. |
| 52 | Qiao Z H, Zhao S, Sheng M L, et al. Metal-induced ordered microporous polymers for fabricating large-area gas separation membranes[J]. Nature Materials, 2019, 18(2): 163-168. |
| 53 | Chen G N, Chen C L, Guo Y N, et al. Solid-solvent processing of ultrathin, highly loaded mixed-matrix membrane for gas separation[J]. Science, 2023, 381(6664): 1350-1356. |
| 54 | Tan X Y, Robijns S, Thür R, et al. Truly combining the advantages of polymeric and zeolite membranes for gas separations[J]. Science, 2022, 378(6625): 1189-1194. |
| 55 | Keerthi A, Goutham S, You Y, et al. Water friction in nanofluidic channels made from two-dimensional crystals[J]. Nature Communications, 2021, 12(1): 3092. |
| 56 | Xie Q, Alibakhshi M A, Jiao S P, et al. Fast water transport in graphene nanofluidic channels[J]. Nature Nanotechnology, 2018, 13(3): 238-245. |
| 57 | Tunuguntla R H, Henley R Y, Yao Y C, et al. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins[J]. Science, 2017, 357(6353): 792-796. |
| 58 | Keerthi A, Geim A K, Janardanan A, et al. Ballistic molecular transport through two-dimensional channels[J]. Nature, 2018, 558(7710): 420-424. |
| 59 | 覃瑶, 张禹萌, 潘雪玲, 等. 限域传递机制初探:以限域状态为切入点描述传递阻力[J]. 化工学报, 2023, 74(1): 74-85. |
| Qin Y, Zhang Y M, Pan X L, et al. Preliminary study on mechanism of transfer in confined space: description of confined transfer resistance based on confined fluid state[J]. CIESC Journal, 2023, 74(1): 74-85. | |
| 60 | Chen X F, Qin Y, Zhu Y D, et al. Accurate prediction of solvent flux in sub-1-nm slit-pore nanosheet membranes[J]. Science Advances, 2024, 10(17): eadl1455. |
| 61 | 高庆伟, 覃瑶, 张禹萌, 等. 限域传质分离机制初探:界面吸附层的“二次限域”效应[J]. 化工学报, 2020, 71(10): 4688-4695. |
| Gao Q W, Qin Y, Zhang Y M, et al. Preliminary study on mechanism of confined mass transfer and separation: “secondary confinement” effect of interfacial adsorption layer[J]. CIESC Journal, 2020, 71(10): 4688-4695. | |
| 62 | Pan X L, Ma Z H, Qin Y, et al. Modeling of alcohol/water separation in graphene-based membranes: the roles of interfacial adsorption and the effective transfer path[J]. Industrial & Engineering Chemistry Research, 2024, 63(14): 6399-6410. |
| 63 | Lu J, Zhang H C, Hou J, et al. Efficient metal ion sieving in rectifying subnanochannels enabled by metal-organic frameworks[J]. Nature Materials, 2020, 19(7): 767-774. |
| 64 | Zhao C, Feng F, Hou J, et al. Unlocking direct lithium extraction in harsh conditions through thiol-functionalized metal-organic framework subnanofluidic membranes[J]. Journal of the American Chemical Society, 2024, 146(20): 14058-14066. |
| 65 | Zuo P P, Ye C C, Jiao Z R, et al. Near-frictionless ion transport within triazine framework membranes[J]. Nature, 2023, 617(7960): 299-305. |
| 66 | Zhao S, Jiang C H, Fan J C, et al. Hydrophilicity gradient in covalent organic frameworks for membrane distillation[J]. Nature Materials, 2021, 20(11): 1551-1558. |
| 67 | Wang M D, Zhang P H, Liang X, et al. Ultrafast seawater desalination with covalent organic framework membranes[J]. Nature Sustainability, 2022, 5: 518-526. |
| 68 | Zhang M C, Guan K C, Shen J, et al. Nanoparticles@rGO membrane enabling highly enhanced water permeability and structural stability with preserved selectivity[J]. AIChE Journal, 2017, 63(11): 5054-5063. |
| 69 | Liang F, Liu Q, Zhao J, et al. Ultrafast water-selective permeation through graphene oxide membrane with water transport promoters[J]. AIChE Journal, 2020, 66(2): e16812. |
| 70 | Yang G, Xie Z L, Cran M, et al. Functionalizing graphene oxide framework membranes with sulfonic acid groups for superior aqueous mixture separation[J]. Journal of Materials Chemistry A, 2019, 7(34): 19682-19690. |
| 71 | Liu G Z, Guo Y N, Chen C L, et al. Eliminating lattice defects in metal-organic framework molecular-sieving membranes[J]. Nature Materials, 2023, 22(6): 769-776. |
| 72 | Liu Y T, Wu H, Li R L, et al. MOF-COF “alloy” membranes for efficient propylene/propane separation[J]. Advanced Materials, 2022, 34(24): 2201423. |
| [1] | Kuangxi LI, Peiqian YU, Jiangyun WANG, Haoran WEI, Zhigang ZHENG, Liuhai FENG. Flow analysis and structure optimization of micro-bubble swirling air flotation device [J]. CIESC Journal, 2024, 75(S1): 223-234. |
| [2] | Huihui XIE, Jiaxin JIANG, Xin WANG, Zheng LI, Xin GUO, Xinran LYU, Lingyun WANG, Yang LIU. Study on transport separation of platinum and palladium by deep eutectic solvent polymer inclusion membrane [J]. CIESC Journal, 2024, 75(S1): 235-243. |
| [3] | Zhi QIU, Ming TAN. Preparation of polyionic liquid membrane and its application in low-sodium and high-potassium healthy soy sauce [J]. CIESC Journal, 2024, 75(S1): 244-250. |
| [4] | Yingyu XU, Guoqiang YANG, Jing PENG, Haining SUN, Zhibing ZHANG. Research on advanced oxidation treatment of coal chemical wastewater using microinterfaces [J]. CIESC Journal, 2024, 75(S1): 283-291. |
| [5] | Lü LIU, Jieru LIU, Liangliang FAN, Liang ZHAO. Study on passive microfluidic method for particle separation based on laminar effect [J]. CIESC Journal, 2024, 75(S1): 67-75. |
| [6] | Zhenghang LUO, Jingyu LI, Weixiong CHEN, Daotong CHONG, Junjie YAN. Numerical simulation of heat transfer characteristic and bubble force analysis of low flow rate vapor condensation under rolling motion [J]. CIESC Journal, 2024, 75(8): 2800-2811. |
| [7] | Qianqian WANG, Bing LI, Weibo ZHENG, Guomin CUI, Bingtao ZHAO, Pingwen MING. Three-dimensional modeling of local dynamic characteristics in hydrogen fuel cells [J]. CIESC Journal, 2024, 75(8): 2812-2820. |
| [8] | Gang ZENG, Lin CHEN, Dong YANG, Haizhuan YUAN, Yanping HUANG. Visualization of local boundary thermal flow field of supercritical CO2 inside a rectangular channel [J]. CIESC Journal, 2024, 75(8): 2831-2839. |
| [9] | Xiaoqiao QIN, Hongbo TAN, Na WEN. Thermodynamic and economic analysis of air separation unit with energy storage and generation [J]. CIESC Journal, 2024, 75(7): 2409-2421. |
| [10] | Wenxuan ZHOU, Zhen LIU, Fujian ZHANG, Zhongqiang ZHANG. Mechanism of water treatment by high permeability-selectivity time dimension membrane method [J]. CIESC Journal, 2024, 75(7): 2583-2593. |
| [11] | Xiaoping LUO, Yuntian HOU, Yijie FAN. Flow boiling heat transfer and temperature uniformity in micro-channel with countercurrent phase separation structure [J]. CIESC Journal, 2024, 75(7): 2474-2485. |
| [12] | Songhong ZHANG, Xinyi ZHAO, Xiaoling LOU, Shaochuan SHEN, Junxian YUN. Separation of lactoperoxidase using cation exchange nano-cryogels [J]. CIESC Journal, 2024, 75(7): 2574-2582. |
| [13] | Zongwei HUO, Yabin NIU, Yanqiu PAN. Behavior of high viscosity oil droplets in oil-water membrane separation and its influencing factors [J]. CIESC Journal, 2024, 75(6): 2262-2273. |
| [14] | Yiqi ZHANG, Xuesong TAN, Wuhuan LI, Quan ZHANG, Changlin MIAO, Xinshu ZHUANG. Efficient fractionation of sugarcane bagasse with phenoxyethanol under mild condition [J]. CIESC Journal, 2024, 75(6): 2274-2282. |
| [15] | Wei WANG, Xu BAI, Xiang ZHAO, Xueliang MA, Wei LIN, Jiuyang YU. Optimization of air flotation cyclone separation conditions based on response surface methodology [J]. CIESC Journal, 2024, 75(5): 1929-1938. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||