CIESC Journal ›› 2024, Vol. 75 ›› Issue (11): 3883-3895.DOI: 10.11949/0438-1157.20241097
• Reviews and monographs • Previous Articles Next Articles
Hongying ZHUO1( ), Zhongzheng ZHAO1,2, Zheng SHEN1, Xiaofeng YANG1(
), Zhongzheng ZHAO1,2, Zheng SHEN1, Xiaofeng YANG1( ), Yanqiang HUANG1
), Yanqiang HUANG1
Received:2024-09-30
Revised:2024-10-29
Online:2024-12-26
Published:2024-11-25
Contact:
Xiaofeng YANG
卓红英1( ), 赵忠正1,2, 沈铮1, 杨小峰1(
), 赵忠正1,2, 沈铮1, 杨小峰1( ), 黄延强1
), 黄延强1
通讯作者:
杨小峰
作者简介:卓红英(1990—),女,博士,助理研究员,zhuohongying@dicp.ac.cn
基金资助:CLC Number:
Hongying ZHUO, Zhongzheng ZHAO, Zheng SHEN, Xiaofeng YANG, Yanqiang HUANG. Research progress on the catalytic conversion of ortho- to para-hydrogen[J]. CIESC Journal, 2024, 75(11): 3883-3895.
卓红英, 赵忠正, 沈铮, 杨小峰, 黄延强. 正-仲氢催化转化研究进展[J]. 化工学报, 2024, 75(11): 3883-3895.
Add to citation manager EndNote|Ris|BibTeX
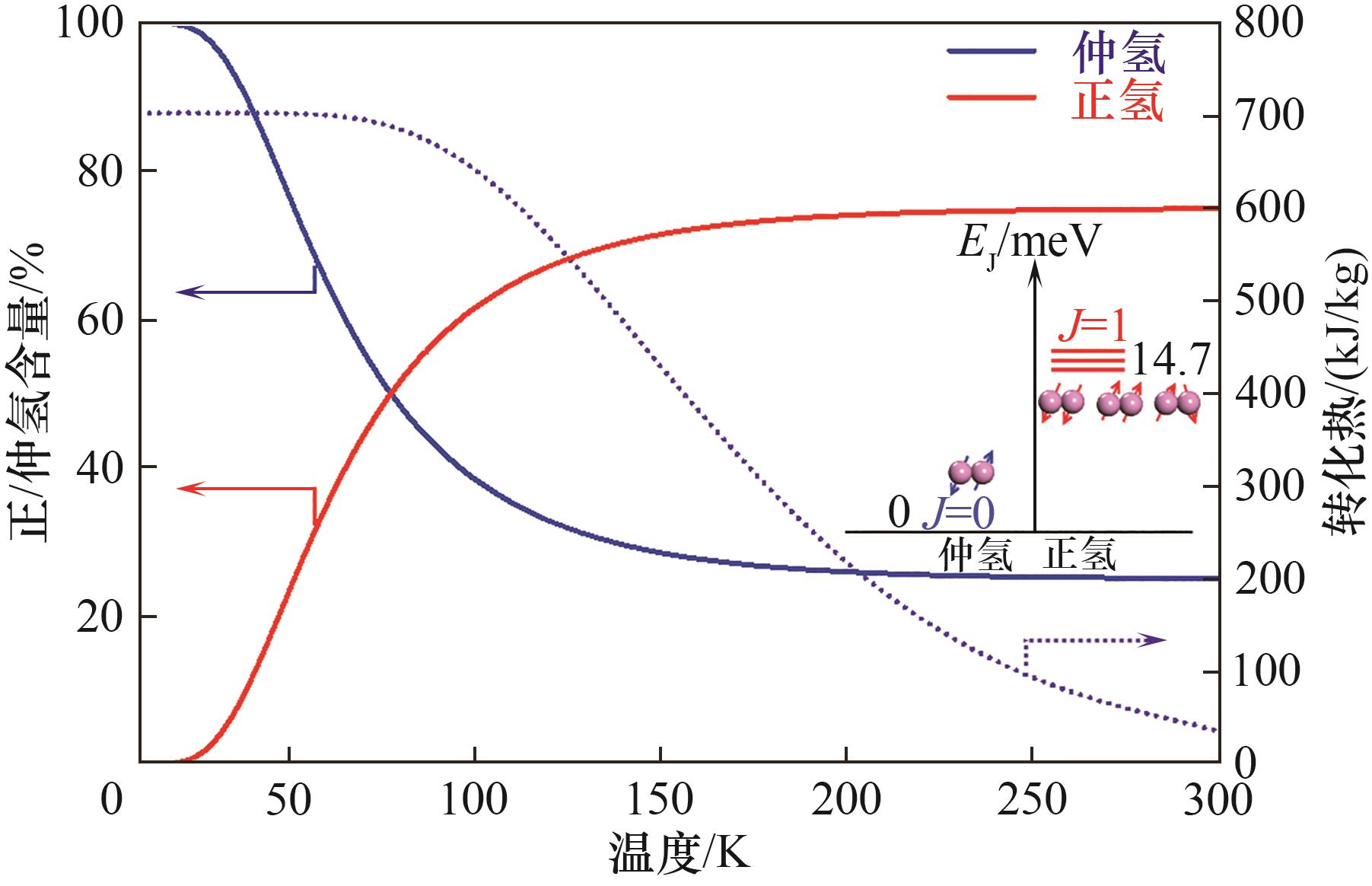
Fig.1 The composition and transformation enthalpy of ortho- to para-H2 as a function of temperature ( inset shows the energy difference of H2 at different nuclear spins)
| 1 | Huang D H, Huzel D K. Modern Engineering for Design of Liquid-Propellant Rocket Engines[M]. Washington DC: American Institute of Aeronautics and Astronautics, 1992. |
| 2 | Wallace J, Ward C. Hydrogen as a fuel[J]. International Journal of Hydrogen Energy, 1983, 8(4): 255-268. |
| 3 | Tiwari S, Pekris M J, Doherty J J. A review of liquid hydrogen aircraft and propulsion technologies[J]. International Journal of Hydrogen Energy, 2024, 57: 1174-1196. |
| 4 | Smith J R, Mastorakos E. An energy systems model of large commercial liquid hydrogen aircraft in a low-carbon future[J]. International Journal of Hydrogen Energy, 2024, 52: 633-654. |
| 5 | He Y C, Hu C Z, Jiang B, et al. Data-driven approach to predict the flow boiling heat transfer coefficient of liquid hydrogen aviation fuel[J]. Fuel, 2022, 324: 124778. |
| 6 | Bracha M. Liquid hydrogen—status and trends as potential aviation fuel[M]//Colpan C O, Kovač A. Sustainable Aviation. Cham: Springer International Publishing, 2022: 23-53. |
| 7 | Furuhama S, Hiruma M, Enomoto Y. Development of a liquid hydrogen car[J]. International Journal of Hydrogen Energy, 1978, 3(1): 61-81. |
| 8 | Peschka W. Liquid hydrogen fueled automotive vehicles in Germany—status and development[J]. International Journal of Hydrogen Energy, 1986, 11(11): 721-728. |
| 9 | Albatayneh A, Juaidi A, Jaradat M, et al. Future of electric and hydrogen cars and trucks: an overview[J]. Energies, 2023, 16(7): 3230. |
| 10 | Ustolin F, Campari A, Taccani R. An extensive review of liquid hydrogen in transportation with focus on the maritime sector[J]. Journal of Marine Science and Engineering, 2022, 10(9): 1222. |
| 11 | Ugurlu A, Oztuna S. How liquid hydrogen production methods affect emissions in liquid hydrogen powered vehicles?[J]. International Journal of Hydrogen Energy, 2020, 45(60): 35269-35280. |
| 12 | Pan A Q, Liu J, Liu Z P, et al. Application of hydrogen energy and review of current conditions[J]. IOP Conference Series: Earth and Environmental Science, 2020, 526(1): 012124. |
| 13 | Škoro G, Lilley S, Bewley R. Neutronics analysis of target, moderators and reflector design for the ISIS TS-1 project[J]. Physica B: Condensed Matter, 2018, 551: 381-385. |
| 14 | Kiyanagi Y, Ooi M, Ogawa H, et al. Development of hydrogen cold moderator systems for a spallation neutron source[J]. Journal of Neutron Research, 2003, 11(1/2): 3-11. |
| 15 | Watanabe N. Neutronics of pulsed spallation neutron sources[J]. Reports on Progress in Physics, 2003, 66(3): 339-381. |
| 16 | Dennison D M, Fowler R H. A note on the specific heat of the hydrogen molecule[J]. Proceedings of the Royal Society A: Mathematical, Physical and Engeering Sciences, 1927, 115(771): 483-486. |
| 17 | Ilisca E. Ortho-para conversion of hydrogen molecules physisorbed on surfaces[J]. Progress in Surface Science, 1992, 41(3): 217-335. |
| 18 | Larsen A H, Simon F E, Swenson C A. The rate of evaporation of liquid hydrogen due to the ortho-para hydrogen conversion[J]. Review of Scientific Instruments, 1948, 19(4): 266-269. |
| 19 | Xu Y F, Bi Y J, Ju Y L. The thermodynamic analysis on the catalytical ortho-para hydrogen conversion during the hydrogen liquefaction process[J]. International Journal of Hydrogen Energy, 2024, 54: 1329-1342. |
| 20 | Zhuzhgov A V, Krivoruchko O P, Isupova L A, et al. Low-temperature conversion of ortho-hydrogen into liquid para-hydrogen: process and catalysts[J]. Catalysis in Industry, 2018, 10(1): 9-19. |
| 21 | Fukutani K, Sugimoto T. Physisorption and ortho-para conversion of molecular hydrogen on solid surfaces[J]. Progress in Surface Science, 2013, 88(4): 279-348. |
| 22 | Ilisca E, Ghiglieno F. Electron exchanges in nuclear spin conversion of hydrogen physisorbed on diamagnetic insulators[J]. The European Physical Journal B, 2014, 87(10): 235. |
| 23 | Ueta H, Fukutani K, Yamakawa K. Fast ortho-to-para conversion of molecular hydrogen in chemisorption and matrix-isolation systems[J]. Frontiers in Chemistry, 2023, 11: 1258035. |
| 24 | Ilisca E. Hydrogen conversion in nanocages[J]. Hydrogen, 2021, 2(2): 160-206. |
| 25 | Xu P, Wen J, Li K, et al. Review of the continuous catalytic ortho-para hydrogen conversion technology for hydrogen liquefaction[J]. International Journal of Hydrogen Energy, 2024, 62: 473-487. |
| 26 | Taylor H S, Sherman A. The ortho: para-hydrogen conversion at surfaces[J]. Journal of the American Chemical Society, 1931, 53(4): 1614-1615. |
| 27 | Taylor H S, Diamond H. The para-hydrogen conversion at paramagnetic surfaces[J]. Journal of the American Chemical Society, 1933, 55(6): 2613-2614. |
| 28 | Zhang X, Karman T, Groenenboom G C, et al. Para-ortho hydrogen conversion: solving a 90-year old mystery[J]. Natural Sciences, 2021, 1(1): 10002. |
| 29 | Weitzel D H, Park O E. Iron catalyst for production of liquid para-hydrogen[J]. 1956, 27(1): 57-58. |
| 30 | Grilly E R. The liquefaction and storage of partially converted liquid hydrogen[J]. 1953, 24(1): 1-4. |
| 31 | Fujiwara M, Niki K, Okano T, et al. Ortho-para conversion of hydrogen molecules on Cr2O3(0001)/Cr(110) surfaces[J]. Journal of Physics: Conference Series, 2010, 200(2): 022038. |
| 32 | Sullivan N S, Zhou D, Edwards C M. Precise and efficient in situ ortho-para-hydrogen converter[J]. Cryogenics, 1990, 30(8): 734-735. |
| 33 | Kim J H, Karng S W, Oh I H, et al. Ortho-para hydrogen conversion characteristics of amorphous and mesoporous Cr2O3 powders at a temperature of 77 K[J]. International Journal of Hydrogen Energy, 2015, 40(41): 14147-14153. |
| 34 | Cunningham C M, Johnston H L. The surface catalysis of the ortho- to para-conversion in liquid hydrogen by paramagnetic oxides on alumina[J]. Journal of the American Chemical Society, 1958, 80(10): 2377-2382. |
| 35 | Schmauch G E, Singleton A H. Technical aspects of ortho-parahydrogen conversion[J]. Industrial & Engineering Chemistry, 1964, 56(5): 20-31. |
| 36 | Das T, Nah I W, Choi J G, et al. Synthesis of iron oxide catalysts using various methods for the spin conversion of hydrogen[J]. Reaction Kinetics, Mechanisms and Catalysis, 2016, 118(2): 669-681. |
| 37 | Das T, Kweon S C, Nah I W, et al. Spin conversion of hydrogen using supported iron catalysts at cryogenic temperature[J]. Cryogenics, 2015, 69: 36-43. |
| 38 | Kim J H, Kang S W, Nah I W, et al. Synthesis and characterization of Fe-modified zeolite for spin conversion of hydrogen at cryogenic temperature[J]. International Journal of Hydrogen Energy, 2015, 40(45): 15529-15533. |
| 39 | 朱楠. 氢正仲转化用铁基催化剂的制备与催化性能研究[D]. 北京: 北京化工大学, 2018. |
| Zhu N. Preparation and performance study of iron oxide catalysts for the spin conversion of hydrogen[D]. Beijing: Beijing University of Chemical Technology, 2018. | |
| 40 | Svadlenak R E, Scott A B. The conversion of ortho- to parahydrogen on iron oxide-zinc oxide catalysts[J]. Journal of the American Chemical Society, 1957, 79(20): 5385-5388. |
| 41 | Zhou H, Li Z Y, Li M S, et al. Study of activation methods for ortho-para hydrogen catalysts in a small isothermal converter based on gas chromatography at LN2 temperature[J]. International Journal of Hydrogen Energy, 2024, 55: 55-64. |
| 42 | Keeler R N, Timmerhaus K D. Poisoning and reactivation of ortho-parahydrogen conversion catalyst[M]//Timmerhaus K D. Advances in Cryogenic Engineering. Boston, MA: Springer US, 1960: 296-306. |
| 43 | 李娜,曹锐霄,魏进,等.铁基正仲氢转化催化剂的影响因素[J/OL].无机材料学报,. |
| Li N, Cao R X, Wei J, et al. Performance influence factor of an iron-based ortho to para hydrogen conversion catalyst[J/OL]. Journal of Inorganic Materials, . | |
| 44 | 杨晓阳, 杨昌乐. 正仲氢转化催化剂性能研究[J]. 化学推进剂与高分子材料, 2018, 16(3): 79-82. |
| Yang X Y, Yang C L. Study on performance of orthohydrogen-parahydrogen converting catalyst[J]. Chemical Propellants & Polymeric Materials, 2018, 16(3): 79-82. | |
| 45 | Barrick P L, Brown L F, Hutchinson H L, et al. Improved ferric oxide gel catalysts for ortho-parahydrogen conversion[M]//Advances in Cryogenic Engineering. Boston, MA: Springer US, 1965: 181-189. |
| 46 | Das T, Kweon S C, Choi J G, et al. Spin conversion of hydrogen over LaFeO3/Al2O3 catalysts at low temperature: synthesis, characterization and activity[J]. International Journal of Hydrogen Energy, 2015, 40(1): 383-391. |
| 47 | Xu H, Bi S H, Xue M Z, et al. Amorphous cobalt iron oxide nanoparticles with high magnetization intensity for spin conversion of hydrogen at 77 K[J]. International Journal of Hydrogen Energy, 2023, 48(81): 31643-31652. |
| 48 | Xu H, Wang J W, Han Y S, et al. Effect of unpaired electron number elements (Al, Cr, Mn) doping in Fe2O3 on ortho to para hydrogen conversion at 77 K[J]. Journal of Energy Storage, 2023, 74: 109512. |
| 49 | Xu H, Wang J W, Han Y S, et al. Ortho- to para-hydrogen spin conversion performance of Ho-Fe2O3 catalytic at 77 K[M]//Springer Proceedings in Physics. Singapore: Springer Nature Singapore, 2024: 186-194. |
| 50 | Yue C Z, Wang J Y, Wang S F, et al. Identification of structural factors in iron oxide triggering ortho-para hydrogen conversion[J]. The Journal of Physical Chemistry C, 2024, 128(30): 12355-12363. |
| 51 | 陈志强, 宋隆, 孙海云, 等. 限域效应提升正仲氢转化催化剂性能研究[J]. 现代化工, 2024, 44(5): 206-211. |
| Chen Z Q, Song L, Sun H Y, et al. Study on performance enhancement of orthohydrogen-parahydrogen conversion catalyst by physical confinement[J]. Modern Chemical Industry, 2024, 44(5): 206-211. | |
| 52 | 陈志强,汪丽,丁明伟,等.封装型α-Fe2O3@SiO2催化剂的制备及其催化正仲氢转化性能评价[J].低碳化学与化工,2024,49(9):106-112. |
| Chen Z Q, Wang L, Ding M W, et al. Preparation of encapsulated α-Fe2O3@SiO2 catalyst and evaluation of its catalytic performance in orthohydrogen-parahydrogen conversion[J]. Low-Carbon Chemistry and Chemical Engineering,2024,49(9):106-112. | |
| 53 | Wakao N, Smith J M, Selwood P W. The low-temperature orthohydrogen conversion over supported oxides and metals[J]. Journal of Catalysis, 1962, 1(1): 62-73. |
| 54 | Zhuzhgov A V, Krivoruchko O P, Isupova L A. Low-temperature conversion of ortho-hydrogen to para-hydrogen over Ni/Al2O3 supported catalysts[J]. Russian Journal of Physical Chemistry A, 2020, 94(1): 58-66. |
| 55 | Juarez A M, Cubric D, King G C. A compact catalytic converter for the production of para-hydrogen[J]. Measurement Science and Technology, 2002, 13(5): N52-N55. |
| 56 | Polyukhov D M, Kudriavykh N A, Gromilov S A, et al. Efficient MOF-catalyzed ortho-para hydrogen conversion for practical liquefaction and energy storage[J]. ACS Energy Letters, 2022, 7(12): 4336-4341. |
| 57 | Boeva O, Antonov A, Zhavoronkova K. Influence of the nature of IB group metals on catalytic activity in reactions of homomolecular hydrogen exchange on Cu, Ag, Au nanoparticles[J]. Catalysis Communications, 2021, 148: 106173. |
| 58 | Boeva O A, Ershov B G, Zhavoronkova K N, et al. Catalytic properties of gold nanoparticles in H2-D2 exchange and ortho-para hydrogen conversion[J]. Doklady Physical Chemistry, 2015, 463(2): 165-167. |
| 59 | Boeva O A, Odintzov A A, Solovov R D, et al. Low-temperature ortho-para hydrogen conversion catalyzed by gold nanoparticles: particle size does not affect the rate[J]. International Journal of Hydrogen Energy, 2017, 42(36): 22897-22902. |
| 60 | Sakurai M, Okano T, Tuzi Y. Ortho-para conversion of n-H2 physisorbed on Ag(111) near two-dimensional condensation conditions[J]. Applied Surface Science, 1988, 33: 245-251. |
| 61 | Turro N J, Martí A A, Chen J Y C, et al. Demonstration of a chemical transformation inside a fullerene. The reversible conversion of the allotropes of H2@C60 [J]. Journal of the American Chemical Society, 2008, 130(32): 10506-10507. |
| 62 | Turro N J, Chen J Y C, Sartori E, et al. The spin chemistry and magnetic resonance of H2@C60. From the Pauli principle to trapping a long lived nuclear excited spin state inside a buckyball[J]. Accounts of Chemical Research, 2010, 43(2): 335-345. |
| 63 | Frunzi M, Jockusch S, Chen J Y C, et al. A photochemical on–off switch for tuning the equilibrium mixture of H2 nuclear spin isomers as a function of temperature[J]. Journal of the American Chemical Society, 2011, 133(36): 14232-14235. |
| 64 | Kosone T, Hori A, Nishibori E, et al. Coordination nano-space as stage of hydrogen ortho-para conversion[J]. Royal Society Open Science, 2015, 2(7): 150006. |
| 65 | Sandler Y L. The ortho-para conversion of hydrogen and deuterium on inhomogeneous paramagnetic surfaces[J]. Canadian Journal of Chemistry, 1954, 32(3): 249-260. |
| 66 | Farkas L, Sandler L. On the heterogeneous ortho-para conversion on paramagnetic crystals[J]. The Journal of Chemical Physics, 1940, 8(3): 248-251. |
| 67 | Harrison L G, McDowell C A, Bawn C E H. The catalysis of the para-hydrogen conversion by the solid free radical αα-diphenyl-β-picryl hydrazyl[J]. Proceedings of the Royal Society of London Series A: Mathematical and Physical Sciences, 1953, 220(1140): 77-90. |
| 68 | Kondow T, Inokuchi H, Wakayama N. Ortho-to-para hydrogen conversion and hydrogen-deuterium exchange in the presence of tetracyanopyrene-cesium complex[J]. 1965, 43(10): 3766-3767. |
| 69 | Matthes J, Pery T, Gründemann S, et al. Bridging the gap between homogeneous and heterogeneous catalysis: ortho/para H2 conversion, hydrogen isotope scrambling, and hydrogenation of olefins by Ir(CO)Cl(PPh3)2 [J]. Journal of the American Chemical Society, 2004, 126(27): 8366-8367. |
| 70 | Muhida R, Setiyanto H, Rahman M M, et al. Ortho-to-para H2 conversion on multiple-decked sandwich clusters of M(C6H6)2 (M=Mn, Fe, Co) induced by an inhomogeneity of spin density distribution[J]. Thin Solid Films, 2006, 509(1/2): 223-226. |
| 71 | Furuya K, Aikawa Y, Hama T, et al. H2 ortho-para spin conversion on inhomogeneous grain surfaces[J]. The Astrophysical Journal, 2019, 882(2): 172. |
| 72 | Ueta H, Watanabe N, Hama T, et al. Surface temperature dependence of hydrogen ortho-para conversion on amorphous solid water[J]. Physical Review Letters, 2016, 116(25): 253201. |
| 73 | Ilisca E. Nuclear spin relaxation, conversion, and polarization of molecular hydrogen in paramagnetic solvents[J]. The Journal of Physical Chemistry C, 2019, 123(27): 16631-16640. |
| 74 | Atkins P W, Clugston M J. Ortho-parahydrogen conversion in paramagnetic solutions[J]. Molecular Physics, 1974, 27(6): 1619-1631. |
| 75 | Selwood P W. Magnetic field effects on the catalyzed nondissociative parahydrogen conversion rate[J]. Journal of Catalysis, 1977, 50(1): 15-23. |
| 76 | Tsuge M, Namiyoshi T, Furuya K, et al. Rapid ortho-to-para nuclear spin conversion of H2 on a silicate dust surface[J]. The Astrophysical Journal, 2021, 908(2): 234. |
| 77 | Hiller M, Lavrov E V, Weber J. Ortho-para conversion of interstitial H2 in Si[J]. Physical Review Letters, 2007, 98(5): 055504. |
| 78 | Strom A I, Fillmore K L, Anderson D T. Hydrogen atom catalyzed ortho-to-para conversion in solid molecular hydrogen[J]. Low Temperature Physics, 2019, 45(6): 676-688. |
| 79 | Abe H, Mizoguchi H, Eguchi R, et al. Exploration of heterogeneous catalyst for molecular hydrogen ortho-para conversion[J]. Exploration, 2024, 4(3): 20230040. |
| 80 | Farkas A. Orthohydrogen, parahydrogen and heavy hydrogen[J]. Nature, 1935, 135: 601-602. |
| 81 | Wigner E. Concerning the paramagnetic conversion of para-ortho hydrogen[J]. Physical Chemistry, 1933, 23: 28. |
| 82 | Farkas L, Sachsse H. Über Die Homogene Katalyse der Para-Orthowasserstoffumwandlung Durch Paramagnetische Stoffe[J]. Z. Phys. Chem. B, 1933, 23: 1. |
| 83 | Sandler Y L. The kinetics of the heterogeneous parahydrogen conversion[J]. The Journal of Chemical Physics, 1953, 21(12): 2243-2244. |
| 84 | Sandler Y L. The adsorption and the magnetic ortho-para conversion of hydrogen on diamagnetic solids (I): Some experiments in surface paramagnetism[J]. The Journal of Physical Chemistry, 1954, 58(1): 54-57. |
| 85 | Arias J A, Selwood P W. The catalyzed o-pH2 conversion and magnetocatalytic effects on EuO and CrO2 [J]. Journal of Catalysis, 1974, 35(2): 273-277. |
| 86 | Ng C F, Selwood P W. Magnetic effects on the ortho-parahydrogen conversion over α-Cr2O3, CoO, and MnO[J]. Journal of Catalysis, 1976, 43(1/2/3): 252-259. |
| 87 | Ishii Y, Sugano S. Ortho-para conversion of hydrogen molecules on magnetic surfaces[J]. Surface Science, 1983, 127(1): 21-34. |
| 88 | Misono M, Selwood P W. Extrinsic field acceleration of the magnetic para hydrogen conversion[J]. Journal of the American Chemical Society, 1968, 90(11): 2977-2978. |
| 89 | Misono M, Selwood P W. Extrinsic field acceleration of the magnetic parahydrogen conversion[J]. Journal of the American Chemical Society, 1969, 91(6): 1300-1303. |
| 90 | Selwood P W. Effect of low magnetic fields on the catalysed parahydrogen conversion rate over certain rare earths[J]. Nature, 1970, 228(5268): 278. |
| 91 | Selwood P W. Extrinsic field conversion of parahydrogen over the rare earths[J]. Journal of Catalysis, 1970, 19(3): 353-359. |
| 92 | Van Cauwelaert F H, Hall W K. Studies of the hydrogen held by solids (Part 17):The ortho-para H2 conversion and H2-D2 exchange reactions over a transition alumina[J]. Trans. Faraday Soc., 1970, 66: 454-468. |
| 93 | Selwood P W. The effect of a weak magnetic field on the rare earth catalyzed parahydrogen conversion rate[J]. Journal of Catalysis, 1971, 22(1): 123-129. |
| 94 | Arias J A, Selwood P W. Parahydrogen conversion over chromiagel near the Néel point[J]. Journal of Catalysis, 1973, 30(2): 255-259. |
| 95 | Berlinsky A J, Hardy W N. Theory of ortho-para conversion and its effect on the NMR spectrum of ordered solid ortho-hydrogen[J]. Physical Review B, 1973, 8(11): 5013-5027. |
| 96 | Arias J A, Selwood P W. The activation of yttria, lutetia, and ytterbia for the ortho-parahydrogen conversion[J]. Journal of Catalysis, 1974, 33(2): 284-288. |
| 97 | Madhusudhan C P, Selwood P W. Extrinsic field effect and absolute para-hydrogen conversion rate on Lutetia[J]. Journal of Catalysis, 1976, 45(1): 106-109. |
| 98 | Selwood P W. The effect of a magnetic field on the catalyzed nondissociative para hydrogen conversion rate[M]//Advances in Catalysis. Amsterdam: Elsevier, 1979: 23-57. |
| 99 | Ilisca E. Introduction to a theory of ortho-para H2 conversion on paramagnetic catalysts: the magnetic field effect[J]. Physical Review Letters, 1978, 40(23): 1535-1538. |
| 100 | Ilisca E, Sugano S. New channel in ortho-para hydrogen conversion[J]. Physical Review Letters, 1986, 57(20): 2590-2593. |
| 101 | Ilisca E. Theory of ortho-para hydrogen conversion catalyzed by "d" electrons[J]. Chemical Physics Letters, 1990, 168(3/4): 289-292. |
| 102 | Ilisca E, Paris S. A charge transfer process in o-p H2 conversion induced by 3d impurities on a perovskite[J]. Surface Science, 1996, 363(1/2/3): 347-353. |
| 103 | Sugimoto T, Fukutani K. Effects of rotational-symmetry breaking on physisorption of ortho- and para-H2 on Ag(111)[J]. Physical Review Letters, 2014, 112(14): 146101. |
| 104 | Diño W A, Kasai H, Okiji A. Orientational effects in dissociative adsorption/associative desorption dynamics of H2(D2) on Cu and Pd[J]. Progress in Surface Science, 2000, 63(3/4/5): 63-134. |
| 105 | Kasai H, Diño W A, Muhida R. Surface science-based reaction design: increasing the ortho-para hydrogen conversion yield via molecular orientation, a case study[J]. Progress in Surface Science, 2003, 72(1/2/3/4): 53-86. |
| 106 | Muhida R, Miura Y, Diño W A, et al. Molecular orientation dependence of ortho-para conversion of a H2 interacting with a metal surface[J]. 2003, 93(1): 644-648. |
| 107 | Muhida R, David M, Rahman M M, et al. Molecular orientation dependence of ortho-para H2 conversion on Fe(OH)3 cluster induced by hyperfine contact interaction[J]. The European Physical Journal D: Atomic, Molecular, Optical and Plasma Physics, 2006, 38(1): 99-101. |
| 108 | Wagner S. Conversion rate of para-hydrogen to ortho-hydrogen by oxygen: implications for PHIP gas storage and utilization[J]. Magnetic Resonance Materials in Physics, Biology and Medicine, 2014, 27(3): 195-199. |
| 109 | Minaev B F, Aagren H. Spin catalysis of ortho-para hydrogen conversion[J]. The Journal of Physical Chemistry, 1995, 99(21): 8936-8940. |
| 110 | Ueta H, Fukutani K. Rotational-energy transfer in H2 ortho-para conversion on a metal surface: interplay between electron and phonon systems[J]. The Journal of Physical Chemistry Letters, 2023, 14(34): 7591-7596. |
| 111 | Buntkowsky G, Walaszek B, Adamczyk A, et al. Mechanism of nuclear spin initiated para-H2 to ortho-H2 conversion[J]. Physical Chemistry Chemical Physics, 2006, 8(16): 1929-1935. |
| 112 | Strzhemechny M A, Hemley R J. New ortho-para conversion mechanism in dense solid hydrogen[J]. Physical Review Letters, 2000, 85(26): 5595-5598. |
| 113 | Fukutani K, Yoshida K, Wilde M, et al. Photostimulated desorption and ortho-para conversion of H2 on Ag surfaces[J]. Physical Review Letters, 2003, 90(9): 096103. |
| 114 | Stewart A T, Squires G L. Analysis of ortho- and para-hydrogen mixtures by the thermal conductivity method[J]. Journal of Scientific Instruments, 1955, 32(1): 26-29. |
| 115 | Raston P L, Kettwich S C, Anderson D T. Infrared studies of ortho-para conversion at Cl-atom and H-atom impurity centers in cryogenic solid hydrogen[J]. Low Temperature Physics, 2010, 36(5): 392-399. |
| 116 | Abouaf-Marguin L, Vasserot A M. Nuclear spin conversion of O2 doped solid normal H2 at 4.2 K: an empirical law to determine the ortho-H2 concentration by infrared absorption spectroscopy[J]. Chemical Physics Letters, 2008, 460(1): 82-85. |
| 117 | Krasch B, Mirz S, Smolinski A, et al. Raman spectroscopy for ortho-para hydrogen catalyst studies[J]. International Journal of Hydrogen Energy, 2023, 48(77): 29952-29961. |
| 118 | Sutherland L M, Knudson J N, Mocko M, et al. Practical in situ determination of ortho-para hydrogen ratios via fiber-optic based Raman spectroscopy[J]. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, 2016, 810: 182-185. |
| 119 | da Silva Falcão B, Jeong K, Al Ghafri S, et al. Ortho- to para-hydrogen catalytic conversion kinetics[J]. International Journal of Hydrogen Energy, 2024, 62: 345-351. |
| 120 | Matsumoto M, Espenson J H. Kinetics of the interconversion of parahydrogen and orthohydrogen catalyzed by paramagnetic complex ions[J]. Journal of the American Chemical Society, 2005, 127(32): 11447-11453. |
| 121 | 俞华栋, 苏嘉南, 王西明. 仲氢含量测量方法研究[J]. 低温与特气, 2024, 42(3): 49-52. |
| Yu H D, Su J N, Wang X M. Research on the measurement method of para hydrogen content[J]. Low Temperature and Specialty Gases, 2024, 42(3): 49-52. | |
| 122 | Romanelli G, Minniti T, Škoro G, et al. Visualization of the catalyzed nuclear-spin conversion of molecular hydrogen using energy-selective neutron imaging[J]. The Journal of Physical Chemistry C, 2019, 123(18): 11745-11751. |
| 123 | Milenko Y Y, Sibileva R M, Strzhemechny M A. Natural ortho-para conversion rate in liquid and gaseous hydrogen[J]. Journal of Low Temperature Physics, 1997, 107(1): 77-92. |
| 124 | Weitzel D H, Blake J H, Konecnik M. Flow conversion kinetics of ortho and parahydrogen[M]//Advances in Cryogenic Engineering. Boston, MA: Springer US, 1960: 286-295. |
| 125 | Wakao N, Selwood P W, Smith J M. Low temperature ortho-para hydrogen conversion-kinetic studies[J]. AIChE Journal, 1962, 8(4): 478-481. |
| 126 | Emmett P H, Harkness R W. The catalytic interconversion of ortho-para hydrogen over iron, platinum and nickel catalysts[J]. Journal of the American Chemical Society, 1935, 57(9): 1624-1631. |
| 127 | Hartl M, Gillis R C, Daemen L, et al. Hydrogen adsorption on two catalysts for the ortho- to parahydrogen conversion: Cr-doped silica and ferric oxide gel[J]. Physical Chemistry Chemical Physics, 2016, 18(26): 17281-17293. |
| 128 | Svensson K, Andersson S. Fast ortho-para conversion of H2 adsorbed at copper surface step atoms[J]. Physical Review Letters, 2007, 98(9): 096105. |
| 129 | Wilhelmsen Ø, Berstad D, Aasen A, et al. Reducing the exergy destruction in the cryogenic heat exchangers of hydrogen liquefaction processes[J]. International Journal of Hydrogen Energy, 2018, 43(10): 5033-5047. |
| 130 | Donaubauer P J, Cardella U, Decker L, et al. Kinetics and heat exchanger design for catalytic ortho-para hydrogen conversion during liquefaction[J]. Chemical Engineering & Technology, 2019, 42(3): 669-679. |
| 131 | Buyanov R. Investigation of the reaction of ortho-hydrogen transformation into para-hydrogen over solid catalysts at a temperature of 78—64 K[J]. Kinetika i Kataliz, 1960, 1(2): 306. |
| 132 | 黄波, 许婧煊. 基于正仲氢多级转化的低温压缩储氢系统研究[J/OL]. 真空与低温, . |
| Huang B, XU J X. Research of cryogenic compressed hydrogen storage system based on multistage ortho-para hydrogen conversion[J/OL]. Vacuum and Cryogenics, . | |
| 133 | 刁希文, 滕越, 赵骞, 等. 正仲氢催化转化性能低温测试装置设计[J]. 低温与超导, 2022, 50(2): 84-88. |
| Diao X W, Teng Y, Zhao Q, et al. Design of cryogenic test device for catalytic conversion performance of ortho-parahydrogen[J]. Cryogenics & Superconductivity, 2022, 50(2): 84-88. | |
| 134 | 刁希文. 宽温区多模式的正仲氢催化转化性能测式平台设计和分析研究[D]. 合肥: 中国科学技术大学, 2022. |
| Diao X W. Design and analysis of multi-mode test plant form for catalytic conversion performance of ortho-para hydrogen in wide temperature range[D]. Hefei: University of Science and Technology of China, 2022. |
| [1] | Huanjuan ZHAO, Yingxin BAO, Kang YU, Jing LIU, Xinming QIAN. Quantitative experimental study on detonation instability of multi-component [J]. CIESC Journal, 2024, 75(S1): 339-348. |
| [2] | Xusheng LIU, Zeyang LI, Yusen YANG, Min WEI. Research progress on electrocatalytic carbon dioxide reduction to gaseous products [J]. CIESC Journal, 2024, 75(7): 2385-2408. |
| [3] | Tingting ZHAO, Lixiang YAN, Fuli TANG, Minzhi XIAO, Ye TAN, Liubin SONG, Zhongliang XIAO, Lingjun LI. Research progress on design strategies and reaction mechanisms of photo-assisted Li-CO2 battery catalysts [J]. CIESC Journal, 2024, 75(5): 1750-1764. |
| [4] | Zhaoxiang ZHANG, Maokun CAI, Zhiying REN, Xiaohong JIA, Fei GUO. Numerical analysis of the effect of temperature and its fluctuations on the vulcanization process of rubber seals [J]. CIESC Journal, 2024, 75(2): 715-726. |
| [5] | Xueyun WANG, Xiaobing YU, Wanwang PENG, Yansong SHEN. Numerical study on combustion zone behaviors of a slagging gasifier [J]. CIESC Journal, 2024, 75(2): 659-674. |
| [6] | Guoyi XIAN, Lifang CHEN, Zhiwen QI. DFT-based study of liquid-phase Beckmann rearrangement mechanism of cyclohexanone oxime [J]. CIESC Journal, 2024, 75(1): 302-311. |
| [7] | Cheng CHENG, Zhongdi DUAN, Haoran SUN, Haitao HU, Hongxiang XUE. Lattice Boltzmann simulation of surface microstructure effect on crystallization fouling [J]. CIESC Journal, 2023, 74(S1): 74-86. |
| [8] | Linzheng WANG, Yubing LU, Ruizhi ZHANG, Yonghao LUO. Analysis on thermal oxidation characteristics of VOCs based on molecular dynamics simulation [J]. CIESC Journal, 2023, 74(8): 3242-3255. |
| [9] | Mengmeng ZHANG, Dong YAN, Yongfeng SHEN, Wencui LI. Effect of electrolyte types on the storage behaviors of anions and cations for dual-ion batteries [J]. CIESC Journal, 2023, 74(7): 3116-3126. |
| [10] | Jin YU, Binbin YU, Xinsheng JIANG. Study on quantification methodology and analysis of chemical effects of combustion control based on fictitious species [J]. CIESC Journal, 2023, 74(3): 1303-1312. |
| [11] | Shijun LIU, Anqing ZHENG, Xiaoli CHEN, Juan FU, Qiucheng SU. Study on pyrolysis characteristics of cellulose-enhanced epoxy resin composites [J]. CIESC Journal, 2023, 74(12): 4968-4978. |
| [12] | Zongpeng LIU, Shaojian HU, Yuning ZHANG, Ling MA, Lei LI, Bencheng WU, Jianhua ZHU. Thermodynamic analysis and kinetics study on synthesis reaction of complex polyolester [J]. CIESC Journal, 2023, 74(11): 4475-4486. |
| [13] | Lei ZHANG, Xiaohui SONG, Jianting ZHANG, Meiling TU, Asan YANG. Reaction kinetics study of tranexamic acid isomerization process [J]. CIESC Journal, 2023, 74(10): 4173-4181. |
| [14] | Ruizhe CHEN, Yongfeng LIU, Chenyang YIN, Long WANG, Lu ZHANG, Jin’ou SONG. Study of the mechanism of pyrolysis of n-hexane initiated by 1-nitropropane [J]. CIESC Journal, 2023, 74(10): 4319-4329. |
| [15] | Chen CHEN, Qian YANG, Yun CHEN, Rui ZHANG, Dong LIU. Chemical kinetic study on coal volatiles combustion for various oxygen concentrations [J]. CIESC Journal, 2022, 73(9): 4133-4146. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||