CIESC Journal ›› 2021, Vol. 72 ›› Issue (1): 555-568.DOI: 10.11949/0438-1157.20201071
• Separation engineering • Previous Articles Next Articles
ZHAO Yu( ),SHI Qi(
),SHI Qi( ),DONG Jinxiang
),DONG Jinxiang
Received:2020-07-31
Revised:2020-10-05
Online:2021-01-05
Published:2021-01-05
Contact:
SHI Qi
通讯作者:
石琪
作者简介:赵宇(1994—),男,硕士研究生,基金资助:CLC Number:
ZHAO Yu, SHI Qi, DONG Jinxiang. Fine adjustment of elliptical windows of ZIFs and performances of adsorptive separation of furfural/5-hydroxymethylfurfural[J]. CIESC Journal, 2021, 72(1): 555-568.
赵宇, 石琪, 董晋湘. ZIFs椭圆形孔窗的精细调控及糠醛/5-羟甲基糠醛吸附分离性能研究[J]. 化工学报, 2021, 72(1): 555-568.
Add to citation manager EndNote|Ris|BibTeX
ZIFs (CCDC number) | Empirical formula | Molecular weight | Crystal system | Space group | Cell parameter a /? | Density/ (g·cm-3) | Window/?2 | SBET / (m2·g-1) | Pore volume/(cm3·g-1) |
|---|---|---|---|---|---|---|---|---|---|
ANA-[Co(eIm)2] (2017658) | C10H14CoN4 | 249.18 | cubic | 26.581(2) | 1.058 | 7.4×4.9/4.2 | 610 | 0.268 | |
ANA-[Co(pIm)2] (2017659) | C12H18CoN4 | 277.23 | cubic | 26.606(2) | 1.173 | 7.5×3.1/4.2 | 333 | 0.139 | |
ANA-[Co(bIm)2] (2017657) | C14H22CoN4 | 305.28 | cubic | 26.340(3) | 1.332 | 7.5×1.3/4.1 | — | — |
Table 1 Crystal structure parameters of ZIFs materials
ZIFs (CCDC number) | Empirical formula | Molecular weight | Crystal system | Space group | Cell parameter a /? | Density/ (g·cm-3) | Window/?2 | SBET / (m2·g-1) | Pore volume/(cm3·g-1) |
|---|---|---|---|---|---|---|---|---|---|
ANA-[Co(eIm)2] (2017658) | C10H14CoN4 | 249.18 | cubic | 26.581(2) | 1.058 | 7.4×4.9/4.2 | 610 | 0.268 | |
ANA-[Co(pIm)2] (2017659) | C12H18CoN4 | 277.23 | cubic | 26.606(2) | 1.173 | 7.5×3.1/4.2 | 333 | 0.139 | |
ANA-[Co(bIm)2] (2017657) | C14H22CoN4 | 305.28 | cubic | 26.340(3) | 1.332 | 7.5×1.3/4.1 | — | — |
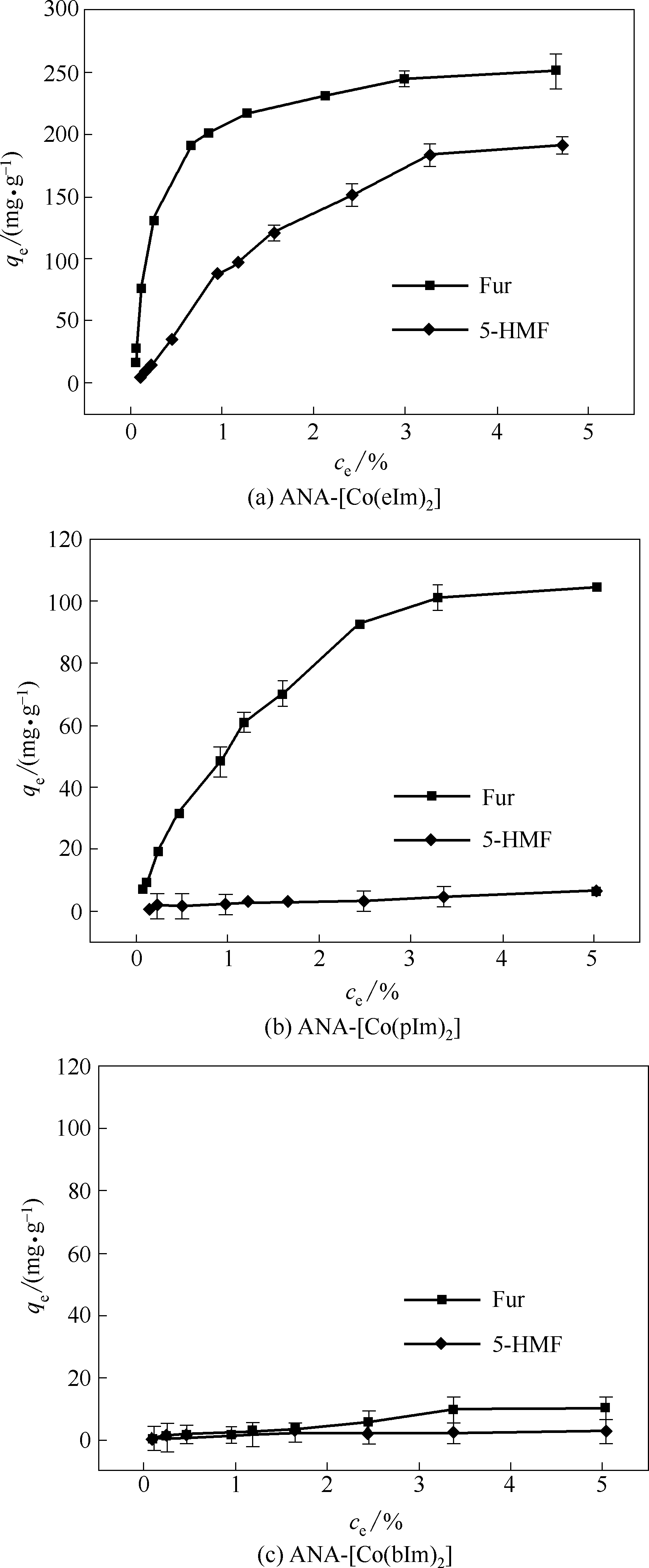
Fig.4 Static adsorption isotherms of ZIFs for single-component Fur and 5-HMF at the temperature of 25℃ (Error bars correspond to the standard deviation at each point)
| ZIFs | Langmuir model | Freundlich model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fur | 5-HMF | Fur | 5-HMF | |||||||||
KL/ (L·mg-1) | qmax/(mg·g-1) | R2 | KL/ (L·mg-1) | qmax/(mg·g-1) | R2 | KF/(mg·g-1) | n | R2 | KF/ (mg·g-1) | n | R2 | |
| ANA-[Co(eIm)2] | 3.144 | 271.8 | 0.981 | 0.367 | 318.1 | 0.981 | 176.153 | 3.108 | 0.841 | 79.946 | 1.569 | 0.941 |
| ANA-[Co(pIm)2] | 0.622 | 145.2 | 0.991 | 0.344 | 9.2 | 0.901 | 52.038 | 1.968 | 0.952 | 2.376 | 1.710 | 0.942 |
| ANA-[Co(bIm)2] | 0.064 | 44.1 | 0.950 | 1.014 | 3.1 | 0.958 | 2.672 | 1.145 | 0.945 | 1.403 | 2.263 | 0.937 |
Table 2 Langmuir adsorption and Freundlich adsorption model for single-component batch adsorption fitting results
| ZIFs | Langmuir model | Freundlich model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fur | 5-HMF | Fur | 5-HMF | |||||||||
KL/ (L·mg-1) | qmax/(mg·g-1) | R2 | KL/ (L·mg-1) | qmax/(mg·g-1) | R2 | KF/(mg·g-1) | n | R2 | KF/ (mg·g-1) | n | R2 | |
| ANA-[Co(eIm)2] | 3.144 | 271.8 | 0.981 | 0.367 | 318.1 | 0.981 | 176.153 | 3.108 | 0.841 | 79.946 | 1.569 | 0.941 |
| ANA-[Co(pIm)2] | 0.622 | 145.2 | 0.991 | 0.344 | 9.2 | 0.901 | 52.038 | 1.968 | 0.952 | 2.376 | 1.710 | 0.942 |
| ANA-[Co(bIm)2] | 0.064 | 44.1 | 0.950 | 1.014 | 3.1 | 0.958 | 2.672 | 1.145 | 0.945 | 1.403 | 2.263 | 0.937 |
| GRM parameter | ANA-[Co(eIm)2] | ANA-[Co(pIm)2] | ANA-[Co(bIm)2] | |||
|---|---|---|---|---|---|---|
| Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | |
| Dax /(cm2·min-1) | 5.730×10-4 | 1.567×10-4 | 4.820×10-4 | 1.773×10-4 | 1.720×10-4 | 4.675×10-4 |
| Kfilm/(cm·min-1) | 2.740×10-2 | 2.360×10-2 | 3.510×10-2 | 1.350×10-2 | 3.490×10-2 | 1.900×10-2 |
| Dpore /(cm2·min-1) | 2.176×10-3 | 2.054×10-4 | 1.534×10-3 | 8.035×10-5 | 8.712×10-5 | 8.006×10-5 |
Table 3 Fitting results of the general rate model (GRM) for single-component dynamic column adsorption
| GRM parameter | ANA-[Co(eIm)2] | ANA-[Co(pIm)2] | ANA-[Co(bIm)2] | |||
|---|---|---|---|---|---|---|
| Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | |
| Dax /(cm2·min-1) | 5.730×10-4 | 1.567×10-4 | 4.820×10-4 | 1.773×10-4 | 1.720×10-4 | 4.675×10-4 |
| Kfilm/(cm·min-1) | 2.740×10-2 | 2.360×10-2 | 3.510×10-2 | 1.350×10-2 | 3.490×10-2 | 1.900×10-2 |
| Dpore /(cm2·min-1) | 2.176×10-3 | 2.054×10-4 | 1.534×10-3 | 8.035×10-5 | 8.712×10-5 | 8.006×10-5 |
| Item | ANA-[Co(eIm)2] /(mg·g-1) | ANA-[Co(pIm)2] /(mg·g-1) | ANA-[Co(bIm)2]/(mg·g-1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2% | 5% | 2% | 5% | 2% | 5% | |||||||
| Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | |
| single-component batch adsorption | 222.3 | 129.3 | 251.4 | 190.8 | 77.7 | 3.3 | 105.1 | 6.5 | 4.4 | 1.9 | 10.1 | 2.8 |
| dynamic column adsorption | ||||||||||||
| single | 212.8 | 125.8 | 245.3 | 195.8 | 76.5 | 3.9 | 93.8 | 7.3 | 4.2 | 0.1 | 9.9 | 1.5 |
| binary | 208.9 | 2.7 | 239.1 | 2.3 | 77.1 | — | 91.7 | — | 4.1 | — | 9.1 | — |
Table 4 Summary of adsorption capacity of single-component batch adsorption and dynamic column adsorption
| Item | ANA-[Co(eIm)2] /(mg·g-1) | ANA-[Co(pIm)2] /(mg·g-1) | ANA-[Co(bIm)2]/(mg·g-1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2% | 5% | 2% | 5% | 2% | 5% | |||||||
| Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | |
| single-component batch adsorption | 222.3 | 129.3 | 251.4 | 190.8 | 77.7 | 3.3 | 105.1 | 6.5 | 4.4 | 1.9 | 10.1 | 2.8 |
| dynamic column adsorption | ||||||||||||
| single | 212.8 | 125.8 | 245.3 | 195.8 | 76.5 | 3.9 | 93.8 | 7.3 | 4.2 | 0.1 | 9.9 | 1.5 |
| binary | 208.9 | 2.7 | 239.1 | 2.3 | 77.1 | — | 91.7 | — | 4.1 | — | 9.1 | — |
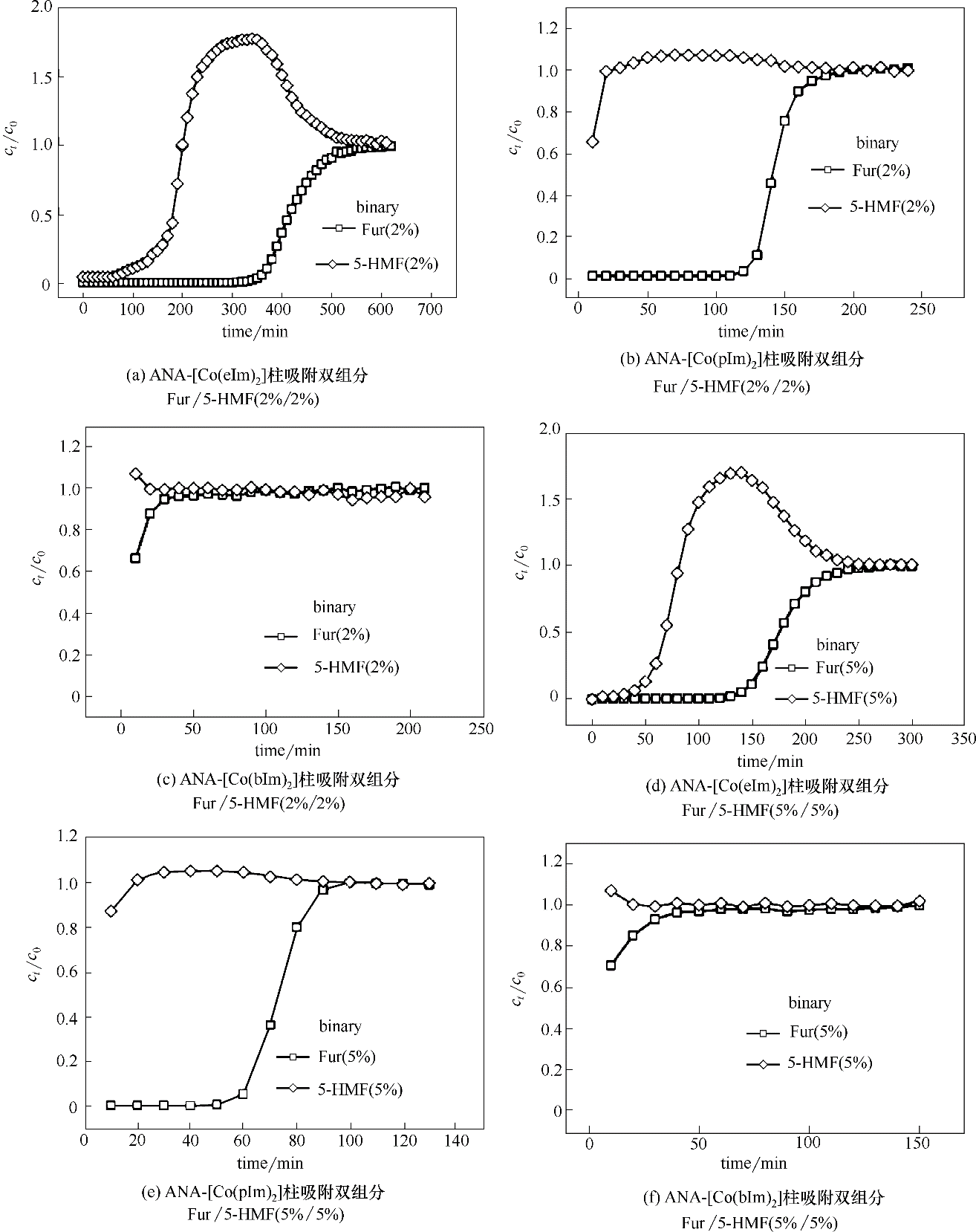
Fig.6 Breakthrough curves of binary-component Fur/5-HMF diluted in water on ZIFs at the temperature of 25℃(length: 25 cm; diameter: 0.4 cm; rate: 0.05 ml·min-1)
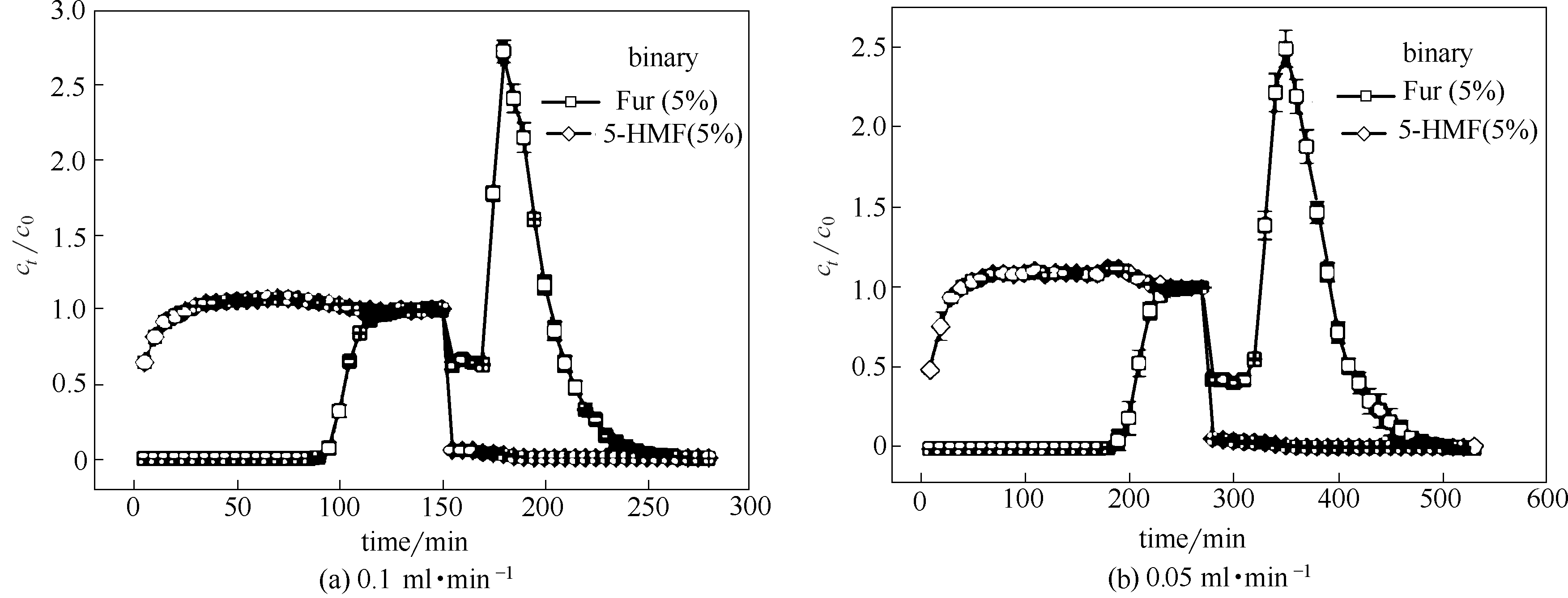
Fig.7 Adsorption-desorption breakthrough curves of binary-component Fur/5-HMF (5%/5%) diluted in water on ANA-[Co(pIm)2] at different flow rates (length: 33 cm, diameter: 0.6 cm, adsorption temperature: 25℃, desorption temperature: 40℃; error bars correspond to the standard deviation at each point)
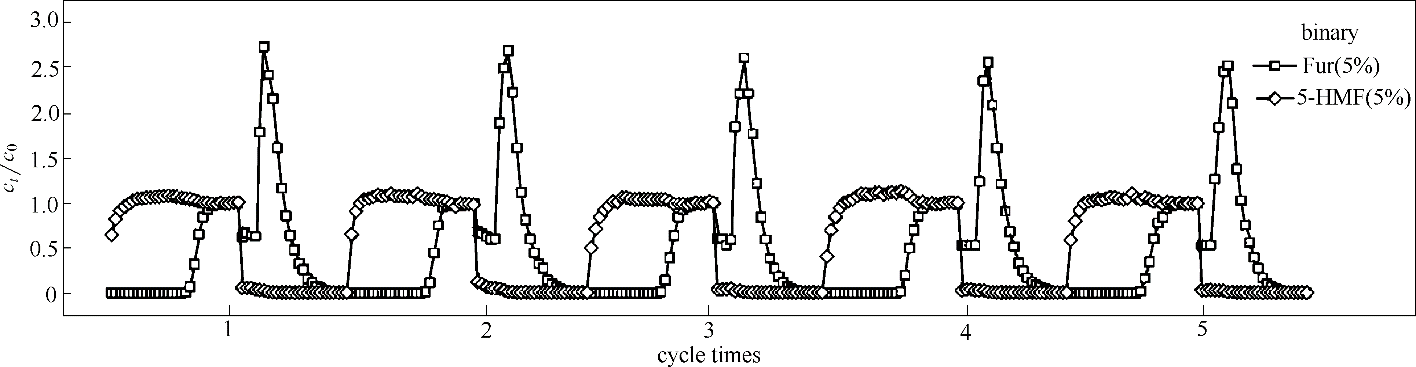
Fig.8 Adsorption-desorption breakthrough curves of binary-component Fur/5-HMF (5%/5%) diluted in water on ANA-[Co(pIm)2] in 5 adsorption-desorption cycles at a flow rate of 0.1 ml·min-1 (length: 33 cm, diameter: 0.6 cm, adsorption temperature: 25℃, desorption temperature: 40℃)
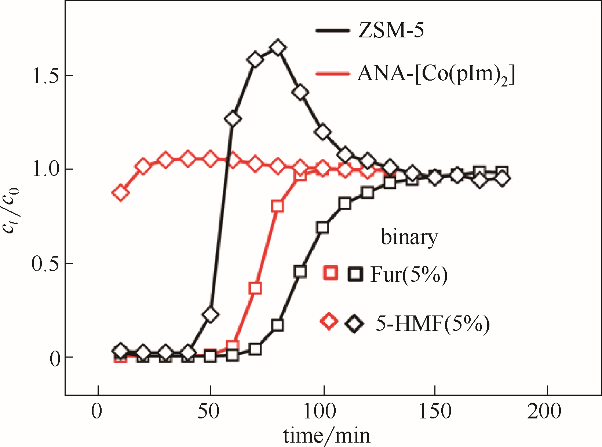
Fig.10 Breakthrough curves of binary-component Fur/5-HMF (5%/5%) diluted in water on ZSM-5 and ANA-[Co(pIm)2] at the temperature of 25℃(length: 25 cm, diameter: 0.4 cm, rate: 0.05 ml·min-1)
| Adsorbent | qFur/(mg·g-1) | q5-HMF /(mg·g-1) | SFur,5-HMF |
|---|---|---|---|
| ZSM-5 | 112.7 | 30.1 | 3.7 |
| MAF-5 | 220.0 | 1.9 | 115.8 |
| ANA-[Co(eIm)2] | 239.1 | 2.3 | 104.0 |
| ANA-[Co(pIm)2] | 91.7 | — | — |
Table 5 Summary of adsorption capacity and selectivity of dynamic column adsorption for ZSM-5, MAF-5[21], ANA-[Co(eIm)2] and ANA-[Co(pIm)2]
| Adsorbent | qFur/(mg·g-1) | q5-HMF /(mg·g-1) | SFur,5-HMF |
|---|---|---|---|
| ZSM-5 | 112.7 | 30.1 | 3.7 |
| MAF-5 | 220.0 | 1.9 | 115.8 |
| ANA-[Co(eIm)2] | 239.1 | 2.3 | 104.0 |
| ANA-[Co(pIm)2] | 91.7 | — | — |
| 1 | Ragauskas A J, Williams C K, Davison B H, et al. The path forward for biofuels and biomaterials[J]. Science, 2006, 311(5760): 484-489. |
| 2 | Zhang X G, Wilson K, Lee A F. Heterogeneously catalyzed hydrothermal processing of C5-C6 sugars[J]. Chemical Reviews, 2016, 116(19): 12328-12368. |
| 3 | Bozell J J, Petersen G R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy's “Top 10” revisited[J]. Green Chemistry, 2010, 12(4): 539-554. |
| 4 | Xu S Q, Pan D H, Wu Y F, et al. Direct conversion of wheat straw components into furan compounds using a highly efficient and reusable SnCl2-PTA/β zeolite catalyst[J]. Industrial & Engineering Chemistry Research, 2019, 58(22): 9276-9285. |
| 5 | Widsten P, Murton K, West M. Production of 5-hydroxymethylfurfural and furfural from a mixed saccharide feedstock in biphasic solvent systems[J]. Industrial Crops & Products, 2018, 119: 237-242. |
| 6 | 聂一凡, 候其东, 李维尊, 等. 糠醛的水解制备和应用研究进展[J]. 化工进展, 2019, 38(5): 2164-2178. |
| Nie Y F, Hou Q D, Li W Z, et al. Advances in production furfural via hydrolysis and application of furfural[J]. Chemical Industry and Engineering Progress, 2019, 38(5): 2164-2178. | |
| 7 | van Putten R J, van der Waal J C, de Jong E D, et al. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources[J]. Chemical Reviews, 2013, 113(3): 1499-1597. |
| 8 | Zhang K, Agrawal M, Harper J, et al. Removal of the fermentation inhibitor, furfural, using activated carbon in cellulosic-ethanol production[J]. Industrial & Engineering Chemistry Research, 2011, 50(24): 14055-14060. |
| 9 | Rajabbeigi N, Ranjan R, Tsapatsis M, et al. Selective adsorption of HMF on porous carbons from fructose/DMSO mixtures[J]. Microporous and Mesoporous Materials, 2012, 158: 253-256. |
| 10 | Jia X M, Li X Y, Liu Z, et al. Adsorption process and mechanism for furfural separation with macroporous resin[J]. Desalination and Water Treatment, 2014, 56(8): 1-11. |
| 11 | Ijzer A C, Vriezekolk E, Rolevink E, et al. Performance analysis of aromatic adsorptive resins for the effective removal of furan derivatives from glucose[J]. Journal of Chemical Technology & Biotechnology, 2015, 90(1): 101-109. |
| 12 | Anbia M, Mohammadi N. A nanoporous adsorbent for removal of furfural from aqueous solutions[J]. Desalination, 2009, 249(1): 150-153. |
| 13 | Ranjan R, Thust S, Gounaris C E, et al. Adsorption of fermentation inhibitors from lignocellulosic biomass hydrolyzates for improved ethanol yield and value-added product recovery[J]. Microporous and Mesoporous Materials, 2009, 122(1): 143-148. |
| 14 | Huang X C, Lin Y Y, Zhang J P, et al. Ligand-directed strategy for zeolite-type metal-organic frameworks: zinc (II) imidazolates with unusual zeolitic topologies[J]. Angewandte Chemie International Edition, 2006, 45(10): 1557-1559. |
| 15 | Banerjee R, Phan A, Wang B, et al. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture[J]. Science, 2008, 319(5865): 939-943. |
| 16 | Li J R, Sculley J P, Zhou H C. Metal-organic frameworks for separations[J]. Chemical Reviews, 2012, 112(2): 869-932. |
| 17 | 王可可, 李亮莎, 黄宏亮, 等. 铪金属-有机骨架材料的孔尺寸调控及其吸附性能[J]. 化工学报, 2014, 65(5): 1696-1705. |
| Wang K K, Li L S, Huang H L, et al. Control of pore size in Hf-based metal-organic frameworks and exploration of their adsorption properties[J]. CIESC Journal, 2014, 65(5): 1696-1705. | |
| 18 | 高春苹, 石琪, 董晋湘.憎水性金属多氮唑骨架材料(MAF-6)对糠醛/水吸附分离性能的研究[J].化工进展, 2017, 36(9): 3429-3435. |
| Gao C P, Shi Q, Dong J X. Study of the adsorptive separation performance of hydrophobic metal azolate framework(MAF-6) for furfural/water system[J]. Chemical Industry and Engineering Progress, 2017, 36(9): 3429-3435. | |
| 19 | Jin H, Li Y S, Liu X L, et al. Recovery of HMF from aqueous solution by zeolitic imidazolate frameworks[J]. Chemical Engineering Science, 2015, 124: 170-178. |
| 20 | Baerlocher C, McCusker L B, Olson D H. Atlas of Zeolite Framework Types[M]. Elsevier, 2007. |
| 21 | Zhao Y, Xu J, Wang J, et al. Adsorptive separation of furfural/5-hydroxymethylfurfural in MAF-5 with ellipsoidal pores[J]. Industrial & Engineering Chemistry Research, 2020, 59 (25): 11734-11742. |
| 22 | Jin H, Li Y S, Yang W S. Adsorption of biomass-derived polyols onto metal-organic frameworks from aqueous solutions[J]. Industrial & Engineering Chemistry Research, 2018, 57(35): 11963-11969. |
| 23 | Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum[J]. Journal of the American Chemical Society, 1918, 40(9): 1361-1403. |
| 24 | Foo K Y, Hameed B H. Insights into the modeling of adsorption isotherm systems[J]. Chemical Engineering Journal, 2010, 156(1): 2-10. |
| 25 | Schute K, Louven Y, Detoni C, et al. Selective liquid phase adsorption of biogenic HMF on hydrophobic spherical activated carbons[J]. Chemie Ingenieur Technik, 2016, 88(3): 355-362. |
| 26 | Yang Q, Runge T. Cross-linked polyethylenimine for selective adsorption and effective recovery of lignocellulose-derived organic acids and aldehydes[J]. ACS Sustainable Chemistry & Engineering, 2018, 7(1): 933-943. |
| 27 | Zheng J Y, Pan B Y, Xiao J X, et al. Experimental and mathematical simulation of noncompetitive and competitive adsorption dynamic of formic acid-levulinic acid-5- hydroxymethylfurfural from single, binary, and ternary systems in a fixed-bed column of SY-01 resin[J]. Industrial & Engineering Chemistry Research, 2018, 57: 8518-8528. |
| 28 | Zhu A X, Lin R B, Qi X L, et al. Zeolitic metal azolate frameworks (MAFs) from ZnO/Zn(OH)2 and monoalkyl-substituted imidazoles and 1, 2, 4-triazoles: efficient syntheses and properties[J]. Microporous and Mesoporous Materials, 2012, 157: 42-49. |
| 29 | 李长海, 石洪仁, 唐洪宇.弱碱性树脂处理β-萘磺酸废水的吸附分离热力学性能[J].化工学报, 2004, 55(7): 1117-1123. |
| Li C H, Shi H R, Tang H Y. Adsorption separation of β-naphthalenesulphonic acid wastewater on weakly basic resin and thermodynamics [J]. Journal of Chemical Industry and Engineering(China), 2004, 55(7): 1117-1123. | |
| 30 | Sulaymon A H, Ahmed K W. Competitive adsorption of furfural and phenolic compounds onto activated carbon in fixed bed column[J]. Environmental Science & Technology, 2008, 42(2): 392-397. |
| 31 | Lin X Q, Qi G X, Shi S L, et al. Estimation of fixed-bed column parameters and mathematical modeling of breakthrough behaviors for adsorption of levulinic acid from aqueous solution using SY-01 resin[J]. Separation and Purification Technology, 2017, 174: 222-231. |
| 32 | Lin X Q, Wu J L, Jin X H, et al. Selective separation of biobutanol from acetone-butanol-ethanol fermentation broth by means of sorption methodology based on a novel macroporous resin[J].Biotechnology Progress, 2012, 28(4): 962-972. |
| 33 | 尚华, 白洪灏, 刘佳奇, 等. CH4-N2在自支撑颗粒型Silicalite-1上的吸附分离及PSA模拟[J]. 化工学报, 2020, 71(5): 2088-2098. |
| Shang H, Bai H H, Liu J Q, et al. PSA simulation and adsorption separation of CH4-N2 by self-supporting pellets Silicalite-1[J]. CIESC Journal, 2020, 71(5): 2088-2098. | |
| 34 | Weil J, Dien B S, Bothast R J, et al. Removal of fermentation inhibitors formed during pretreatment of biomass by polymeric adsorbents[J]. Industrial & Engineering Chemistry Research, 2002, 41(24): 6132-6138. |
| 35 | Schute K, Louven Y, Detoni C, et al. Selective liquid phase adsorption of biogenic HMF on hydrophobic spherical activated carbons[J]. Chemie Ingenieur Technik, 2016, 88(3): 355-362. |
| [1] | Congqi HUANG, Yimei WU, Jianye CHEN, Shuangquan SHAO. Simulation study of thermal management system of alkaline water electrolysis device for hydrogen production [J]. CIESC Journal, 2023, 74(S1): 320-328. |
| [2] | Yaxin ZHAO, Xueqin ZHANG, Rongzhu WANG, Guo SUN, Shanjing YAO, Dongqiang LIN. Removal of monoclonal antibody aggregates with ion exchange chromatography by flow-through mode [J]. CIESC Journal, 2023, 74(9): 3879-3887. |
| [3] | Jiali ZHENG, Zhihui LI, Xinqiang ZHAO, Yanji WANG. Kinetics of ionic liquid catalyzed synthesis of 2-cyanofuran [J]. CIESC Journal, 2023, 74(9): 3708-3715. |
| [4] | Yan GAO, Peng WU, Chao SHANG, Zejun HU, Xiaodong CHEN. Preparation of magnetic agarose microspheres based on a two-fluid nozzle and their protein adsorption properties [J]. CIESC Journal, 2023, 74(8): 3457-3471. |
| [5] | Jiayi ZHANG, Jiali HE, Jiangpeng XIE, Jian WANG, Yu ZHAO, Dongqiang ZHANG. Research progress of pervaporation technology for N-methylpyrrolidone recovery in lithium battery production [J]. CIESC Journal, 2023, 74(8): 3203-3215. |
| [6] | Bingchun SHENG, Jianguo YU, Sen LIN. Study on lithium resource separation from underground brine with high concentration of sodium by aluminum-based lithium adsorbent [J]. CIESC Journal, 2023, 74(8): 3375-3385. |
| [7] | Ruihang ZHANG, Pan CAO, Feng YANG, Kun LI, Peng XIAO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Analysis of key parameters affecting product purity of natural gas ethane recovery process via ZIF-8 nanofluid [J]. CIESC Journal, 2023, 74(8): 3386-3393. |
| [8] | Lei XING, Chunyu MIAO, Minghu JIANG, Lixin ZHAO, Xinya LI. Optimal design and performance analysis of downhole micro gas-liquid hydrocyclone [J]. CIESC Journal, 2023, 74(8): 3394-3406. |
| [9] | Shuang LIU, Linzhou ZHANG, Zhiming XU, Suoqi ZHAO. Study on molecular level composition correlation of viscosity of residual oil and its components [J]. CIESC Journal, 2023, 74(8): 3226-3241. |
| [10] | Zhaolun WEN, Peirui LI, Zhonglin ZHANG, Xiao DU, Qiwang HOU, Yegang LIU, Xiaogang HAO, Guoqing GUAN. Design and optimization of cryogenic air separation process with dividing wall column based on self-heat regeneration [J]. CIESC Journal, 2023, 74(7): 2988-2998. |
| [11] | Jie WANG, Xiaolin QIU, Ye ZHAO, Xinyang LIU, Zhongqiang HAN, Yong XU, Wenhan JIANG. Preparation and properties of polyelectrolyte electrostatic deposition modified PHBV antioxidant films [J]. CIESC Journal, 2023, 74(7): 3068-3078. |
| [12] | Ji CHEN, Ze HONG, Zhao LEI, Qiang LING, Zhigang ZHAO, Chenhui PENG, Ping CUI. Study on coke dissolution loss reaction and its mechanism based on molecular dynamics simulations [J]. CIESC Journal, 2023, 74(7): 2935-2946. |
| [13] | Yuanliang ZHANG, Xinqi LUAN, Weige SU, Changhao LI, Zhongxing ZHAO, Liqin ZHOU, Jianmin CHEN, Yan HUANG, Zhenxia ZHAO. Study on selective extraction of nicotine by ionic liquids composite extractant and DFT calculation [J]. CIESC Journal, 2023, 74(7): 2947-2956. |
| [14] | Jinming GAO, Yujiao GUO, Chenglin E, Chunxi LU. Study on the separation characteristics of a downstream gas-liquid vortex separator in a closed hood [J]. CIESC Journal, 2023, 74(7): 2957-2966. |
| [15] | Kuikui HAN, Xianglong TAN, Jinzhi LI, Ting YANG, Chun ZHANG, Yongfen ZHANG, Hongquan LIU, Zhongwei YU, Xuehong GU. Four-channel hollow fiber MFI zeolite membrane for the separation of xylene isomers [J]. CIESC Journal, 2023, 74(6): 2468-2476. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||