CIESC Journal ›› 2021, Vol. 72 ›› Issue (10): 5319-5329.DOI: 10.11949/0438-1157.20210378
• Biochemical engineering and technology • Previous Articles Next Articles
Hui ZHOU1( ),Zhifeng TIAN2,Xiaowei TANG2,Zhilong XIU1(
),Zhifeng TIAN2,Xiaowei TANG2,Zhilong XIU1( )
)
Received:2021-03-15
Revised:2021-05-18
Online:2021-10-05
Published:2021-10-05
Contact:
Zhilong XIU
通讯作者:
修志龙
作者简介:周惠(1994—),女,硕士研究生,CLC Number:
Hui ZHOU,Zhifeng TIAN,Xiaowei TANG,Zhilong XIU. Urease-driven preparation of calcium carbonate micro-nanoparticles with different polymorphs[J]. CIESC Journal, 2021, 72(10): 5319-5329.
周惠,田志锋,唐小微,修志龙. 脲酶驱动不同晶型碳酸钙微纳米颗粒的制备[J]. 化工学报, 2021, 72(10): 5319-5329.
Add to citation manager EndNote|Ris|BibTeX
| 脲酶溶液 | 脲酶活性/(U/L) | 蛋白质含量/(g/L) | 比活性/(U/g) |
|---|---|---|---|
| fermentation broth | 3.12 ± 0.02 | 0.77 ± 0.03 | 4.06 ± 0.12 |
| supernatant | 1.24 ± 0.01 | 0.29 ± 0.04 | 4.28 ± 0.12 |
| bacterial cell | 3.75 ± 0.16 | 0.62 ± 0.02 | 6.05 ± 0.01 |
| crude enzyme solution | 5.75 ± 0.08 | 0.87 ± 0.03 | 6.63 ± 0.21 |
Table 1 The results of urease activity in different solutions
| 脲酶溶液 | 脲酶活性/(U/L) | 蛋白质含量/(g/L) | 比活性/(U/g) |
|---|---|---|---|
| fermentation broth | 3.12 ± 0.02 | 0.77 ± 0.03 | 4.06 ± 0.12 |
| supernatant | 1.24 ± 0.01 | 0.29 ± 0.04 | 4.28 ± 0.12 |
| bacterial cell | 3.75 ± 0.16 | 0.62 ± 0.02 | 6.05 ± 0.01 |
| crude enzyme solution | 5.75 ± 0.08 | 0.87 ± 0.03 | 6.63 ± 0.21 |
| 脲酶溶液 | 球霰石 | 方解石 |
|---|---|---|
| 发酵液 | 88.10% | 11.90% |
| 上清液 | 100.00% | 0 |
| 菌体 | 0 | 100.00% |
| 粗酶液 | 53.26% | 46.74% |
Table 2 Proportion of vaterite and calcite by urease in different solutions
| 脲酶溶液 | 球霰石 | 方解石 |
|---|---|---|
| 发酵液 | 88.10% | 11.90% |
| 上清液 | 100.00% | 0 |
| 菌体 | 0 | 100.00% |
| 粗酶液 | 53.26% | 46.74% |
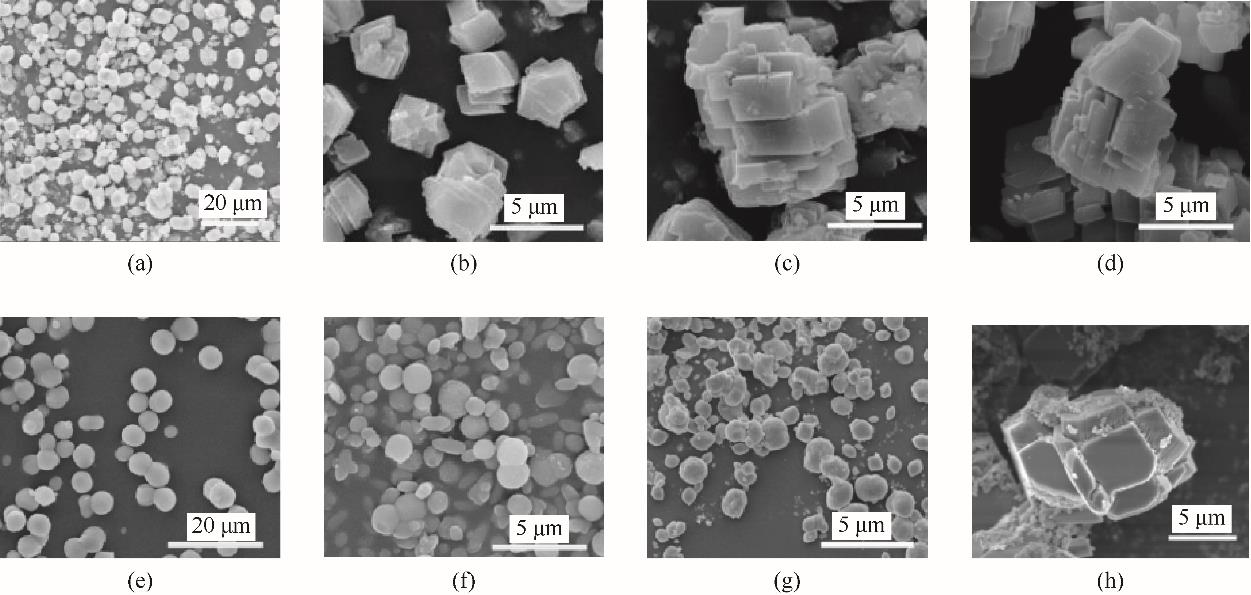
Fig.9 SEM of calcite and vaterite at different time(a)—(d) were the crystal morphology of the control group at <1 min, 30 min, 7 d and 14 d, respectively; (e)—(h) were the crystal morphology of the pure vaterite group at <1 min, 30 min, 7 d and 14 d, respectively
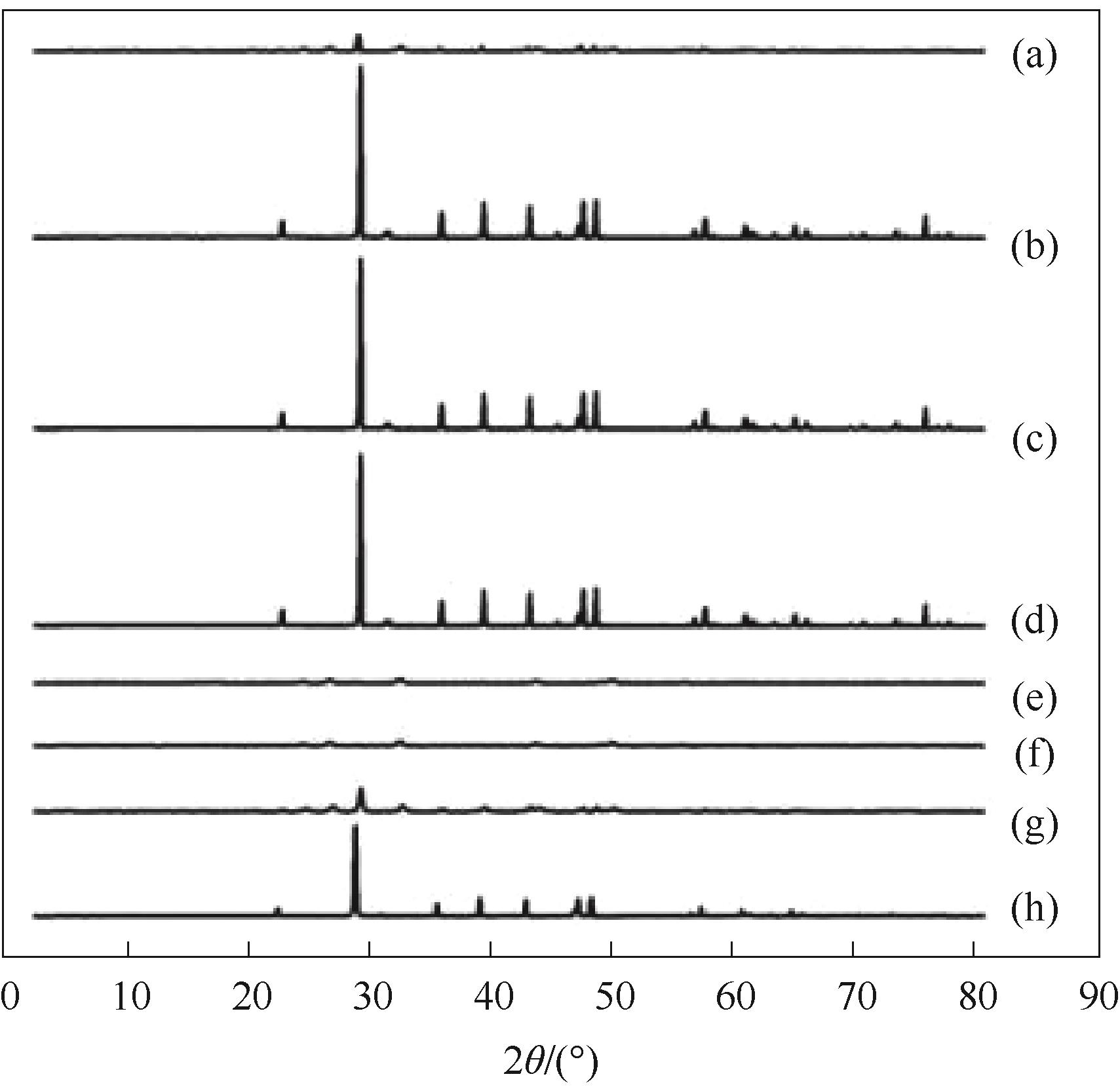
Fig.10 XRD of control group and pure vaterite group at different time(a) control group, < 1 min; (b) control group, 30 min; (c) control group, 7 d; (d) control group, 14 d; (e) vaterite group, < 1 min; (f) vaterite group, 30 min; (g) vaterite group, 7 d; (h) vaterite group, 14 d
| 晶体类型 | <1 min | 30 min | 7 d | 14 d | |
|---|---|---|---|---|---|
| 对照组 | 方解石 | 96.81% | 100% | 100% | 100% |
| 球霰石 | 3.19% | 0 | 0 | 0 | |
| 纯球霰石组 | 方解石 | 0 | 0 | 29.21% | 100% |
| 球霰石 | 100% | 100% | 70.78% | 0 | |
Table 3 Proportion of vaterite and calcite in control group and pure vaterite group at different time
| 晶体类型 | <1 min | 30 min | 7 d | 14 d | |
|---|---|---|---|---|---|
| 对照组 | 方解石 | 96.81% | 100% | 100% | 100% |
| 球霰石 | 3.19% | 0 | 0 | 0 | |
| 纯球霰石组 | 方解石 | 0 | 0 | 29.21% | 100% |
| 球霰石 | 100% | 100% | 70.78% | 0 | |
| 1 | Patil V J, Patil U D, Kulkarni R D, et al. Synthesis of nano CaCO3/acrylic co-polymer latex composites for interior decorative paints[J]. Polymer Composites, 2018, 39(4): 1350-1360. |
| 2 | Al-Attar F, Al-Samhan M. Nano CaCO3 incorporation with polypropylene to reduce film water vapor permeability for packaging application[J]. Asian Journal of Scientific Research, 2020, 13(4): 275-283. |
| 3 | Wang H M, Chen Y Z, Zhang Z J. Enhanced ink-absorption performance of inkjet printing paper-based patterns with core-shell-structure CaCO3@SiO2 pigments[J]. Nordic Pulp & Paper Research Journal, 2019, 34(4): 525-533. |
| 4 | Morsy F A, El-Sheikh S M, Barhoum A. Nano-silica and SiO2/CaCO3 nanocomposite prepared from semi-burned rice straw ash as modified papermaking fillers[J]. Arabian Journal of Chemistry, 2019, 12(7): 1186-1196. |
| 5 | 王训遒, 蒋登高. 纳米碳酸钙复合丙烯酸涂料的流变性[J]. 化工学报, 2007, 58(1): 238-247. |
| Wang X Q, Jiang D G. Rheological property of nanometer calcium carbonate composite acrylic coatings[J]. Journal of Chemical Industry and Engineering (China), 2007, 58(1): 238-247. | |
| 6 | Boyjoo Y, Pareek V K, Liu J. Synthesis of micro and nano-sized calcium carbonate particles and their applications[J]. Journal of Materials Chemistry A, 2014, 2(35): 14270-14288. |
| 7 | Gong L F, Qian S H, Wang W, et al. Influence of nano-additives (nano-PTFE and nano-CaCO3) on tribological properties of food-grade aluminum-based grease[J]. Tribology International, 2021, 160: 107014. |
| 8 | Maleki Dizaj S, Sharifi S, Ahmadian E, et al. An update on calcium carbonate nanoparticles as cancer drug/gene delivery system[J]. Expert Opinion on Drug Delivery, 2019, 16(4): 331-345. |
| 9 | Trofimov A, Ivanova A, Zyuzin M, et al. Porous inorganic carriers based on silica, calcium carbonate and calcium phosphate for controlled/modulated drug delivery: fresh outlook and future perspectives[J]. Pharmaceutics, 2018, 10(4): 167. |
| 10 | Wang S, Ni D Z, Yue H, et al. Exploration of antigen induced CaCO3 nanoparticles for therapeutic vaccine[J]. Small, 2018, 14(14): 1704272. |
| 11 | Batool A, Valiyaveettil S. Coprecipitation—an efficient method for removal of polymer nanoparticles from water[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(35): 13481-13487. |
| 12 | Zhang X D, Shi D Y, Li X, et al. Nanoscale dispersing of zero-valent iron on CaCO3 and their significant synergistic effect in high performance removal of lead[J]. Chemosphere, 2019, 224: 390-397. |
| 13 | Fathy M, Zayed M A, Moustafa Y M. Synthesis and applications of CaCO3/HPC core-shell composite subject to heavy metals adsorption processes[J]. Heliyon, 2019, 5(8): e02215. |
| 14 | Akiyama M, Kawasaki S. Biogeochemical simulation of microbially induced calcite precipitation with Pararhodobacter sp. strain SO1[J]. Acta Geotechnica, 2019, 14(3): 685-696. |
| 15 | Wen K J, Li Y, Amini F, et al. Impact of bacteria and urease concentration on precipitation kinetics and crystal morphology of calcium carbonate[J]. Acta Geotechnica, 2020, 15(1): 17-27. |
| 16 | Zheng T W. Bacteria-induced facile biotic calcium carbonate precipitation[J]. Journal of Crystal Growth, 2021, 563: 126096. |
| 17 | Trushina D B, Bukreeva T V, Antipina M N. Size-controlled synthesis of vaterite calcium carbonate by the mixing method: aiming for nanosized particles[J]. Crystal Growth & Design, 2016, 16(3): 1311-1319. |
| 18 | Oral Ç M, Ercan B. Influence of pH on morphology, size and polymorph of room temperature synthesized calcium carbonate particles[J]. Powder Technology, 2018, 339: 781-788. |
| 19 | Wei W, Ma G H, Hu G, et al. Preparation of hierarchical hollow CaCO3 particles and the application as anticancer drug carrier[J]. Journal of the American Chemical Society, 2008, 130(47): 15808-15810. |
| 20 | Polat S. Experimental investigations on the effects of asparagine and serine on the polymorphism of calcium carbonate[J]. Advanced Powder Technology, 2020, 31(10): 4282-4291. |
| 21 | 张一江, 柳鑫华, 陈智慧, 等. L-半胱氨酸改性聚环氧琥珀酸的合成及其阻垢缓蚀性能[J]. 化工学报, 2016, 67(10): 4344-4355. |
| Zhang Y J, Liu X H, Chen Z H, et al. Synthesis of L-cysteine modified polyepoxysuccinic acid and evaluation of its inhibition on scale deposition and corrosion[J]. CIESC Journal, 2016, 67(10): 4344-4355. | |
| 22 | Liu X, Li K X, Wu C Q, et al. Egg white-assisted preparation of inorganic functional materials: a sustainable, eco-friendly, low-cost and multifunctional method[J]. Ceramics International, 2019, 45(18): 23869-23889. |
| 23 | Yang D, Yan Y, Yang X, et al. A basic protein, N25, from a mollusk modifies calcium carbonate morphology and shell biomineralization[J]. Journal of Biological Chemistry, 2019, 294(21): 8371-8383. |
| 24 | Wei Y, Xu H, Xu S M, et al. Synthesis and characterization of calcium carbonate on three kinds of microbial cells templates[J]. Journal of Crystal Growth, 2020, 547: 125755. |
| 25 | Singh A, Singh S K. The impact of various drug ferrying additives on phase transitions behavior of calcite, vaterite and aragonite[J]. Phase Transitions, 2019, 92(11): 990-1008. |
| 26 | Bastrzyk A, Fiedot-Toboła M, Polowczyk I, et al. Effect of a lipopeptide biosurfactant on the precipitation of calcium carbonate[J]. Colloids and Surfaces B: Biointerfaces, 2019, 174: 145-152. |
| 27 | Chaparro-Acuña S P, Becerra-Jiménez M L, Martínez-Zambrano J J, et al. Soil bacteria that precipitate calcium carbonate: mechanism and applications of the process[J]. Acta Agronómica, 2018, 67(2): 277-288. |
| 28 | Seifan M, Berenjian A. Application of microbially induced calcium carbonate precipitation in designing bio self-healing concrete[J]. World Journal of Microbiology and Biotechnology, 2018, 34(11): 1-15. |
| 29 | Ortega-Villamagua E, Gudiño-Gomezjurado M, Palma-Cando A. Microbiologically induced carbonate precipitation in the restoration and conservation of cultural heritage materials[J]. Molecules, 2020, 25(23): 5499. |
| 30 | Ghosh T, Bhaduri S, Montemagno C, et al. Sporosarcina pasteurii can form nanoscale calcium carbonate crystals on cell surface[J]. PLoS One, 2019, 14(1): e0210339. |
| 31 | Zhang W C, Ju Y, Zong Y W, et al. In situ real-time study on dynamics of microbially induced calcium carbonate precipitation at a single-cell level[J]. Environmental Science & Technology, 2018, 52(16): 9266-9276. |
| 32 | Zehner J, Røyne A, Wentzel A, et al. Microbial-induced calcium carbonate precipitation: an experimental toolbox for in situ and real-time investigation of micro-scale pH evolution[J]. RSC Advances, 2020, 10(35): 20485-20493. |
| 33 | Zehner J, Røyne A, Sikorski P. Calcite seed-assisted microbial induced carbonate precipitation(MICP)[J]. PLoS One, 2021, 16(2): e0240763. |
| 34 | Gorospe C M, Han S H, Kim S G, et al. Effects of different calcium salts on calcium carbonate crystal formation by Sporosarcina pasteurii KCTC 3558[J]. Biotechnology and Bioprocess Engineering, 2013, 18(5): 903-908. |
| 35 | Whiffin V S. Microbial CaCO3 precipitation for the production of biocement[D]. Western Australia: Murdoch University, 2004. |
| 36 | Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding[J]. Analytical Biochemistry, 1976, 72(1/2): 248-254. |
| 37 | Seepma S Y M H, Ruiz-Hernandez S E, Nehrke G, et al. Controlling CaCO3 particle size with {Ca2+}: {CO32-} ratios in aqueous environments[J]. Crystal Growth & Design, 2021, 21(3): 1576-1590. |
| 38 | Tobler D J, Cuthbert M O, Greswell R B, et al. Comparison of rates of ureolysis between Sporosarcina pasteurii and an indigenous groundwater community under conditions required to precipitate large volumes of calcite[J]. Geochimica et Cosmochimica Acta, 2011, 75(11): 3290-3301. |
| 39 | Aizenberg J, Black A J, Whitesides G M. Oriented growth of calcite controlled by self-assembled monolayers of functionalized alkanethiols supported on gold and silver[J]. Journal of the American Chemical Society, 1999, 121(18): 4500-4509. |
| 40 | Deng H, Wang S, Wang X, et al. Two competitive nucleation mechanisms of calcium carbonate biomineralization in response to surface functionality in low calcium ion concentration solution[J]. Regenerative Biomaterials, 2015, 2(3): 187-195. |
| 41 | Fang P A, Conway J F, Margolis H C, et al. Hierarchical self-assembly of amelogenin and the regulation of biomineralization at the nanoscale[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(34): 14097-14102. |
| 42 | Meldrum F C, Cölfen H. Controlling mineral morphologies and structures in biological and synthetic systems[J]. Chemical Reviews, 2008, 108(11): 4332-4432. |
| 43 | Massi M, Ogden M I, Jones F. Investigating vaterite phase stabilisation by a tetrazole molecule during calcium carbonate crystallisation[J]. Journal of Crystal Growth, 2012, 351(1): 107-114. |
| 44 | 李云钊, 宋兴福, 孙玉柱, 等. 反应-萃取-结晶过程制备碳酸钙的晶型转变与结晶机理[J]. 化工学报, 2015, 66(10): 4007-4015. |
| Li Y Z, Song X F, Sun Y Z, et al. Polymorph transformation and formation mechanism of calcium carbonate during reactive extraction-crystallization process[J]. CIESC Journal, 2015, 66(10): 4007-4015. | |
| 45 | Sergeeva A, Sergeev R, Lengert E, et al. Composite magnetite and protein containing CaCO3 crystals. external manipulation and vaterite→calcite recrystallization-mediated release performance[J]. ACS Applied Materials & Interfaces, 2015, 7(38): 21315-21325. |
| 46 | Fang Z, He W, Tu T, et al. An efficient and green pathway for continuous Friedel-Crafts acylation over α-Fe2O3 and CaCO3 nanoparticles prepared in the microreactors[J]. Chemical Engineering Journal, 2018, 331: 443-449. |
| [1] | Yepin CHENG, Daqing HU, Yisha XU, Huayan LIU, Hanfeng LU, Guokai CUI. Application of ionic liquid-based deep eutectic solvents for CO2 conversion [J]. CIESC Journal, 2023, 74(9): 3640-3653. |
| [2] | Rubin ZENG, Zhongjie SHEN, Qinfeng LIANG, Jianliang XU, Zhenghua DAI, Haifeng LIU. Study of the sintering mechanism of Fe2O3 nanoparticles based on molecular dynamics simulation [J]. CIESC Journal, 2023, 74(8): 3353-3365. |
| [3] | Lingding MENG, Ruqing CHONG, Feixue SUN, Zihui MENG, Wenfang LIU. Immobilization of carbonic anhydrase on modified polyethylene membrane and silica [J]. CIESC Journal, 2023, 74(8): 3472-3484. |
| [4] | Xudong YU, Qi LI, Niancu CHEN, Li DU, Siying REN, Ying ZENG. Phase equilibria and calculation of aqueous ternary system KCl + CaCl2 + H2O at 298.2, 323.2, and 348.2 K [J]. CIESC Journal, 2023, 74(8): 3256-3265. |
| [5] | Chongda DUAN, Xiaowei YAO, Jiahua ZHU, Jing SUN, Nan HU, Guangyue LI. Effects of environmental factors on calcium carbonate precipitation induced by Klebsiella aerogenes [J]. CIESC Journal, 2023, 74(8): 3543-3553. |
| [6] | Yaxin CHEN, Hang YUAN, Guanzhang LIU, Lei MAO, Chun YANG, Ruifang ZHANG, Guangya ZHANG. Advances in enzyme self-immobilization mediated by protein nanocages [J]. CIESC Journal, 2023, 74(7): 2773-2782. |
| [7] | Xiaoling TANG, Jiarui WANG, Xuanye ZHU, Renchao ZHENG. Biosynthesis of chiral epichlorohydrin by halohydrin dehalogenase based on Pickering emulsion system [J]. CIESC Journal, 2023, 74(7): 2926-2934. |
| [8] | Tan ZHANG, Guang LIU, Jinping LI, Yuhan SUN. Performance regulation strategies of Ru-based nitrogen reduction electrocatalysts [J]. CIESC Journal, 2023, 74(6): 2264-2280. |
| [9] | Yong LI, Jiaqi GAO, Chao DU, Yali ZHAO, Boqiong LI, Qianqian SHEN, Husheng JIA, Jinbo XUE. Construction of Ni@C@TiO2 core-shell dual-heterojunctions for advanced photo-thermal catalytic hydrogen generation [J]. CIESC Journal, 2023, 74(6): 2458-2467. |
| [10] | Juhui CHEN, Qian ZHANG, Lingfeng SHU, Dan LI, Xin XU, Xiaogang LIU, Chenxi ZHAO, Xifeng CAO. Study on flow characteristics of nanoparticles in a rotating fluidized bed based on DEM method [J]. CIESC Journal, 2023, 74(6): 2374-2381. |
| [11] | Lei MAO, Guanzhang LIU, Hang YUAN, Guangya ZHANG. Efficient preparation of carbon anhydrase nanoparticles capable of capturing CO2 and their characteristics [J]. CIESC Journal, 2023, 74(6): 2589-2598. |
| [12] | Lanhe ZHANG, Qingyi LAI, Tiezheng WANG, Xiaozhuo GUAN, Mingshuang ZHANG, Xin CHENG, Xiaohui XU, Yanping JIA. Effect of H2O2 on nitrogen removal and sludge properties in SBR [J]. CIESC Journal, 2023, 74(5): 2186-2196. |
| [13] | Zijian WANG, Ming KE, Jiahan LI, Shuting LI, Jinru SUN, Yanbing TONG, Zhiping ZHAO, Jiaying LIU, Lu REN. Progress in preparation and application of short b-axis ZSM-5 molecular sieve [J]. CIESC Journal, 2023, 74(4): 1457-1473. |
| [14] | Tianhao BAI, Xiaowen WANG, Mengzi YANG, Xinwei DUAN, Jie MI, Mengmeng WU. Study on release and inhibition behavior of COS during high-temperature gas desulfurization process using Zn-based oxide derived from hydrotalcite [J]. CIESC Journal, 2023, 74(4): 1772-1780. |
| [15] | Lufan JIA, Yiying WANG, Yuman DONG, Qinyuan LI, Xin XIE, Hao YUAN, Tao MENG. Aqueous two-phase system based adherent droplet microfluidics for enhanced enzymatic reaction [J]. CIESC Journal, 2023, 74(3): 1239-1246. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||