CIESC Journal ›› 2023, Vol. 74 ›› Issue (4): 1703-1711.DOI: 10.11949/0438-1157.20230087
• Energy and environmental engineering • Previous Articles Next Articles
Zhen LONG1,2,3,4( ), Jinhang WANG1,2,3,4,5, Yong HE1,2,3,4(
), Jinhang WANG1,2,3,4,5, Yong HE1,2,3,4( ), Deqing LIANG1,2,3,4(
), Deqing LIANG1,2,3,4( )
)
Received:2023-02-08
Revised:2023-03-30
Online:2023-06-02
Published:2023-04-05
Contact:
Yong HE, Deqing LIANG
龙臻1,2,3,4( ), 王谨航1,2,3,4,5, 何勇1,2,3,4(
), 王谨航1,2,3,4,5, 何勇1,2,3,4( ), 梁德青1,2,3,4(
), 梁德青1,2,3,4( )
)
通讯作者:
何勇,梁德青
作者简介:龙臻(1986—),女,博士,副研究员,longzhen@ms.giec.ac.cn
基金资助:CLC Number:
Zhen LONG, Jinhang WANG, Yong HE, Deqing LIANG. Characteristics study on hydrates formation from gas mixture under ionic liquid together with kinetic hydrate inhibitors[J]. CIESC Journal, 2023, 74(4): 1703-1711.
龙臻, 王谨航, 何勇, 梁德青. 离子液体与动力学抑制剂作用下混合气体水合物生成特性研究[J]. 化工学报, 2023, 74(4): 1703-1711.
Add to citation manager EndNote|Ris|BibTeX
| 体系 | 水合物相组分浓度/%(mol) | 耗气量/mmol | 气相组分浓度/%(mol) | ||||
|---|---|---|---|---|---|---|---|
| CH4 | C2H6 | C3H8 | CH4 | C2H6 | C3H8 | ||
| 纯水 | 83.77 | 10.13 | 6.1 | 86.5 | 99.41 | 0.54 | 0.05 |
| [BMP][BF4] | 85.07 | 8.92 | 6.01 | 86.4 | 98.23 | 1.65 | 0.12 |
| PVPK90 | 83.05 | 10.48 | 6.47 | 81.2 | 99.16 | 0.77 | 0.07 |
| PVCap | 82.80 | 10.57 | 6.63 | 78.4 | 98.91 | 0.97 | 0.12 |
| PVPK90+[BMP][BF4] | 83.77 | 9.99 | 6.24 | 84.4 | 99.06 | 0.88 | 0.06 |
| PVPCap+[BMP][BF4] | 52.78 | 23.37 | 23.85 | 20.2 | 96.82 | 2.83 | 0.32 |
Table 1 Gas compositions in vapor phase and hydrate phase at the end of reaction and total gas consumption in the presence of water or various inhibitors
| 体系 | 水合物相组分浓度/%(mol) | 耗气量/mmol | 气相组分浓度/%(mol) | ||||
|---|---|---|---|---|---|---|---|
| CH4 | C2H6 | C3H8 | CH4 | C2H6 | C3H8 | ||
| 纯水 | 83.77 | 10.13 | 6.1 | 86.5 | 99.41 | 0.54 | 0.05 |
| [BMP][BF4] | 85.07 | 8.92 | 6.01 | 86.4 | 98.23 | 1.65 | 0.12 |
| PVPK90 | 83.05 | 10.48 | 6.47 | 81.2 | 99.16 | 0.77 | 0.07 |
| PVCap | 82.80 | 10.57 | 6.63 | 78.4 | 98.91 | 0.97 | 0.12 |
| PVPK90+[BMP][BF4] | 83.77 | 9.99 | 6.24 | 84.4 | 99.06 | 0.88 | 0.06 |
| PVPCap+[BMP][BF4] | 52.78 | 23.37 | 23.85 | 20.2 | 96.82 | 2.83 | 0.32 |
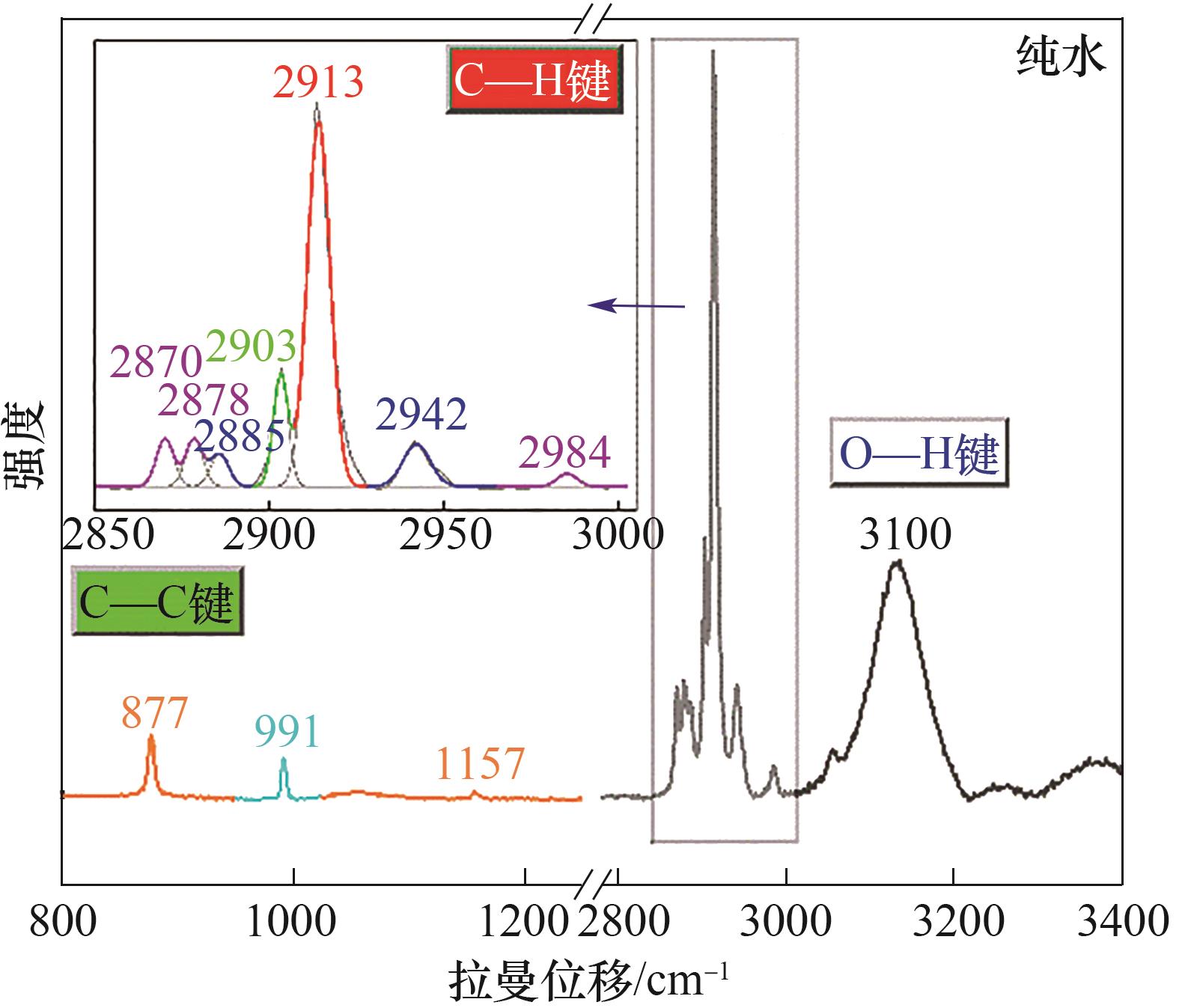
Fig.6 Typical Raman spectrum of CH4/C2H6/C3H8 hydrate formed with pure water (800—1200 cm-1: C—C region; 2800—3000 cm-1: C—H region; 3000—3400 cm-1: O—H region)
| 客体 分子 | 客体分子直径/Å | sⅠ型水合物 | sⅡ型水合物 | ||
|---|---|---|---|---|---|
| 512 | 51262 | 512 | 51264 | ||
| CH4 | 4.36 | 0.855 | 0.744 | 0.868 | 0.655 |
| C2H6 | 5.5 | 1.08 | 0.939 | 1.1 | 0.826 |
| C3H8 | 6.28 | 1.23 | 1.07 | 1.25 | 0.943 |
Table 2 Ratios of molecular diameters to cage diameters for some guest molecules[1]
| 客体 分子 | 客体分子直径/Å | sⅠ型水合物 | sⅡ型水合物 | ||
|---|---|---|---|---|---|
| 512 | 51262 | 512 | 51264 | ||
| CH4 | 4.36 | 0.855 | 0.744 | 0.868 | 0.655 |
| C2H6 | 5.5 | 1.08 | 0.939 | 1.1 | 0.826 |
| C3H8 | 6.28 | 1.23 | 1.07 | 1.25 | 0.943 |
| 1 | Sloan E D, Koh C A. Clathrate Hydrates of Natural Gases[M]. 3rd ed. Boca Raton, FL: CRC Press/Taylor & Francis, 2008. |
| 2 | Song Y C, Yang L, Zhao J F, et al. The status of natural gas hydrate research in China: a review[J]. Renewable and Sustainable Energy Reviews, 2014, 31: 778-791. |
| 3 | Boswell R, Collett T S. Current perspectives on gas hydrate resources[J]. Energy & Environmental Science, 2011, 4(4): 1206-1215. |
| 4 | Hammerschmidt E G. Formation of gas hydrates in natural gas transmission lines[J]. Industrial & Engineering Chemistry, 1934, 26(8): 851-855. |
| 5 | 张剑波, 王志远, 刘书杰, 等. 深水气井测试过程中水合物流动障碍防治方法[J]. 石油勘探与开发, 2020, 47(6): 1256-1264. |
| Zhang J B, Wang Z Y, Liu S J, et al. A method for preventing hydrates from blocking flow during deep-water gas well testing[J]. Petroleum Exploration and Development, 2020, 47(6): 1256-1264. | |
| 6 | Wang Y H, Fan S S, Lang X M. Reviews of gas hydrate inhibitors in gas-dominant pipelines and application of kinetic hydrate inhibitors in China[J]. Chinese Journal of Chemical Engineering, 2019, 27(9): 2118-2132. |
| 7 | 李锐, 宁伏龙, 张凌, 等. 低剂量水合物抑制剂的研究进展[J]. 石油化工, 2018, 47(2): 203-210. |
| Li R, Ning F L, Zhang L, et al. Progress in the research of the low dosage hydrate inhibitors[J]. Petrochemical Technology, 2018, 47(2): 203-210. | |
| 8 | 陈玉川, 史博会, 李文庆, 等. 水合物动力学抑制剂的作用机理研究进展[J]. 化工进展, 2018, 37(5): 1726-1743. |
| Chen Y C, Shi B H, Li W Q, et al. Progress of influence mechanism of kinetic hydrate inhibitors[J]. Chemical Industry and Engineering Progress, 2018, 37(5): 1726-1743. | |
| 9 | 董三宝, 田茂琳, 徐遥远, 等. 天然气水合物防聚剂研究进展[J]. 广东化工, 2021, 48(10): 82-85. |
| Dong S B, Tian M L, Xu Y Y, et al. Progress in the investigation of natural gas hydrate anti-agglomerants[J]. Guangdong Chemical Industry, 2021, 48(10): 82-85. | |
| 10 | Salmin D C, Estanga D, Koh C A. Review of gas hydrate anti-agglomerant screening techniques[J]. Fuel, 2022, 319: 122862. |
| 11 | Kelland M A, Dirdal E G, Ree L H S. Solvent synergists for improved kinetic hydrate inhibitor performance of poly(N-vinylcaprolactam)[J]. Energy & Fuels, 2020, 34(2): 1653-1663. |
| 12 | Xu S R, Fan S S, Fang S T, et al. Excellent synergy effect on preventing CH4 hydrate formation when glycine meets polyvinylcaprolactam[J]. Fuel, 2017, 206: 19-26. |
| 13 | Lee W, Shin J Y, Cha J H, et al. Inhibition effect of ionic liquids and their mixtures with poly(N-vinylcaprolactam) on methane hydrate formation[J]. Journal of Industrial and Engineering Chemistry, 2016, 38: 211-216. |
| 14 | Rogers R D, Seddon K R. Ionic liquids: solvents of the future?[J]. Science, 2003, 302(5646): 792-793. |
| 15 | Xiao C W, Adidharma H. Dual function inhibitors for methane hydrate[J]. Chemical Engineering Science, 2009, 64(7): 1522-1527. |
| 16 | Xiao C W, Wibisono N, Adidharma H. Dialkylimidazolium halide ionic liquids as dual function inhibitors for methane hydrate[J]. Chemical Engineering Science, 2010, 65(10): 3080-3087. |
| 17 | Zare M, Kondori J, Zendehboudi S, et al. PC-SAFT/UNIQUAC model assesses formation condition of methane hydrate in the presence of imidazolium-based ionic liquid systems[J]. Fuel, 2020, 266: 116757. |
| 18 | Tariq M, Connor E, Thompson J, et al. Doubly dual nature of ammonium-based ionic liquids for methane hydrates probed by rocking-rig assembly[J]. RSC Advances, 2016, 6(28): 23827-23836. |
| 19 | Cha J H, Ha C, Kang S P, et al. Thermodynamic inhibition of CO2 hydrate in the presence of morpholinium and piperidinium ionic liquids[J]. Fluid Phase Equilibria, 2016, 413: 75-79. |
| 20 | Long Z, Zhou X B, Shen X D, et al. Phase equilibria and dissociation enthalpies of methane hydrate in imidazolium ionic liquid aqueous solutions[J]. Industrial & Engineering Chemistry Research, 2015, 54(46): 11701-11708. |
| 21 | Nasir Q, Suleman H, Elsheikh Y A. A review on the role and impact of various additives as promoters/inhibitors for gas hydrate formation[J]. Journal of Natural Gas Science and Engineering, 2020, 76: 103211. |
| 22 | Farhadian A, Shadloo A, Zhao X, et al. Challenges and advantages of using environmentally friendly kinetic gas hydrate inhibitors for flow assurance application: a comprehensive review[J]. Fuel, 2023, 336: 127055. |
| 23 | Kim K S, Kang J W, Kang S P. Tuning ionic liquids for hydrate inhibition[J]. Chemical Communications, 2011, 47(22): 6341-6343. |
| 24 | Kang S P, Kim E S, Shin J Y, et al. Unusual synergy effect on methane hydrate inhibition when ionic liquid meets polymer[J]. RSC Advances, 2013, 3(43): 19920-19923. |
| 25 | Shen X D, Zhou X B, Liang D Q. Kinetic effects of ionic liquids on methane hydrate[J]. Energy & Fuels, 2019, 33(2): 1422-1432. |
| 26 | Shen X D, Shi L L, Long Z, et al. Experimental study on the kinetic effect of N-butyl-N-methylpyrrolidinium bromide on CO2 hydrate[J]. Journal of Molecular Liquids, 2016, 223: 672-677. |
| 27 | 任俊杰, 龙臻, 梁德青. 离子液体与PVP K90复合抑制剂对甲烷水合物的生成影响[J]. 化工学报, 2020, 71(11): 5256-5264. |
| Ren J J, Long Z, Liang D Q. Effect of complex inhibitors containing ionic liquids and PVP K90 on formation of methane hydrate[J]. CIESC Journal, 2020, 71(11): 5256-5264. | |
| 28 | Ren J J, Lu Z L, Long Z, et al. Experimental study on the kinetic effect of N-butyl-N-methylpyrrolidinium tetrafluoroborate and poly(N-vinyl-caprolactam) on CH4 hydrate formation[J]. RSC Advances, 2020, 10(26): 15320-15327. |
| 29 | Lee D, Go W, Seo Y. Experimental and computational investigation of methane hydrate inhibition in the presence of amino acids and ionic liquids[J]. Energy, 2019, 182: 632-640. |
| 30 | Del Villano L, Kelland M A. An investigation into the kinetic hydrate inhibitor properties of two imidazolium-based ionic liquids on Structure Ⅱ gas hydrate[J]. Chemical Engineering Science, 2010, 65(19): 5366-5372. |
| 31 | Kang S P, Jung T, Lee J W. Macroscopic and spectroscopic identifications of the synergetic inhibition of an ionic liquid on hydrate formations[J]. Chemical Engineering Science, 2016, 143: 270-275. |
| 32 | Qureshi M F, Atilhan M, Altamash T, et al. Gas hydrate prevention and flow assurance by using mixtures of ionic liquids and synergent compounds: combined kinetics and thermodynamic approach[J]. Energy & Fuels, 2016, 30(4): 3541-3548. |
| 33 | Long Z, Zhou X B, Lu Z L, et al. Kinetic inhibition performance of N-vinyl caprolactam/isopropylacrylamide copolymers on methane hydrate formation[J]. Energy, 2022, 242: 123056. |
| 34 | Redlich O, Kwong J N S. On the thermodynamics of solutions; an equation of state; fugacities of gaseous solutions[J]. Chemical Reviews, 1949, 44(1): 233-244. |
| 35 | Chen G J, Guo T M. A new approach to gas hydrate modelling[J]. Chemical Engineering Journal, 1998, 71(2): 145-151. |
| 36 | Uchida T, Moriwaki M, Takeya S, et al. Two-step formation of methane-propane mixed gas hydrates in a batch-type reactor[J]. AIChE Journal, 2004, 50(2): 518-523. |
| 37 | Uchida T, Takeya S, Kamata Y, et al. Spectroscopic measurements on binary, ternary, and quaternary mixed-gas molecules in clathrate structures[J]. Industrial & Engineering Chemistry Research, 2007, 46(14): 5080-5087. |
| 38 | Kumar R, Linga P, Moudrakovski I, et al. Structure and kinetics of gas hydrates from methane/ethane/propane mixtures relevant to the design of natural gas hydrate storage and transport facilities[J]. AIChE Journal, 2008, 54(8): 2132-2144. |
| 39 | Daraboina N, Ripmeester J, Walker V K, et al. Natural gas hydrate formation and decomposition in the presence of kinetic inhibitors (3): Structural and compositional changes[J]. Energy & Fuels, 2011, 25(10): 4398-4404. |
| 40 | Cha M J, Shin K, Seo Y, et al. Catastrophic growth of gas hydrates in the presence of kinetic hydrate inhibitors[J]. The Journal of Physical Chemistry A, 2013, 117(51): 13988-13995. |
| 41 | Sharifi H, Englezos P. Accelerated hydrate crystal growth in the presence of low dosage additives known as kinetic hydrate inhibitors[J]. Journal of Chemical & Engineering Data, 2015, 60(2): 336-342. |
| 42 | Takeya S, Ripmeester J A. Anomalous preservation of CH4 hydrate and its dependence on the morphology of hexagonal ice[J]. ChemPhysChem, 2010, 11(1): 70-73. |
| 43 | Subramanian S, Sloan E D. Trends in vibrational frequencies of guests trapped in clathrate hydrate cages[J]. The Journal of Physical Chemistry B, 2002, 106(17): 4348-4355. |
| 44 | Yagasaki T, Matsumoto M, Tanaka H. Adsorption mechanism of inhibitor and guest molecules on the surface of gas hydrates[J]. Journal of the American Chemical Society, 2015, 137(37): 12079-12085. |
| 45 | Xu J F, Li L W, Liu J X, et al. The molecular mechanism of the inhibition effects of PVCaps on the growth of sI hydrate: an unstable adsorption mechanism[J]. Physical Chemistry Chemical Physics, 2018, 20(12): 8326-8332. |
| 46 | Castillo-Borja F, Bravo-Sánchez U I. Molecular dynamics simulation study of the performance of different inhibitors for methane hydrate growth[J]. Journal of Molecular Liquids, 2021, 337: 116510. |
| [1] | Qi WANG, Bin ZHANG, Xiaoxin ZHANG, Hujian WU, Haitao ZHAN, Tao WANG. Synthesis of isoxepac and 2-ethylanthraquinone catalyzed by chloroaluminate-triethylamine ionic liquid/P2O5 [J]. CIESC Journal, 2023, 74(S1): 245-249. |
| [2] | Ruimin CHE, Wenqiu ZHENG, Xiaoyu WANG, Xin LI, Feng XU. Research progress on homogeneous processing of cellulose in ionic liquids [J]. CIESC Journal, 2023, 74(9): 3615-3627. |
| [3] | Minghao SONG, Fei ZHAO, Shuqing LIU, Guoxuan LI, Sheng YANG, Zhigang LEI. Multi-scale simulation and study of volatile phenols removal from simulated oil by ionic liquids [J]. CIESC Journal, 2023, 74(9): 3654-3664. |
| [4] | Shaoqi YANG, Shuheng ZHAO, Lungang CHEN, Chenguang WANG, Jianjun HU, Qing ZHOU, Longlong MA. Hydrodeoxygenation of lignin-derived compounds to alkanes in Raney Ni-protic ionic liquid system [J]. CIESC Journal, 2023, 74(9): 3697-3707. |
| [5] | Junfeng LU, Huaiyu SUN, Yanlei WANG, Hongyan HE. Molecular understanding of interfacial polarization and its effect on ionic liquid hydrogen bonds [J]. CIESC Journal, 2023, 74(9): 3665-3680. |
| [6] | Jiali ZHENG, Zhihui LI, Xinqiang ZHAO, Yanji WANG. Kinetics of ionic liquid catalyzed synthesis of 2-cyanofuran [J]. CIESC Journal, 2023, 74(9): 3708-3715. |
| [7] | Lei WU, Jiao LIU, Changcong LI, Jun ZHOU, Gan YE, Tiantian LIU, Ruiyu ZHU, Qiuli ZHANG, Yonghui SONG. Catalytic microwave pyrolysis of low-rank pulverized coal for preparation of high value-added modified bluecoke powders containing carbon nanotubes [J]. CIESC Journal, 2023, 74(9): 3956-3967. |
| [8] | Meisi CHEN, Weida CHEN, Xinyao LI, Shangyu LI, Youting WU, Feng ZHANG, Zhibing ZHANG. Advances in silicon-based ionic liquid microparticle enhanced gas capture and conversion [J]. CIESC Journal, 2023, 74(9): 3628-3639. |
| [9] | Yepin CHENG, Daqing HU, Yisha XU, Huayan LIU, Hanfeng LU, Guokai CUI. Application of ionic liquid-based deep eutectic solvents for CO2 conversion [J]. CIESC Journal, 2023, 74(9): 3640-3653. |
| [10] | Lizhi WANG, Qiancheng HANG, Yeling ZHENG, Yan DING, Jiaji CHEN, Qing YE, Jinlong LI. Separation of methyl propionate + methanol azeotrope using ionic liquid entrainers [J]. CIESC Journal, 2023, 74(9): 3731-3741. |
| [11] | Jie CHEN, Yongsheng LIN, Kai XIAO, Chen YANG, Ting QIU. Study on catalytic synthesis of sec-butanol by tunable choline-based basic ionic liquids [J]. CIESC Journal, 2023, 74(9): 3716-3730. |
| [12] | Zehao MI, Er HUA. DFT and COSMO-RS theoretical analysis of SO2 absorption by polyamines type ionic liquids [J]. CIESC Journal, 2023, 74(9): 3681-3696. |
| [13] | Rubin ZENG, Zhongjie SHEN, Qinfeng LIANG, Jianliang XU, Zhenghua DAI, Haifeng LIU. Study of the sintering mechanism of Fe2O3 nanoparticles based on molecular dynamics simulation [J]. CIESC Journal, 2023, 74(8): 3353-3365. |
| [14] | Xudong YU, Qi LI, Niancu CHEN, Li DU, Siying REN, Ying ZENG. Phase equilibria and calculation of aqueous ternary system KCl + CaCl2 + H2O at 298.2, 323.2, and 348.2 K [J]. CIESC Journal, 2023, 74(8): 3256-3265. |
| [15] | Linqi YAN, Zhenlei WANG. Multi-step predictive soft sensor modeling based on STA-BiLSTM-LightGBM combined model [J]. CIESC Journal, 2023, 74(8): 3407-3418. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||