CIESC Journal ›› 2025, Vol. 76 ›› Issue (7): 3172-3184.DOI: 10.11949/0438-1157.20241452
• Reviews and monographs • Previous Articles Next Articles
Jiali WANG1,2( ), Fang LIU1,2, Wei CHEN1,3(
), Fang LIU1,2, Wei CHEN1,3( ), Xiaoying ZHANG4, Shengting LI4, Tian TIAN2, Xiangyu XIN2, Guang LIU2(
), Xiaoying ZHANG4, Shengting LI4, Tian TIAN2, Xiangyu XIN2, Guang LIU2( ), Yufei SONG1,3(
), Yufei SONG1,3( )
)
Received:2024-12-16
Revised:2025-02-17
Online:2025-08-13
Published:2025-07-25
Contact:
Wei CHEN, Guang LIU, Yufei SONG
王佳丽1,2( ), 刘芳1,2, 陈伟1,3(
), 刘芳1,2, 陈伟1,3( ), 张晓英4, 李生廷4, 田甜2, 信翔宇2, 刘光2(
), 张晓英4, 李生廷4, 田甜2, 信翔宇2, 刘光2( ), 宋宇飞1,3(
), 宋宇飞1,3( )
)
通讯作者:
陈伟,刘光,宋宇飞
作者简介:王佳丽(2001—),女,硕士研究生,wangjl0130@163.com
基金资助:CLC Number:
Jiali WANG, Fang LIU, Wei CHEN, Xiaoying ZHANG, Shengting LI, Tian TIAN, Xiangyu XIN, Guang LIU, Yufei SONG. Recent advances in magnesium-based nanocomposites via in-situ template-confined synthesis[J]. CIESC Journal, 2025, 76(7): 3172-3184.
王佳丽, 刘芳, 陈伟, 张晓英, 李生廷, 田甜, 信翔宇, 刘光, 宋宇飞. 模板限域原位制备镁基纳米复合材料进展[J]. 化工学报, 2025, 76(7): 3172-3184.
Add to citation manager EndNote|Ris|BibTeX
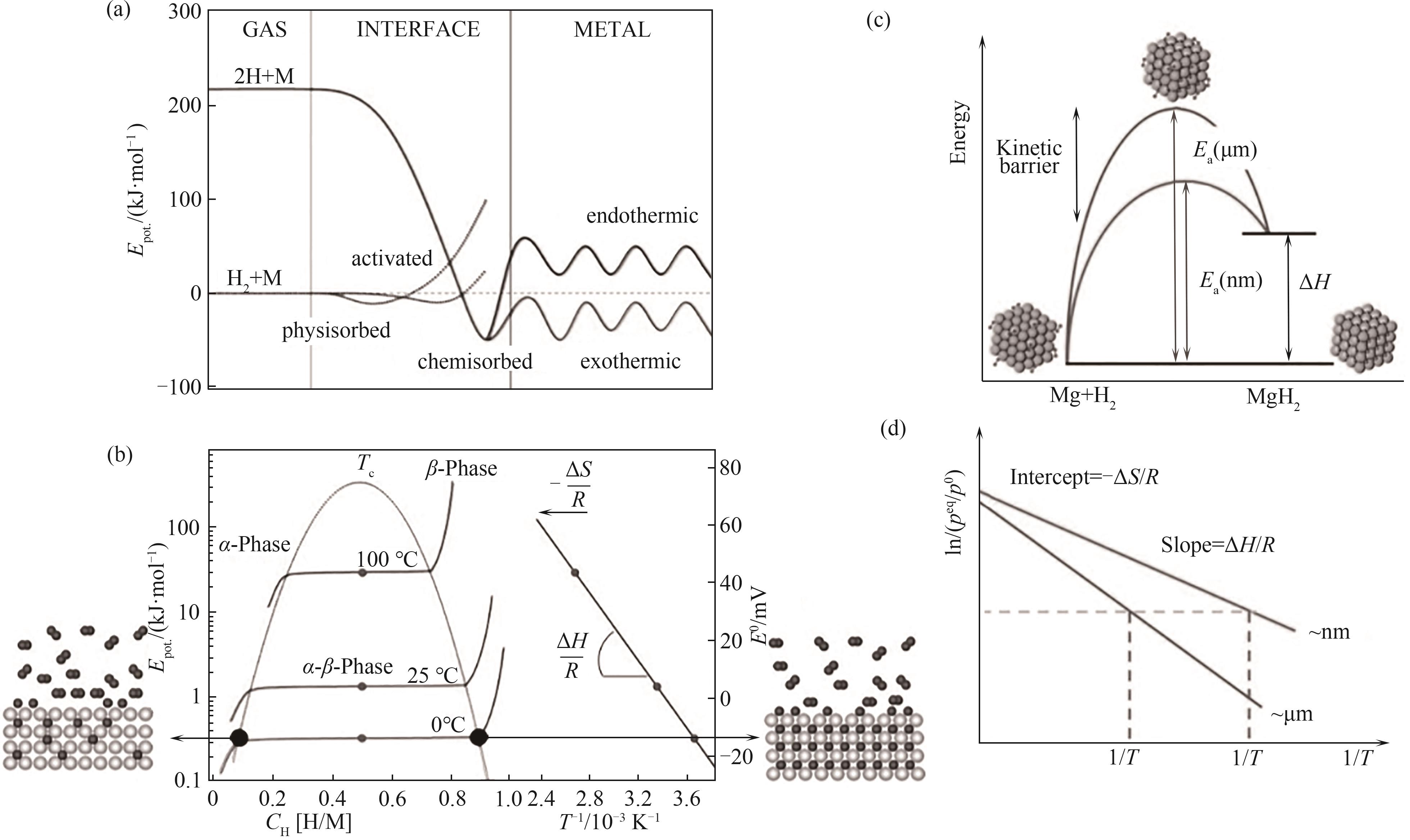
Fig. 1 Kinetic (a) and thermodynamic (b) schematics of magnesium hydride [10]; Improvement principle of kinetics (c) and thermodynamics (d) in hydrogen storage process of magnesium hydride by nanization[11]
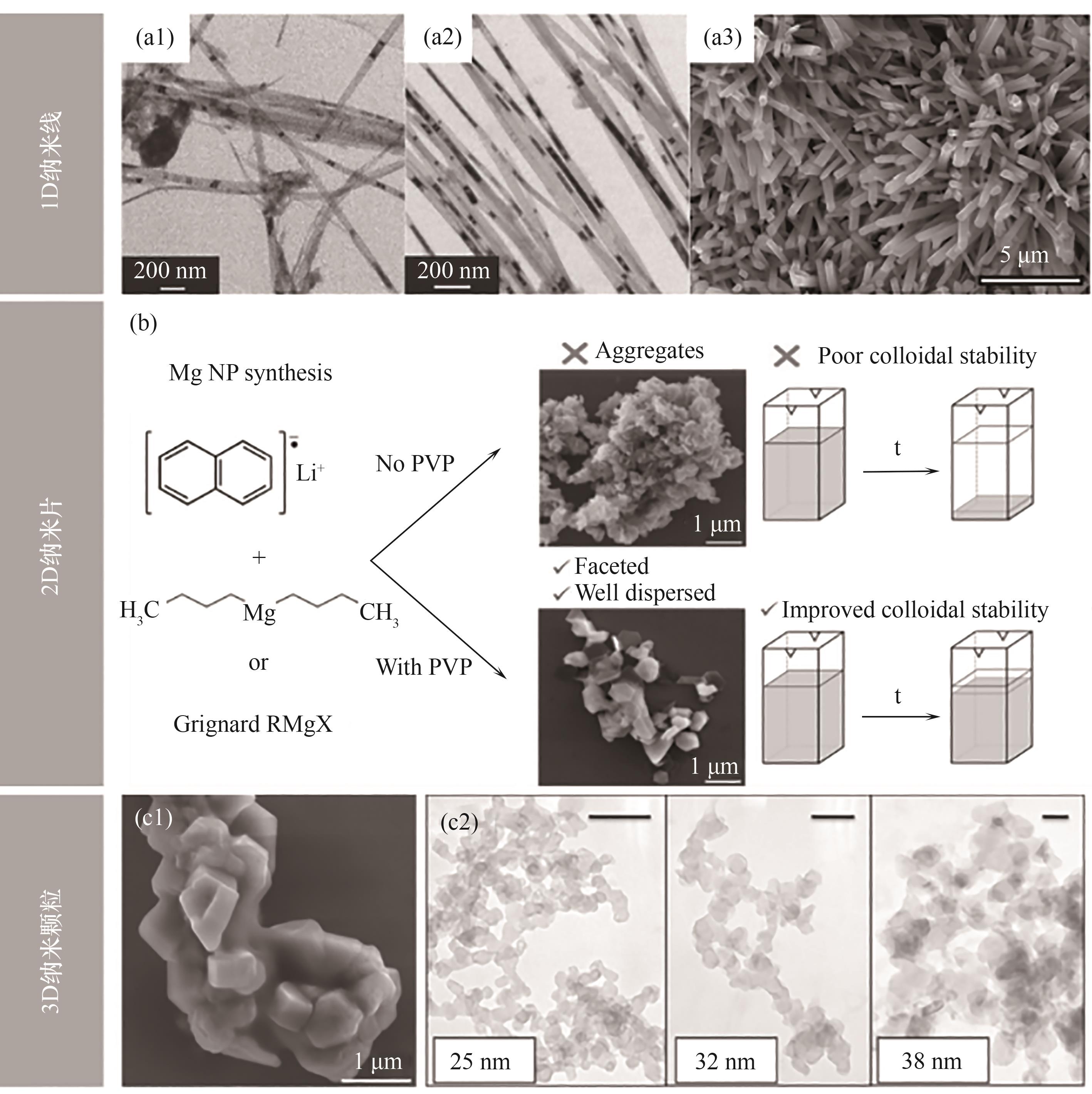
Fig.4 (a1,a2) TEM images of Mg nanofibers[33]; (a3) SEM image of Mg nanowires prepared from MeMgCl[34]; (b) Diagram of Mg with different morphologies prepared by different synthetic raw materials and end-sealing agents[35]; (c1) SEM image of Mg NPs synthesized in the presence of SDS[35]; (c2) TEM images of Mg nanocrystals with different particle sizes[36]
| 材料 | 制备方法 | Mg/MgH2尺寸/nm | 起始脱氢温度/℃ | 吸放氢焓值ΔHabs/des/(kJ·mol-1) | 吸放氢活化能Eab/de/(kJ·mol-1) | 储氢量/%(质量) | 文献 |
|---|---|---|---|---|---|---|---|
| HCNs/Mg | 化学还原 | 5~15 | — | -65.86/76.73 Bulk:-74.7/74.06 | — | 4.35 | [ |
| Fe/Mg | 化学还原 | 3~10(5) | 约325(DSC) | ΔHdes = -59.9 ± 1.9 | Ede = 137 ± 6 | 约2.67 | [ |
| Mg-Ti | 化学还原 | 40~60(50) | 约300(DSC) | -73.0 ± 1.8/75.8 ± 4.7 | Ede = 50.2 | 6.2 | [ |
| Mg-Ni | 化学还原 | 10~20 | 约244(DSC) | -70.0/70.7 | 57.4/139.1 | 6.0 | [ |
| Mg@Pt | 化学还原 | 3(Pt) | 287.5 | — | 82.4/152.8 | 6.5 | [ |
| Mg@Ti@Ni | 化学还原 | 50~600 | 340(DSC) | -67.12/69.84 | Ede = 63.7 | 6.27 | [ |
| Mg-Ni-TiS2 | 化学还原 | 70(Mg-Ni) | — | -71.13/72.25 | Eab = 79.4 ± 0.9 | 约4.7 | [ |
| Mg/ZIF-67 | 化学还原 | — | 约289 | -67.5/77.8 | Ede = 161.73 | 5.1 | [ |
| 0.6 MgH2@CMK-3 | 浸渍加氢 | 1~2 | 50 | ΔHdes = 55.4 | — | 5.5 | [ |
| MgH2@BCNTs | 浸渍加氢 | 15~20 | 220 | ΔHdes = 68.92 | Ede = 97.97 | 5.79 | [ |
| MgH2@CoS-NBs | 浸渍加氢 | 5~10 | 约282(DSC) | -65.6/68.1 | 57.4/120.8 | 3.23 | [ |
| Mg@Ni-MOF | 浸渍加氢 | 3 | 约305(DSC) | -65.7/69.7 | 41.5/144.7 | 2.7 | [ |
| MgH2/TiO2 | 溶剂热法 | — | 180 | — | — | 3.4 | [ |
| MHCH-5 | 溶剂热法 | 约5.5 | 约140 | -46.9/49.1 | 31/43 | 6.63 | [ |
| Mg75(TiC0.6)12@C | 气相沉积 | <50 | — | — | 54.7/56.5 | 5.2 | [ |
Table 1 Hydrogen storage properties of MgH2 limited by different materials (Unless otherwise stated, the initial dehydrogenation temperatures are determined through TPD tests)
| 材料 | 制备方法 | Mg/MgH2尺寸/nm | 起始脱氢温度/℃ | 吸放氢焓值ΔHabs/des/(kJ·mol-1) | 吸放氢活化能Eab/de/(kJ·mol-1) | 储氢量/%(质量) | 文献 |
|---|---|---|---|---|---|---|---|
| HCNs/Mg | 化学还原 | 5~15 | — | -65.86/76.73 Bulk:-74.7/74.06 | — | 4.35 | [ |
| Fe/Mg | 化学还原 | 3~10(5) | 约325(DSC) | ΔHdes = -59.9 ± 1.9 | Ede = 137 ± 6 | 约2.67 | [ |
| Mg-Ti | 化学还原 | 40~60(50) | 约300(DSC) | -73.0 ± 1.8/75.8 ± 4.7 | Ede = 50.2 | 6.2 | [ |
| Mg-Ni | 化学还原 | 10~20 | 约244(DSC) | -70.0/70.7 | 57.4/139.1 | 6.0 | [ |
| Mg@Pt | 化学还原 | 3(Pt) | 287.5 | — | 82.4/152.8 | 6.5 | [ |
| Mg@Ti@Ni | 化学还原 | 50~600 | 340(DSC) | -67.12/69.84 | Ede = 63.7 | 6.27 | [ |
| Mg-Ni-TiS2 | 化学还原 | 70(Mg-Ni) | — | -71.13/72.25 | Eab = 79.4 ± 0.9 | 约4.7 | [ |
| Mg/ZIF-67 | 化学还原 | — | 约289 | -67.5/77.8 | Ede = 161.73 | 5.1 | [ |
| 0.6 MgH2@CMK-3 | 浸渍加氢 | 1~2 | 50 | ΔHdes = 55.4 | — | 5.5 | [ |
| MgH2@BCNTs | 浸渍加氢 | 15~20 | 220 | ΔHdes = 68.92 | Ede = 97.97 | 5.79 | [ |
| MgH2@CoS-NBs | 浸渍加氢 | 5~10 | 约282(DSC) | -65.6/68.1 | 57.4/120.8 | 3.23 | [ |
| Mg@Ni-MOF | 浸渍加氢 | 3 | 约305(DSC) | -65.7/69.7 | 41.5/144.7 | 2.7 | [ |
| MgH2/TiO2 | 溶剂热法 | — | 180 | — | — | 3.4 | [ |
| MHCH-5 | 溶剂热法 | 约5.5 | 约140 | -46.9/49.1 | 31/43 | 6.63 | [ |
| Mg75(TiC0.6)12@C | 气相沉积 | <50 | — | — | 54.7/56.5 | 5.2 | [ |
| [1] | Hussain S A, Razi F, Hewage K, et al. The perspective of energy poverty and 1st energy crisis of green transition[J]. Energy, 2023, 275: 127487. |
| [2] | Zhao X, Ma X, Chen B, et al. Challenges toward carbon neutrality in China: strategies and countermeasures[J]. Resources, Conservation and Recycling, 2022, 176: 105959. |
| [3] | 李灿. 绿色氢能在"双碳"战略中的作用[J]. 科技导报, 2024, 42(15): 1-2. |
| Li C. The role of green hydrogen energy in dual carbon strategy[J]. Science & Technology Review, 2024, 42(15): 1-2. | |
| [4] | 徐立军, 苏昕, 朱迪, 等. "双碳"目标下氢能产业技术发展分析[J]. 新疆大学学报(自然科学版中英文), 2024, 41(4): 385-407. |
| Xu L J, Su X, Zhu D, et al. Analysis of the technological development of the hydrogen energy industry in the context of dual-carbon targets[J]. Journal of Xinjiang University (Natural Science Edition in Chinese and English), 2024, 41(4): 385-407. | |
| [5] | Osman A I, Mehta N, Elgarahy A M, et al. Hydrogen production, storage, utilisation and environmental impacts: a review[J]. Environmental Chemistry Letters, 2022, 20(1): 153-188. |
| [6] | Yan X R, Zheng W G, Wei Y J, et al. Current status and economic analysis of green hydrogen energy industry chain[J]. Processes, 2024, 12(2): 315. |
| [7] | Xu Y H, Zhou Y, Li Y T, et al. Research progress and application prospects of solid-state hydrogen storage technology[J]. Molecules, 2024, 29(8): 1767. |
| [8] | 毕秋艳, 党力, 曹海莲, 等. 青海盐湖镁资源开发与利用研究进展[J]. 盐湖研究, 2022, 30(1): 101-109. |
| Bi Q Y, Dang L, Cao H L, et al. Development and utilization of magnesium resources in Qinghai salt lakes[J]. Journal of Salt Lake Research, 2022, 30(1): 101-109. | |
| [9] | Ding Z, Li Y T, Yang H, et al. Tailoring MgH2 for hydrogen storage through nanoengineering and catalysis[J]. Journal of Magnesium and Alloys, 2022, 10(11): 2946-2967. |
| [10] | Züttel A. Materials for hydrogen storage[J]. Materials Today, 2003, 6(9): 24-33. |
| [11] | Ren L, Li Y H, Zhang N, et al. Nanostructuring of Mg-based hydrogen storage materials: recent advances for promoting key applications[J]. Nano-Micro Letters, 2023, 15(1): 93. |
| [12] | Duan C W, Su Z H, Tian Y T, et al. Mechanochemical assisted hydrogenation of Mg-CNTs-Ni: kinetics modeling and reaction mechanism[J]. Chemical Engineering Journal, 2022, 441: 136059. |
| [13] | Zhang Q Y, Huang Y K, Xu L, et al. Highly dispersed MgH2 nanoparticle-graphene nanosheet composites for hydrogen storage[J]. ACS Applied Nano Materials, 2019, 2(6): 3828-3835. |
| [14] | Guo F H, Zhang T B, Shi L M, et al. Hydrogen absorption/desorption cycling performance of Mg-based alloys with in-situ formed Mg2Ni and LaH x (x = 2, 3) nanocrystallines[J]. Journal of Magnesium and Alloys, 2023, 11(4): 1180-1192. |
| [15] | Gao H, Shao Y, Shi R, et al. Effect of few-layer Ti3C2T x supported nano-Ni via self-assembly reduction on hydrogen storage performance of MgH2 [J]. ACS Applied Materials & Interfaces, 2020, 12(42): 47684-47694. |
| [16] | Lan Z Q, Hong F F, Shi W T, et al. Effect of MOF-derived carbon-nitrogen nanosheets co-doped with nickel and titanium dioxide nanoparticles on hydrogen storage performance of MgH2 [J]. Chemical Engineering Journal, 2023, 468: 143692. |
| [17] | Meng Y, Zhang J, Ju S L, et al. Understanding and unlocking the role of V in boosting the reversible hydrogen storage performance of MgH2 [J]. Journal of Materials Chemistry A, 2023, 11(18): 9762-9771. |
| [18] | Wan L F, Liu Y S, Cho E S, et al. Atomically thin interfacial suboxide key to hydrogen storage performance enhancements of magnesium nanoparticles encapsulated in reduced graphene oxide[J]. Nano Letters, 2017, 17(9): 5540-5545. |
| [19] | Zhang L T, Tian G B, Wu F Y, et al. The influence of grain size and catalytic doping on magnesium hydride nanocrystals for hydrogen storage[J]. Journal of Physics and Chemistry of Solids, 2023, 178: 111335. |
| [20] | Cheung S, Deng W Q, Van Duin A C T, et al. ReaxFF(MgH) reactive force field for magnesium hydride systems[J]. The Journal of Physical Chemistry A, 2005, 109(5): 851-859. |
| [21] | Vajeeston P, Sartori S, Ravindran P, et al. MgH2 in carbon scaffolds: a combined experimental and theoretical investigation[J]. The Journal of Physical Chemistry C, 2012, 116(40): 21139-21147. |
| [22] | 欧阳柳章, 叶素云, 朱敏. MmM5/Mg复合薄膜的显微结构与储氢性能的关系[J]. 电子显微学报, 2009, 28(4): 356-360. |
| Ouyang L Z, Ye S Y, Zhu M. Microstructure and hydrogen storage properties of MmNi3.5(CoAlMn)1.5/Mg multi-layers[J]. Journal of Chinese Electron Microscopy Society, 2009, 28(4): 356-360. | |
| [23] | Huang L J, Shi S T, Cui J, et al. Thermally-assisted milling and hydrogenolysis for synthesizing ultrafine MgH2 with destabilized thermodynamics[J]. Nanotechnology, 2021, 32(28): 285402. |
| [24] | Wang Y Q, Lan Z Q, Huang X, et al. Study on catalytic effect and mechanism of MOF (MOF = ZIF-8, ZIF-67, MOF-74) on hydrogen storage properties of magnesium[J]. International Journal of Hydrogen Energy, 2019, 44(54): 28863-28873. |
| [25] | Tan D Z, Peng C, Zhang Q G. Microstructural characteristics and hydrogen storage properties of the Mg-Ni-TiS2 nanocomposite prepared by a solution-based method[J]. International Journal of Hydrogen Energy, 2023, 48(44): 16756-16768. |
| [26] | Lu C, Ma Y L, Li F, et al. Visualization of fast "hydrogen pump" in core-shell nanostructured Mg@Pt through hydrogen-stabilized Mg3Pt[J]. Journal of Materials Chemistry A, 2019, 7(24): 14629-14637. |
| [27] | Cho Y, Kang S, Wood B C, et al. Heteroatom-doped graphenes as actively interacting 2D encapsulation media for Mg-based hydrogen storage[J]. ACS Applied Materials & Interfaces, 2022, 14(18): 20823-20834. |
| [28] | Wang Y R, Chen X W, Zhang H Y, et al. Heterostructures built in metal hydrides for advanced hydrogen storage reversibility[J]. Advanced Materials, 2020, 32(31): 2002647. |
| [29] | Hu M M, Xie X B, Chen M, et al. TiCX-decorated Mg nanoparticles confined in carbon shell: preparation and catalytic mechanism for hydrogen storage[J]. Journal of Alloys and Compounds, 2020, 817: 152813. |
| [30] | Rieke R D, Hudnall P M. Activated metals. Ⅰ. Preparation of highly reactive magnesium metal[J]. Journal of the American Chemical Society, 1972, 94(20): 7178-7179. |
| [31] | Rieke R D, Bales S E. Activated metals. Ⅳ. Preparation and reactions of highly reactive magnesium metal[J]. Journal of the American Chemical Society, 1974, 96(6): 1775-1781. |
| [32] | Aguey-Zinsou K F, Ares-Fernández J R. Synthesis of colloidal magnesium: a near room temperature store for hydrogen[J]. Chemistry of Materials, 2008, 20(2): 376-378. |
| [33] | Sun Y H, Aguey-Zinsou K F. Synthesis of magnesium nanofibers by electroless reduction and their hydrogen interaction properties[J]. Particle & Particle Systems Characterization, 2017, 34(4): 1600276. |
| [34] | Viyannalage L, Lee V, Dennis R V, et al. From Grignard's reagents to well-defined Mg nanostructures: distinctive electrochemical and solution reduction routes[J]. Chemical Communications, 2012, 48(42): 5169-5171. |
| [35] | Wayman T M R, Lomonosov V, Ringe E. Capping agents enable well-dispersed and colloidally stable metallic magnesium nanoparticles[J]. The Journal of Physical Chemistry C, Nanomaterials and Interfaces, 2024, 128(11): 4666-4676. |
| [36] | Norberg N S, Arthur T S, Fredrick S J, et al. Size-dependent hydrogen storage properties of Mg nanocrystals prepared from solution[J]. Journal of the American Chemical Society, 2011, 133(28): 10679-10681. |
| [37] | Liu W, Aguey-Zinsou K F. Size effects and hydrogen storage properties of Mg nanoparticles synthesised by an electroless reduction method[J]. Journal of Materials Chemistry A, 2014, 2(25): 9718-9726. |
| [38] | Jia Y, Yao X D. Carbon scaffold modified by metal (Ni) or non-metal (N) to enhance hydrogen storage of MgH2 through nanoconfinement[J]. International Journal of Hydrogen Energy, 2017, 42(36): 22933-22941. |
| [39] | Mao J F, Zou J X, Lu C, et al. Hydrogen storage and hydrolysis properties of core-shell structured Mg-MF x (M=V, Ni, La and Ce) nano-composites prepared by arc plasma method[J]. Journal of Power Sources, 2017, 366: 131-142. |
| [40] | Xia G L, Zhang L J, Chen X W, et al. Carbon hollow nanobubbles on porous carbon nanofibers: an ideal host for high-performance sodium-sulfur batteries and hydrogen storage[J]. Energy Storage Materials, 2018, 14: 314-323. |
| [41] | Zhang J G, Zhu Y F, Lin H J, et al. Metal hydride nanoparticles with ultrahigh structural stability and hydrogen storage activity derived from microencapsulated nanoconfinement[J]. Advanced Materials, 2017, 29(24): 1700760. |
| [42] | Jangid M K, Sharma S S, Ray J, et al. Structural, optical and electrical characterizations of Mg/Ti/Ni multilayer thin films deposited by DC magnetron sputtering for hydrogen storage[J]. International Journal of Hydrogen Energy, 2023, 48(96): 37921-37929. |
| [43] | Reddy G L N, Kumar S. Hydrogen storage studies in Pd/Ti/Mg films[J]. International Journal of Hydrogen Energy, 2018, 43(5): 2840-2849. |
| [44] | Abdul Majid N A, Watanabe J, Notomi M. Improved desorption temperature of magnesium hydride via multi-layering Mg/Fe thin film[J]. International Journal of Hydrogen Energy, 2021, 46(5): 4181-4187. |
| [45] | Setijadi E J, Boyer C, Aguey-Zinsou K F. Switching the thermodynamics of MgH2 nanoparticles through polystyrene stabilisation and oxidation[J]. RSC Advances, 2014, 4(75): 39934-39940. |
| [46] | Song M Y, Choi E, Kwak Y J. Synthesis of a Mg-based alloy with a hydrogen-storage capacity of over 7wt% by adding a polymer CMC via transformation-involving milling[J]. Materials Research Bulletin, 2018, 108: 23-31. |
| [47] | Song M Y, Choi E, Kwak Y J. Preparation of a Mg-based alloy with a high hydrogen-storage capacity by adding a polymer CMC via milling in a hydrogen atmosphere[J]. International Journal of Hydrogen Energy, 2019, 44(7): 3779-3789. |
| [48] | Song M Y, Kwak Y J. Hydrogen storage properties of Mg alloy prepared by incorporating polyvinylidene fluoride via reactive milling[J]. Korean Journal of Metals and Materials, 2018, 56(12): 878-884. |
| [49] | Cao H J, Georgopanos P, Capurso G, et al. Air-stable metal hydride-polymer composites of Mg(NH2)2-LiH and TPX™[J]. Materials Today Energy, 2018, 10: 98-107. |
| [50] | Makridis S S, Gkanas E I, Panagakos G, et al. Polymer-stable magnesium nanocomposites prepared by laser ablation for efficient hydrogen storage[J]. International Journal of Hydrogen Energy, 2013, 38(26): 11530-11535. |
| [51] | Rafatnejad M, Raygan S, Sefidmooy Azar M. Investigation of dehydrogenation performance and air stability of MgH2-PMMA nanostructured composite prepared by direct high-energy ball-milling[J]. Materials for Renewable and Sustainable Energy, 2020, 9(2): 14. |
| [52] | Ruminski A M, Bardhan R, Brand A, et al. Synergistic enhancement of hydrogen storage and air stability via Mg nanocrystal-polymer interfacial interactions[J]. Energy & Environmental Science, 2013, 6(11): 3267-3271. |
| [53] | Jeon K J, Moon H R, Ruminski A M, et al. Air-stable magnesium nanocomposites provide rapid and high-capacity hydrogen storage without using heavy-metal catalysts[J]. Nature Materials, 2011, 10(4): 286-290. |
| [54] | Liang H, Chen D D, Chen M F, et al. Study of the synthesis of PMMA-Mg nanocomposite for hydrogen storage application[J]. International Journal of Hydrogen Energy, 2020, 45(7): 4743-4753. |
| [55] | Shinde S S, Kim D H, Yu J Y, et al. Self-assembled air-stable magnesium hydride embedded in 3-D activated carbon for reversible hydrogen storage[J]. Nanoscale, 2017, 9(21): 7094-7103. |
| [56] | Jia Y, Sun C H, Cheng L N, et al. Destabilization of Mg-H bonding through nano-interfacial confinement by unsaturated carbon for hydrogen desorption from MgH2 [J]. Physical Chemistry Chemical Physics, 2013, 15(16): 5814-5820. |
| [57] | Liu M J, Zhao S C, Xiao X Z, et al. Novel 1D carbon nanotubes uniformly wrapped nanoscale MgH2 for efficient hydrogen storage cycling performances with extreme high gravimetric and volumetric capacities[J]. Nano Energy, 2019, 61: 540-549. |
| [58] | Liu Y N, Zou J X, Zeng X Q, et al. Study on hydrogen storage properties of Mg nanoparticles confined in carbon aerogels[J]. International Journal of Hydrogen Energy, 2013, 38(13): 5302-5308. |
| [59] | Huang Y Q, Xia G L, Chen J, et al. One-step uniform growth of magnesium hydride nanoparticles on graphene[J]. Progress in Natural Science: Materials International, 2017, 27(1): 81-87. |
| [60] | Ali W, Qin Y Y, Khan N A, et al. Highly air-stable magnesium hydrides encapsulated by nitrogen-doped graphene nanospheres with favorable hydrogen storage kinetics[J]. Chemical Engineering Journal, 2024, 480: 148163. |
| [61] | Xia G L, Tan Y B, Chen X W, et al. Monodisperse magnesium hydride nanoparticles uniformly self-assembled on graphene[J]. Advanced Materials, 2015, 27(39): 5981-5988. |
| [62] | Cho E S, Ruminski A M, Aloni S, et al. Graphene oxide/metal nanocrystal multilaminates as the atomic limit for safe and selective hydrogen storage[J]. Nature Communications, 2016, 7(1): 10804. |
| [63] | Cho E S, Ruminski A M, Liu Y S, et al. Hierarchically controlled inside-out doping of Mg nanocomposites for moderate temperature hydrogen storage[J]. Advanced Functional Materials, 2017, 27(47): 1704316. |
| [64] | Lyu J Z, Kudiiarov V, Svyatkin L, et al. On the catalytic mechanism of 3D and 4D transition-metal-based materials on the hydrogen sorption properties of Mg/MgH2 [J]. Catalysts, 2023, 13(3): 519. |
| [65] | Liu H, Wang Z Z, Zhang J Z, et al. Recent advances in hydrogen storage of MgH2 doped by Ni[J]. IOP Conference Series: Earth and Environmental Science, 2019, 267(2): 022042. |
| [66] | Ding S J, Qiao Y Q, Cai X C, et al. Catalytic mechanisms of nickel nanoparticles for the improved dehydriding kinetics of magnesium hydride[J]. Journal of Magnesium and Alloys, 2024, 12(10): 4278-4288. |
| [67] | Li F, Huang Z N, Wang Y Q, et al. Effect of MOF-derived nanoparticle-cumulated flower-like CoFe@C coated composites on hydrogenation/dehydrogenation performance of MgH2 [J]. Chemical Engineering Journal, 2024, 485: 150008. |
| [68] | Cui J, Liu J W, Wang H, et al. Mg-TM (TM: Ti, Nb, V, Co, Mo or Ni) core-shell like nanostructures: synthesis, hydrogen storage performance and catalytic mechanism[J]. Journal of Materials Chemistry A, 2014, 2(25): 9645-9655. |
| [69] | Bu F Q, Wajid A, Yang N, et al. Fabrication of amorphous TiO2 hydrogen channels and graphene wrappers to enhance the hydrogen storage properties of MgH2 with extremely high cycle stability[J]. Journal of Materials Chemistry A, 2024, 12(20): 12190-12197. |
| [70] | Ren L, Zhu W, Li Y H, et al. Oxygen vacancy-rich 2D TiO2 nanosheets: a bridge toward high stability and rapid hydrogen storage kinetics of nano-confined MgH2 [J]. Nano-Micro Letters, 2022, 14(1): 144. |
| [71] | Zhu L, Liao Y X, Zhong Y J, et al. Improved reversible dehydrogenation performance of MgH2 by the synergistic effects of porous boron nitride and NbF5 [J]. Journal of Energy Storage, 2020, 29: 101418. |
| [72] | Bolarin J A, Zou R, Li Z, et al. MXenes for magnesium-based hydrides: a review[J]. Applied Materials Today, 2022, 29: 101570. |
| [73] | Zhou D S, Zhao D L, Sun H F, et al. Two-dimensional material MXene and its derivatives enhance the hydrogen storage properties of MgH2: a review and summary[J]. International Journal of Hydrogen Energy, 2024, 71: 279-297. |
| [74] | Liu H Z, Duan X Q, Wu Z Y, et al. Exfoliation of compact layered Ti2VAlC2 MAX to open layered Ti2VC2 MXene towards enhancing the hydrogen storage properties of MgH2 [J]. Chemical Engineering Journal, 2023, 468: 143688. |
| [75] | Lan Z Q, Fu H, Zhao R L, et al. Roles of in situ-formed NbN and Nb2O5 from N-doped Nb2C MXene in regulating the re/hydrogenation and cycling performance of magnesium hydride[J]. Chemical Engineering Journal, 2022, 431: 133985. |
| [76] | Zhu W, Ren L, Lu C, et al. Nanoconfined and in situ catalyzed MgH2 self-assembled on 3D Ti3C2 MXene folded nanosheets with enhanced hydrogen sorption performances[J]. Acs Nano, 2021, 15(11): 18494-18504. |
| [77] | Zhou D S, Sun H F, Guo S H, et al. Hydrogen storage properties of Mg-based alloys modified with metal-organic frameworks and carbon-based porous materials: a review and summary[J]. International Journal of Hydrogen Energy, 2024, 57: 1373-1388. |
| [78] | Ma Z W, Zhang Q Y, Panda S, et al. In situ catalyzed and nanoconfined magnesium hydride nanocrystals in a Ni-MOF scaffold for hydrogen storage[J]. Sustainable Energy & Fuels, 2020, 4(9): 4694-4703. |
| [79] | Xing X F, Zhang X J, Wei M X, et al. Nanoencapsulated MgBu2@ZIF-67 to construct high loading Mg-Co@C nanocomposites: breaking through the barrier of room temperature onset dehydrogenation[J]. Small, 2024, 20(44): 2402982. |
| [80] | Xing X F, Wang Y X, Zhang Z, et al. MgBu2 nanosheets encapsulated in ZIF-8 for producing carbon scaffold in situ nanoconfined Mg hydrogen storage materials: record-high loading and decreased dehydrogenation enthalpy[J]. Nano Energy, 2024, 127: 109740. |
| [81] | Ren L, Zhu W, Zhang Q Y, et al. MgH2 confinement in MOF-derived N-doped porous carbon nanofibers for enhanced hydrogen storage[J]. Chemical Engineering Journal, 2022, 434: 134701. |
| [82] | Ma Z W, Panda S, Zhang Q Y, et al. Improving hydrogen sorption performances of MgH2 through nanoconfinement in a mesoporous CoS nano-boxes scaffold[J]. Chemical Engineering Journal, 2021, 406: 126790. |
| [83] | Han D J, Kwon C, Cho Y, et al. Tailoring hierarchical pore structures in carbon scaffolds for hydrogen storage of nanoconfined magnesium[J]. Chemical Engineering Journal, 2024, 481: 148451. |
| [84] | Liu W, Setijadi E J, Aguey-Zinsou K F. Tuning the thermodynamic properties of MgH2 at the nanoscale via a catalyst or destabilizing element coating strategy[J]. The Journal of Physical Chemistry C, 2014, 118(48): 27781-27792. |
| [85] | Liu Y N, Zou J X, Zeng X Q, et al. A co-precipitated Mg-Ti nano-composite with high capacity and rapid hydrogen absorption kinetics at room temperature[J]. RSC Advances, 2014, 4(81): 42764-42771. |
| [86] | Liu Y N, Zou J X, Zeng X Q, et al. Hydrogen storage properties of a Mg-Ni nanocomposite coprecipitated from solution[J]. The Journal of Physical Chemistry C, 2014, 118(32): 18401-18411. |
| [87] | Lu C, Zou J X, Shi X Y, et al. Synthesis and hydrogen storage properties of core-shell structured binary Mg@Ti and ternary Mg@Ti@Ni composites[J]. International Journal of Hydrogen Energy, 2017, 42(4): 2239-2247. |
| [1] | Wei SU, Dahai ZHAO, Xu JIN, Zhongyan LIU, Jing LI, Xiaosong ZHANG. Delaying condensation frosting using biphilic surfaces coupled with spatial control of liquid desiccant [J]. CIESC Journal, 2025, 76(S1): 140-151. |
| [2] | Haolei DUAN, Haoyuan CHEN, Kunfeng LIANG, Lin WANG, Bin CHEN, Yong CAO, Chenguang ZHANG, Shuopeng LI, Dengyu ZHU, Yaru HE, Dapeng YANG. Performance analysis and comprehensive evaluation of thermal management system schemes with low GWP refrigerants [J]. CIESC Journal, 2025, 76(S1): 54-61. |
| [3] | Songyuan GUO, Xiaoqing ZHOU, Wubing MIAO, Bin WANG, Rui ZHUAN, Qingtai CAO, Chengcheng CHEN, Guang YANG, Jingyi WU. Numerical study on characteristics of pressurized discharge in liquid oxygen tank equipped with porous plate in the ascent period of rocket [J]. CIESC Journal, 2025, 76(S1): 62-74. |
| [4] | Tengfei ZHU, Ye LIU. Performance analysis of low GWP refrigerant used in new energy vehicle air conditioning [J]. CIESC Journal, 2025, 76(S1): 343-350. |
| [5] | Di WU, Bin HU, Jiatong JIANG. Experimental study and application analysis of R1233zd(E) high temperature heat pump [J]. CIESC Journal, 2025, 76(S1): 377-383. |
| [6] | Liang GUO, Ye CHEN, Qiming JIA, Xiujuan XIE. Simulated and experimental investigations on self-pressurization of liquid helium storage tank [J]. CIESC Journal, 2025, 76(7): 3561-3571. |
| [7] | Zeming DONG, Juwei LOU, Nan WANG, Liangqi CHEN, Jiangfeng WANG, Pan ZHAO. Research on thermodynamic properties of supercritical compressed carbon dioxide energy storage system with waste heat recovery [J]. CIESC Journal, 2025, 76(7): 3477-3486. |
| [8] | Yu GONG, Shengli WANG, Jinju SUN, Kuo HAI, Wen HUANG. Thermodynamic model and exploration of micro multi-stage compressor inflation system [J]. CIESC Journal, 2025, 76(7): 3626-3638. |
| [9] | Lin LI, Mingmei WANG, Erwei SONG, Wenwen WANG, Yaochang ZHANG, Erqiang WANG. Thermodynamic analysis and optimization of isoprene/n-pentane separation process [J]. CIESC Journal, 2025, 76(6): 2549-2558. |
| [10] | Yihao JIN, Junxin LUO, Zhangmao HU, Wei WANG, Qian YIN. Experimental investigation on hydrophilic functionalized MgSO4/expanded vermiculite composites for water adsorption and heat storage [J]. CIESC Journal, 2025, 76(4): 1852-1862. |
| [11] | Junde ZHAO, Aiguo ZHOU, Yanlin CHEN, Jiale ZHENG, Tianshu GE. Current status of energy consumption of adsorption CO2 direct air capture [J]. CIESC Journal, 2025, 76(4): 1375-1390. |
| [12] | Wenzhi DAI, Xiongjian SHEN, Xiaobo SONG, Xinle YANG. Environmental analysis of biomass double-stage evaporation double-regenerative organic Rankine cycle system [J]. CIESC Journal, 2025, 76(3): 1230-1242. |
| [13] | Hanbin WANG, Shuai HU, Fenglei BI, Junsen LI, Laibin HE. Desorption performance analysis of a metal hydride reactor with novel corrugated fins based on finite element method [J]. CIESC Journal, 2025, 76(1): 221-230. |
| [14] | Juhui CHEN, Tong SU, Dan LI, Liwei CHEN, Wensheng LYU, Fanqi MENG. Study on the heat transfer characteristics of microchannels under the action of fin-shaped spoilers [J]. CIESC Journal, 2024, 75(9): 3122-3132. |
| [15] | Xinyue LU, Ruiying CHEN, Xiaxue JIANG, Hairui LIANG, Ge GAO, Zhengfang YE. Comparative study on liquid air energy storage system and liquid carbon dioxide energy storage system coupled with liquefied natural gas cold energy [J]. CIESC Journal, 2024, 75(9): 3297-3309. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||