CIESC Journal ›› 2021, Vol. 72 ›› Issue (3): 1382-1391.DOI: 10.11949/0438-1157.20200876
• Catalysis, kinetics and reactors • Previous Articles Next Articles
ZHANG Fangfang1( ),HAN Min1,ZHAO Juan1,LING Lixia1(
),HAN Min1,ZHAO Juan1,LING Lixia1( ),ZHANG Riguang2,WANG Baojun2
),ZHANG Riguang2,WANG Baojun2
Received:2020-07-03
Revised:2020-09-03
Online:2021-03-05
Published:2021-03-05
Contact:
LING Lixia
张芳芳1( ),韩敏1,赵娟1,凌丽霞1(
),韩敏1,赵娟1,凌丽霞1( ),章日光2,王宝俊2
),章日光2,王宝俊2
通讯作者:
凌丽霞
作者简介:张芳芳(1993—),女,硕士研究生,基金资助:CLC Number:
ZHANG Fangfang, HAN Min, ZHAO Juan, LING Lixia, ZHANG Riguang, WANG Baojun. DFT study on reduction of NO over Pd atom anchored on single-vacancy graphene[J]. CIESC Journal, 2021, 72(3): 1382-1391.
张芳芳, 韩敏, 赵娟, 凌丽霞, 章日光, 王宝俊. 单空缺石墨烯负载的Pd单原子催化剂上NO还原的密度泛函理论研究[J]. 化工学报, 2021, 72(3): 1382-1391.
Add to citation manager EndNote|Ris|BibTeX
| Reaction | H | H′ | H″ | N | O | Q(H+N+O) |
|---|---|---|---|---|---|---|
NO N+O N+O | -0.254 | -0.451 | -0.705 | |||
NO+H NOH NOH | 0.298 | -0.164 | -0.306 | -0.172 | ||
NO+H HNO HNO | -0.023 | -0.012 | -0.15 | -0.185 | ||
HNO NH+O NH+O | 0.216 | -0.424 | -0.466 | -0.674 | ||
HNO+H NHOH NHOH | -0.138 | 0.196 | -0.011 | -0.298 | -0.251 | |
HNO+H NH2O NH2O | 0.037 | 0.203 | -0.1 | -0.384 | -0.244 | |
NH2O NH2+O NH2+O | 0.203 | 0.198 | -0.516 | -0.512 | -0.627 | |
NH2O N+H2O N+H2O | 0.338 | 0.264 | -0.505 | -0.574 | -0.477 | |
NH2O+H NH2OH NH2OH | 0.093 | 0.229 | 0.244 | -0.127 | -0.461 | -0.022 |
Table 1 The Mulliken charge of each atom of the adsorbed species in the transition state structure of each elementary reaction and the charge of the entire adsorbed species
| Reaction | H | H′ | H″ | N | O | Q(H+N+O) |
|---|---|---|---|---|---|---|
NO N+O N+O | -0.254 | -0.451 | -0.705 | |||
NO+H NOH NOH | 0.298 | -0.164 | -0.306 | -0.172 | ||
NO+H HNO HNO | -0.023 | -0.012 | -0.15 | -0.185 | ||
HNO NH+O NH+O | 0.216 | -0.424 | -0.466 | -0.674 | ||
HNO+H NHOH NHOH | -0.138 | 0.196 | -0.011 | -0.298 | -0.251 | |
HNO+H NH2O NH2O | 0.037 | 0.203 | -0.1 | -0.384 | -0.244 | |
NH2O NH2+O NH2+O | 0.203 | 0.198 | -0.516 | -0.512 | -0.627 | |
NH2O N+H2O N+H2O | 0.338 | 0.264 | -0.505 | -0.574 | -0.477 | |
NH2O+H NH2OH NH2OH | 0.093 | 0.229 | 0.244 | -0.127 | -0.461 | -0.022 |
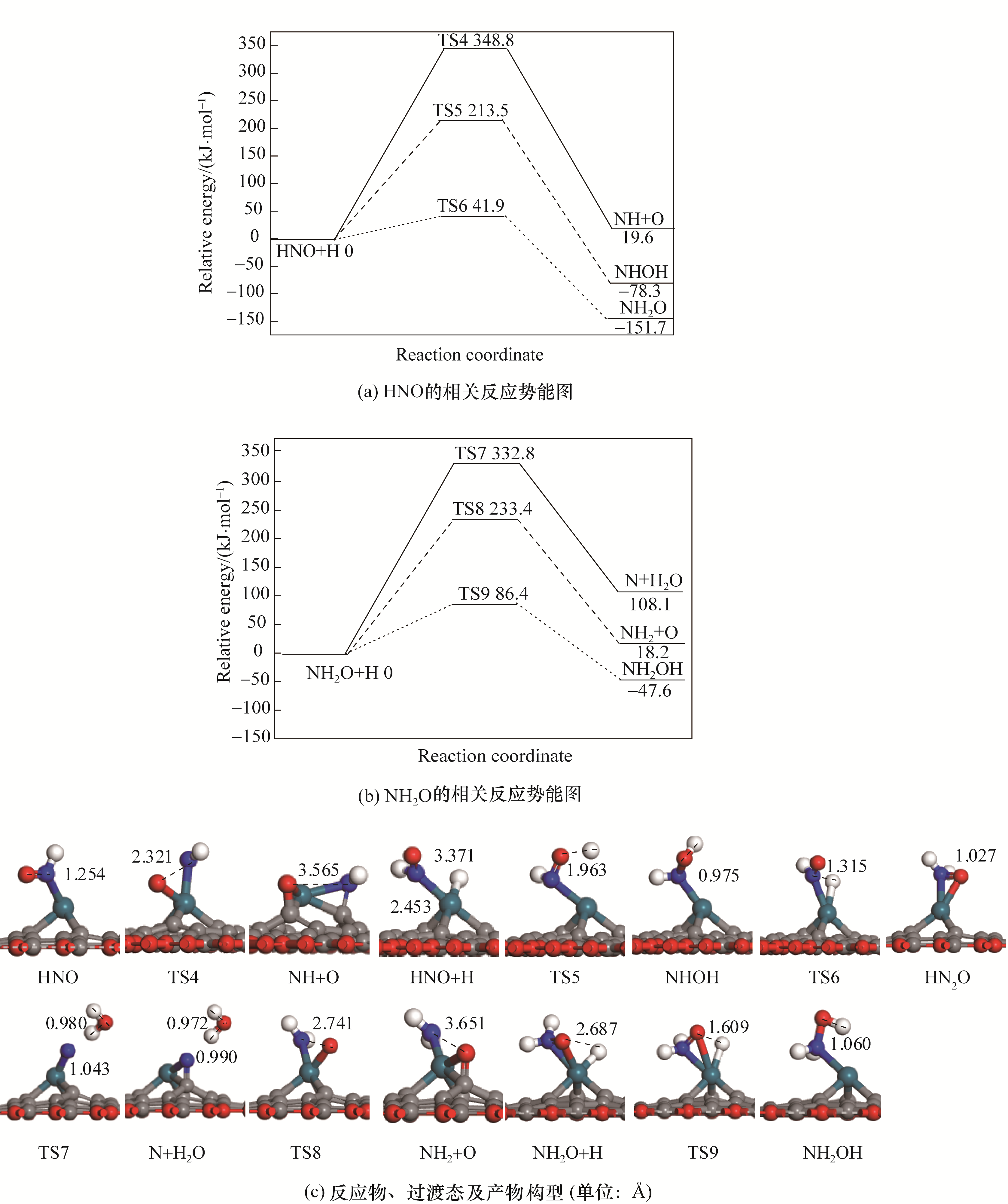
Fig.3 Potential energy diagram of related intermediates in the reduction of NO together with corresponding configurations of initial states, transition states and final states
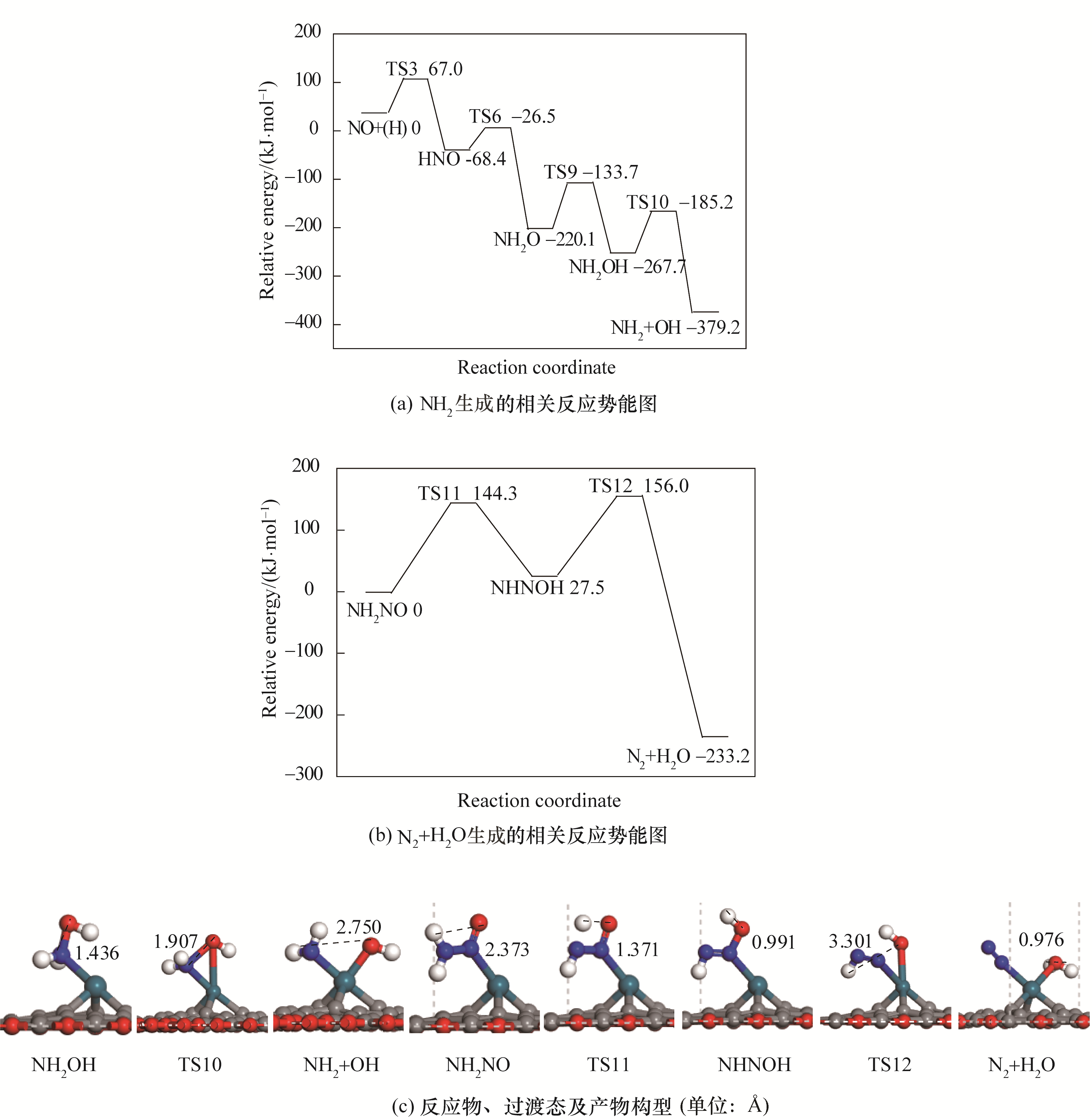
Fig.4 Potential energy diagram of correlated reactions of the formation of N2 and H2O together with corresponding configurations of initial states, transition states and final states
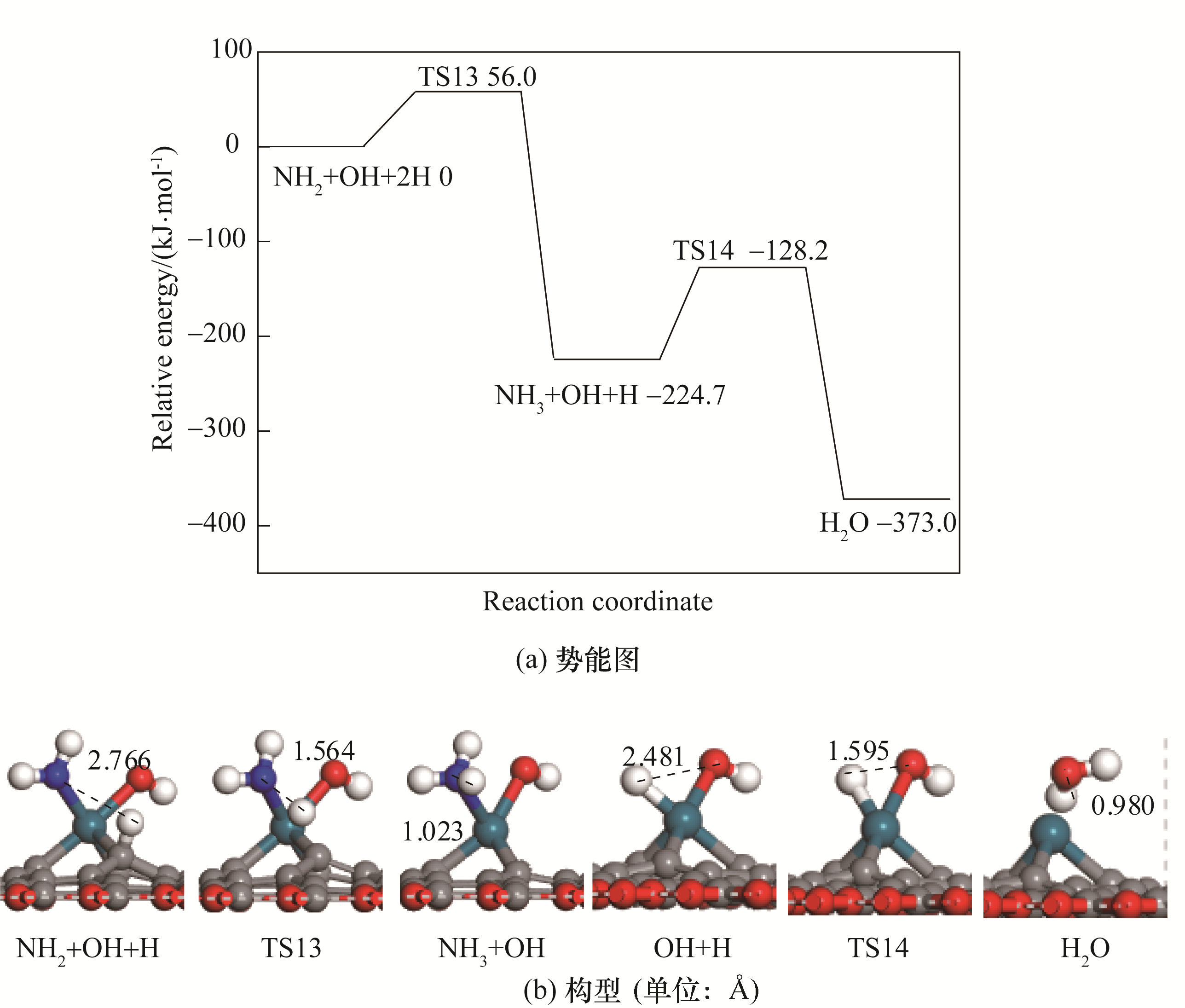
Fig. 5 Potential energy diagram of correlated reactions of the formation of NH3 together with corresponding configurations of initial states, transition states and final states
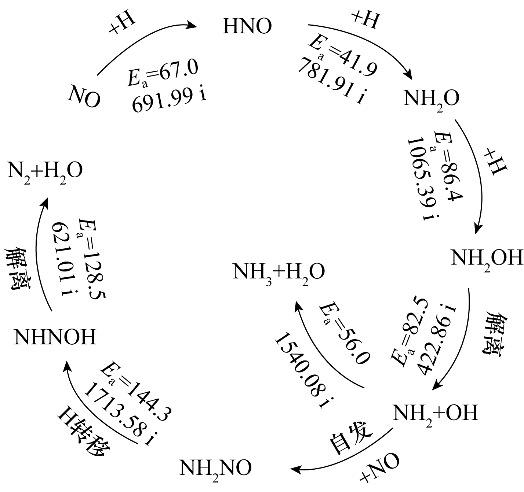
Fig.6 The formation pathways of N2 and NH3 and the energy barrier (kJ·mol-1) for each elementary step and virtual frequency corresponding to the transition state(cm-1)
| 1 | Jabłońska M, Palkovits R. It is no laughing matter: nitrous oxide formation in diesel engines and advances in its abatement over rhodium-based catalysts[J]. Catalysis Science & Technology, 2016, 6: 7671-7687. |
| 2 | Tsukahara H, Ishida T, Mayumi M. Gas-phase oxidation of nitric oxide: chemical kinetics and rate constant[J]. Nitric Oxide, 1999, 3(3): 191-198. |
| 3 | Garda G R, Castellani N J. Isocyanate (NCO) evidence in the CO + NO reaction over palladium[J]. Applied Catalysis A: General, 2015, 494: 48-56. |
| 4 | Herman G S, Peden C H F, Schmieg S J, et al. A comparison of the NO-CO reaction over Rh(100), Rh(110) and Rh(111)[J]. Catalysis Letters, 1999, 62(2): 131-138. |
| 5 | Fink T, Dath J P, Bassett M R, et al. The mechanism of the “explosive” NO + CO reaction on Pt(100): experiments and mathematical modeling[J]. Surface Science, 1991, 245: 96-110. |
| 6 | Fujiwara K, Müller U, Pratsinis S E. Pd subnano-clusters on TiO2 for solar-light removal of NO[J]. ACS Catalysis, 2016, 6(3): 1887-1893. |
| 7 | Fujiwara K, Pratsinis S E. Atomically dispersed Pd on nanostructured TiO2 for NO removal by solar light[J]. AIChE Journal, 2017, 63(1): 139-146. |
| 8 | Chitpakdee C, Junkaew A, Maitarad P, et al. Understanding the role of Ru dopant on selective catalytic reduction of NO with NH3 over Ru-doped CeO2 catalyst[J]. Chemical Engineering Journal, 2019, 369: 124-133. |
| 9 | Wang J G, Liu C J. Density functional theory study of methane activation over PdO/HZSM-5[J]. Journal of Molecular Catalysis A: Chemical, 2006, 247(1/2):199-205. |
| 10 | Yu M, Wang Z Y. Understanding catalytic reactions over zeolites: a density functional theory study of selective catalytic reduction of NOx by NH3 over Cu-SAPO-34[J]. ACS Catalysis, 2016, 6: 7882-7891. |
| 11 | Chen P R, Khetan A, Jabłońska M, et al. Local dynamics of copper active sites in zeolite catalysts for selective catalytic reduction of NOx with NH3[J]. Applied Catalysis B: Environmental, 2018, 237: 263-272. |
| 12 | Begum P, Gogoi P, Mishra B K, et al. Theoretical insight of nitric oxide adsorption on neutral and charged Pdn (n = 1—5) clusters[J]. International Journal of Quantum Chemistry, 2015, 115(13): 837-845. |
| 13 | Loffreda D, Simon D, Sautet P. Structure sensitivity for NO dissociation on palladium and rhodium surfaces[J]. Journal of Catalysis, 2003, 213(2): 211-225. |
| 14 | Gao Y, Zhang L M, Kong C C, et al. NO adsorption and dissociation on palladium clusters: the importance of charged state and metal doping[J]. Chemical Physics Letters, 2016, 658: 7-11. |
| 15 | Pei G X, Liu X Y, Yang X, et al. Performance of Cu-alloyed Pd single-atom catalyst for semihydrogenation of acetylene under simulated front-end conditions[J]. ACS Catalysis, 2017, 7(2): 1491-1500. |
| 16 | Shi Y, Zhao C, Wei H, et al. Single-atom catalysis in mesoporous photovoltaics: the principle of utility maximization[J]. Advanced Materials, 2014, 26(48): 8147-8153. |
| 17 | Paredis K, Ono L K, Behafarid F, et al. Evolution of the structure and chemical state of Pd nanoparticles during the in situ catalytic reduction of NO with H2[J]. Journal of the American Chemical Society, 2011, 133(34): 13455-13464. |
| 18 | Yin Z, Li C, Su Y, et al. Investigation of reaction mechanisms of NO with CO on Pd1/MgO and Pd4/MgO catalysts[J]. Chemical Physics, 2012, 395: 108-114. |
| 19 | Ding W C, Gu X K, Su H Y, et al. Single Pd atom embedded in CeO2(111) for NO reduction with CO: a first-principles study[J]. The Journal of Physical Chemistry C, 2014, 118(23): 12216-12223. |
| 20 | Esrafili M D, Saeidi N. Catalytic reduction of NO by CO molecules over Ni-doped graphene: a DFT investigation[J]. New Journal of Chemistry, 2017, 41: 13149-13155. |
| 21 | 金燕, 杨倩, 赵文斌, 等. 石墨烯化学气相沉积法可控制备的催化反应体系研究[J]. 化工学报, 2020, 71(6): 2564-2585. |
| Jin Y, Yang Q, Zhao W B, et al. Catalytic reaction system for controllable synthesis of graphene with chemical vapor deposition[J]. CIESC Journal, 2020, 71(6): 2564-2585. | |
| 22 | Jia T T, Lu C H, Zhang Y F, et al. A comparative study of CO catalytic oxidation on Pd-anchored graphene oxide and Pd-embedded vacancy graphene[J]. Journal of Nanoparticle Research, 2014, 16(2): 1-11. |
| 23 | Zhao J X, Chen Y, Fu H G. Si-embedded graphene: an efficient and metal-free catalyst for CO oxidation by N2O or O2[J]. Theoretical Chemistry Accounts, 2012, 131(6): 1-11. |
| 24 | Yang W, Gao Z, Liu X. Directly catalytic reduction of NO without NH3 by single atom iron catalyst: a DFT calculation[J]. Fuel, 2019, 243: 262-270. |
| 25 | Esrafili M D. Exploring reaction mechanisms for the reduction of NO molecules over Al- or Si-anchored graphene oxide: a metal-free approach[J]. ChemistrySelect, 2018, 3: 12072-12079. |
| 26 | Esrafili M D, Dinparast L. A DFT study on the catalytic hydrogenation of CO2 to formic acid over Ti-doped graphene nanoflake[J]. Chemical Physics Letters, 2017, 682: 49-54. |
| 27 | Liu Z B, Li S F, Yang J, et al. Ultrafast construction of oxygen-containing scaffold over graphite for trapping Ni2+ into single atom catalysts[J]. ACS Nano, 2020, 14(9): 11622-11669. |
| 28 | Han Y, Wang Y G, Chen W, et al. Hollow N-doped carbon spheres with isolated cobalt single atomic sites: superior electrocatalysts for oxygen reduction[J]. Journal of the American Chemical Society, 2017, 139(48): 17269-17272. |
| 29 | Luo D, Zhang G, Liu J, et al. Evaluation criteria for reduced graphene oxide[J]. The Journal of Physical Chemistry C, 2011, 115(23): 11327-11335. |
| 30 | Shen J, Hu Y, Shi M, et al. Fast and facile preparation of graphene oxide and reduced graphene oxide nanoplatelets[J]. Chemistry of Materials, 2009, 21(15): 3514-3520. |
| 31 | Gómez-Navarro C, Meyer J C, Sundaram R S, et al. Atomic structure of reduced graphene oxide[J]. Nano Letters, 2010, 10(4): 1144-1148. |
| 32 | Erickson K, Erni R, Lee Z, et al. Determination of the local chemical structure of graphene oxide and reduced graphene oxide[J]. Advanced Materials, 2010, 22(40): 4467-4472. |
| 33 | Hu Y, Griffiths K, Norton P R. Surface science studies of selective catalytic reduction of NO: progress in the last ten years[J]. Surface Science, 2009, 603(10/11/12): 1740-1750. |
| 34 | 曹蕃, 苏胜, 向军, 等. SCR反应过程中NO/NH3在γ-Al2O3表面吸附特性[J]. 化工学报, 2014, 65(10): 4056-4062. |
| Cao F, Su S, Xiang J, et al. NO/NH3 adsorption properties on γ-Al2O3(110) surface during SCR process[J]. CIESC Journal, 2014, 65(10): 4056-4062. | |
| 35 | Wen H, Huai L, Jin X, et al. Mechanism of nitric oxide reduction by hydrogen on Ni(110) and Ir/Ni(110): first principles and microkinetic modeling[J]. The Journal of Physical Chemistry C, 2019, 123: 4825-4836. |
| 36 | Carlsson J M, Scheffler M. Structural, electronic, and chemical properties of nanoporous carbon[J]. Physical Review Letters, 2006, 96(4): 046806. |
| 37 | Lu Y, Zhou M, Zhang C, et al. Metal-embedded graphene: a possible catalyst with high activity[J]. The Journal of Physical Chemistry C, 2009, 113(47): 20156-20160. |
| 38 | Ling L, Feng X, Cao Y, et al. The catalytic CO oxidative coupling to dimethyl oxalate on Pd clusters anchored on defected graphene: a theoretical study[J]. Molecular Catalysis, 2018, 453: 100-112. |
| 39 | Fan L L, Ling L X, Wang B J, et al. The adsorption of mercury species and catalytic oxidation of Hg0 on the metal-loaded activated carbon[J]. Applied Catalysis A: General, 2016, 520: 13-23. |
| 40 | Esrafili M D, Nurazar R, Vessally E. Application of Si-doped graphene as a metal-free catalyst for decomposition of formic acid: a theoretical study[J]. International Journal of Quantum Chemistry, 2015, 115(17): 1153-1160. |
| 41 | Ling L, Cao Y, Zhao Z, et al. Density functional theory calculations and analysis for the reduction of NO by H2 on Pd6/TiO2[J]. Computational Materials Science, 2018, 149: 182-190. |
| 42 | Farberow C A, Dumesic J A, Mavrikakis M. Density functional theory calculations and analysis of reaction pathways for reduction of nitric oxide by hydrogen on Pt(111)[J]. ACS Catalysis, 2014, 4(10): 3307-3319. |
| 43 | Chen Y, Liu Y J, Wang H X, et al. Silicon-doped graphene: an effective and metal-free catalyst for NO reduction to N2O?[J]. ACS Applied Materials & Interfaces, 2013, 5(13): 5994-6000. |
| 44 | Wang Y Y, Zhang D J, Yu Z Y, et al. Mechanism of N2O formation during NO reduction on the Au(111) Surface[J]. The Journal of Physical Chemistry C, 2010, 114: 2711-2716. |
| 45 | Ling L, Zhao Z, Feng X. Insight into the reduction of NO by H2 on the stepped Pd(211) surface[J]. The Journal of Physical Chemistry C, 2017, 121: 16399-16414. |
| 46 | Huai L Y, He C Z, Wang H, et al. NO dissociation and reduction by H2 on Pd(111): a first-principles study[J]. Journal of Catalysis, 2015, 322: 73-83. |
| 47 | Bryliakova A A, Matveev A V, Tapilin V M, et al. NO + H2 reaction over Pd(110): TPD, TPR and DFT study[J]. Molecular Catalysis, 2018, 448: 53-62. |
| 48 | Huai L, Su T, Wen H, et al. NO reduction by H2 on the Rh(111) and Rh(221) surfaces: a mechanistic and kinetic study[J]. The Journal of Physical Chemistry C, 2016, 120: 5410-5419. |
| 49 | Yang M, Yuan H, Wang H. Insights into the selective catalytic reduction of NO by NH3 over Mn3O4(110): a DFT study coupled with microkinetic analysis[J]. Science China Chemistry, 2018, 61: 457-467. |
| 50 | Zheng H, Song W, Zhou Y. Mechanistic study of selective catalytic reduction of NOx with NH3 over Mn-TiO2: a combination of experimental and DFT Study[J]. The Journal of Physical Chemistry C, 2017, 121(36): 19859-19871. |
| [1] | Xin YANG, Wen WANG, Kai XU, Fanhua MA. Simulation analysis of temperature characteristics of the high-pressure hydrogen refueling process [J]. CIESC Journal, 2023, 74(S1): 280-286. |
| [2] | Congqi HUANG, Yimei WU, Jianye CHEN, Shuangquan SHAO. Simulation study of thermal management system of alkaline water electrolysis device for hydrogen production [J]. CIESC Journal, 2023, 74(S1): 320-328. |
| [3] | Baomin DAI, Qilong WANG, Shengchun LIU, Jianing ZHANG, Xinhai LI, Fandi ZONG. Thermodynamic performance analysis of combined cooling and heating system based on combination of CO2 with the zeotropic refrigerant assisted subcooled [J]. CIESC Journal, 2023, 74(S1): 64-73. |
| [4] | Zehao MI, Er HUA. DFT and COSMO-RS theoretical analysis of SO2 absorption by polyamines type ionic liquids [J]. CIESC Journal, 2023, 74(9): 3681-3696. |
| [5] | Yihao ZHANG, Zhenlei WANG. Fault detection using grouped support vector data description based on maximum information coefficient [J]. CIESC Journal, 2023, 74(9): 3865-3878. |
| [6] | Junfeng LU, Huaiyu SUN, Yanlei WANG, Hongyan HE. Molecular understanding of interfacial polarization and its effect on ionic liquid hydrogen bonds [J]. CIESC Journal, 2023, 74(9): 3665-3680. |
| [7] | Ke LI, Jian WEN, Biping XIN. Study on influence mechanism of vacuum multi-layer insulation coupled with vapor-cooled shield on self-pressurization process of liquid hydrogen storage tank [J]. CIESC Journal, 2023, 74(9): 3786-3796. |
| [8] | Manzheng ZHANG, Meng XIAO, Peiwei YAN, Zheng MIAO, Jinliang XU, Xianbing JI. Working fluid screening and thermodynamic optimization of hazardous waste incineration coupled organic Rankine cycle system [J]. CIESC Journal, 2023, 74(8): 3502-3512. |
| [9] | Feifei YANG, Shixi ZHAO, Wei ZHOU, Zhonghai NI. Sn doped In2O3 catalyst for selective hydrogenation of CO2 to methanol [J]. CIESC Journal, 2023, 74(8): 3366-3374. |
| [10] | Bin LI, Zhenghu XU, Shuang JIANG, Tianyong ZHANG. Clean and efficient synthesis of accelerator CBS by hydrogen peroxide catalytic oxidation method [J]. CIESC Journal, 2023, 74(7): 2919-2925. |
| [11] | Xiaoyang LIU, Jianliang YU, Yujie HOU, Xingqing YAN, Zhenhua ZHANG, Xianshu LYU. Effect of spiral microchannel on detonation propagation of hydrogen-doped methane [J]. CIESC Journal, 2023, 74(7): 3139-3148. |
| [12] | Ming DONG, Jinliang XU, Guanglin LIU. Molecular dynamics study on heterogeneous characteristics of supercritical water [J]. CIESC Journal, 2023, 74(7): 2836-2847. |
| [13] | Xiaowen ZHOU, Jie DU, Zhanguo ZHANG, Guangwen XU. Study on the methane-pulsing reduction characteristics of Fe2O3-Al2O3 oxygen carrier [J]. CIESC Journal, 2023, 74(6): 2611-2623. |
| [14] | Yong LI, Jiaqi GAO, Chao DU, Yali ZHAO, Boqiong LI, Qianqian SHEN, Husheng JIA, Jinbo XUE. Construction of Ni@C@TiO2 core-shell dual-heterojunctions for advanced photo-thermal catalytic hydrogen generation [J]. CIESC Journal, 2023, 74(6): 2458-2467. |
| [15] | Xiqing ZHANG, Yanting WANG, Yanhong XU, Shuling CHANG, Tingting SUN, Ding XUE, Lihong ZHANG. Effect of Mg content on isobutane dehydrogenation properties over nanosheets supported Pt-In catalysts [J]. CIESC Journal, 2023, 74(6): 2427-2435. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||