CIESC Journal ›› 2021, Vol. 72 ›› Issue (7): 3768-3779.DOI: 10.11949/0438-1157.20201818
• Biochemical engineering and technology • Previous Articles Next Articles
YANG Ruixiong1( ),ZHENG Xin1,LU Tao1,ZHAO Yuze1,YANG Qinghua1,LU Yinghua1,2,3,HE Ning1,2,LING Xueping1,2,3(
),ZHENG Xin1,LU Tao1,ZHAO Yuze1,YANG Qinghua1,LU Yinghua1,2,3,HE Ning1,2,LING Xueping1,2,3( )
)
Received:2020-12-15
Revised:2021-02-27
Online:2021-07-05
Published:2021-07-05
Contact:
LING Xueping
杨瑞雄1( ),郑鑫1,陆涛1,赵誉泽1,杨庆华1,卢英华1,2,3,何宁1,2,凌雪萍1,2,3(
),郑鑫1,陆涛1,赵誉泽1,杨庆华1,卢英华1,2,3,何宁1,2,凌雪萍1,2,3( )
)
通讯作者:
凌雪萍
作者简介:杨瑞雄(1996—),女,硕士研究生,基金资助:CLC Number:
YANG Ruixiong, ZHENG Xin, LU Tao, ZHAO Yuze, YANG Qinghua, LU Yinghua, HE Ning, LING Xueping. Effects of substitution of ER domains on the synthesis of eicosapentaenoic acid in Schizochytrium limacinum SR21[J]. CIESC Journal, 2021, 72(7): 3768-3779.
杨瑞雄, 郑鑫, 陆涛, 赵誉泽, 杨庆华, 卢英华, 何宁, 凌雪萍. 烯酰还原酶基因的替换对裂殖壶菌合成二十碳五烯酸的影响[J]. 化工学报, 2021, 72(7): 3768-3779.
Add to citation manager EndNote|Ris|BibTeX

Fig.5 Biomass (a), total lipids yield (b) and lipids content (c) in the wild strain and B-sh-ER strains; Biomass (d), total lipids yield (e) and lipids content (f) in the wild strain and C-sh-ER strains
| Fatty acid | Composition/% | ||
|---|---|---|---|
| Wild strain | B-sh-ER strain | C-sh-ER strain | |
| C14:0 | 3.39 ± 0.05 | 3.59 ± 0.13 | 4.09 ± 0.30 |
| C14:1 | 1.20 ± 0.02 | 1.81 ± 0.11a | 1.69 ± 0.14a |
| C16:0 | 53.02 ± 0.60 | 53.88 ± 0.71 | 55.38 ± 0.10 |
| C18:0 | 1.75 ± 0.01 | 2.61 ± 0.15a | 2.04 ± 0.16 |
| ARA | 0.16 ± 0.02 | 0.37 ± 0.01a | 0.53 ± 0.08a |
| EPA | 0.42 ± 0.01 | 0.78 ± 0.01b | 0.37 ± 0.02 |
| DPA | 6.51 ± 0.03 | 6.08 ± 0.10 | 4.45 ± 0.18a |
| DHA | 29.76 ± 0.25 | 27.63 ± 0.77 | 29.52 ± 0.62 |
| EPA×100/DHA | 1.61 ± 0.03 | 2.82 ± 0.03b | 1.25 ± 0.07a |
| SFAs | 58.15 ± 0.65 | 60.08 ± 0.69 | 61.51 ± 0.57a |
| PUFAs | 37.45 ± 0.27 | 34.71 ± 0.70a | 34.87 ± 0.32a |
Table 1 Fatty acid composition of wild strains, C-sh-ER strains and B-sh-ER strains
| Fatty acid | Composition/% | ||
|---|---|---|---|
| Wild strain | B-sh-ER strain | C-sh-ER strain | |
| C14:0 | 3.39 ± 0.05 | 3.59 ± 0.13 | 4.09 ± 0.30 |
| C14:1 | 1.20 ± 0.02 | 1.81 ± 0.11a | 1.69 ± 0.14a |
| C16:0 | 53.02 ± 0.60 | 53.88 ± 0.71 | 55.38 ± 0.10 |
| C18:0 | 1.75 ± 0.01 | 2.61 ± 0.15a | 2.04 ± 0.16 |
| ARA | 0.16 ± 0.02 | 0.37 ± 0.01a | 0.53 ± 0.08a |
| EPA | 0.42 ± 0.01 | 0.78 ± 0.01b | 0.37 ± 0.02 |
| DPA | 6.51 ± 0.03 | 6.08 ± 0.10 | 4.45 ± 0.18a |
| DHA | 29.76 ± 0.25 | 27.63 ± 0.77 | 29.52 ± 0.62 |
| EPA×100/DHA | 1.61 ± 0.03 | 2.82 ± 0.03b | 1.25 ± 0.07a |
| SFAs | 58.15 ± 0.65 | 60.08 ± 0.69 | 61.51 ± 0.57a |
| PUFAs | 37.45 ± 0.27 | 34.71 ± 0.70a | 34.87 ± 0.32a |
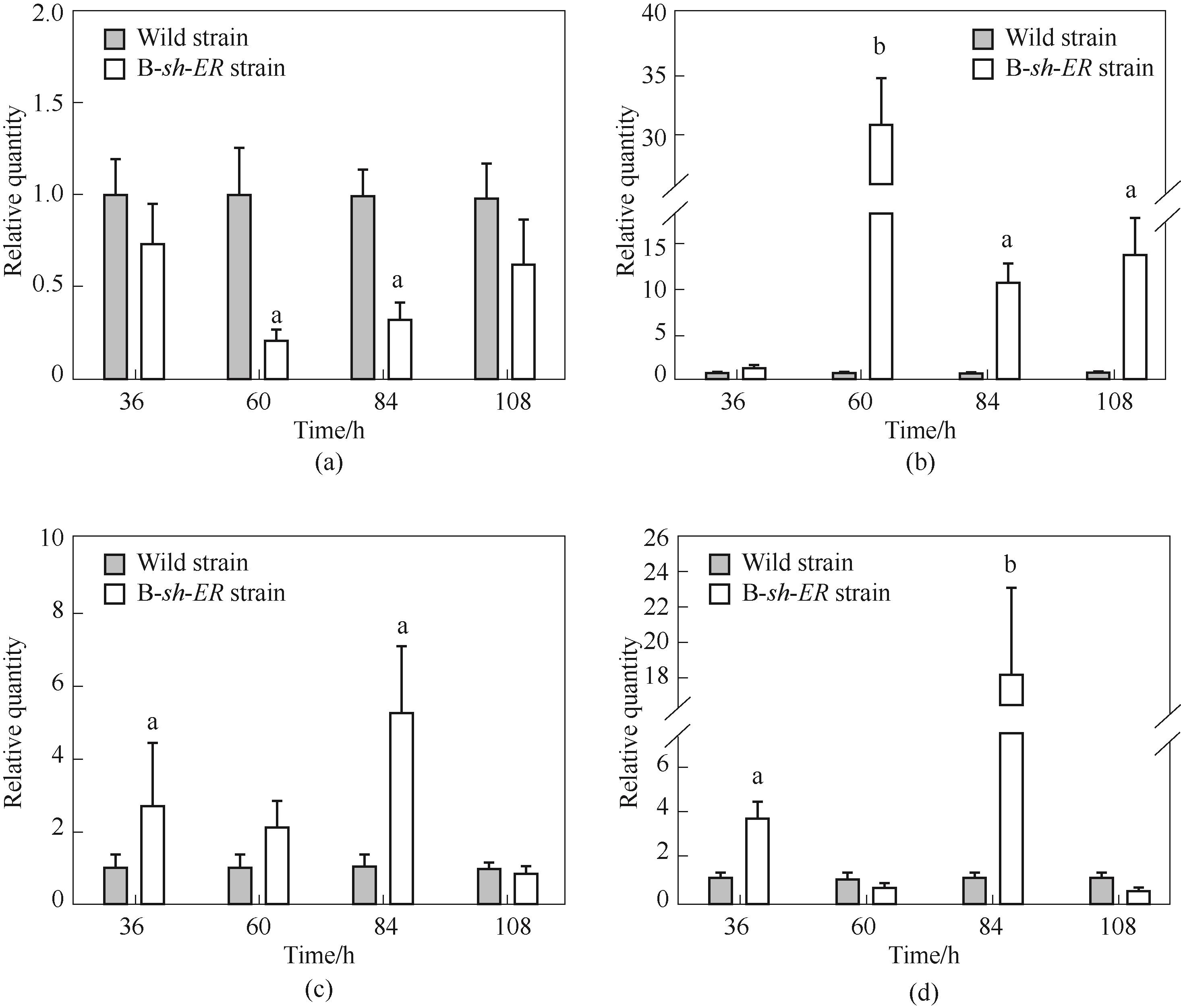
Fig.6 The transcription level of related gene in B-sh-ER strain(a) Schizochytrium limacinum SR21 enoyl-reductase gene ORFB-ER; (b) Shewanella sp. SCRC2738 enoyl-reductase gene sh-ER; (c) Schizochytrium limacinum SR21 enoyl-reductase gene ORFC-ER; (d) acyl transferase (AT) gene
| Lipid profile | Wild strain | B-sh-ER strain |
|---|---|---|
| total lipid/(g/L) | 56.90 ± 1.36 | 73.24 ± 1.09b |
| PUFAs/% | 42.34 ± 0.76 | 32.02 ± 1.21a |
| DHA/% | 33.22 ± 0.38 | 25.10 ± 0.68a |
| DPA/% | 8.37 ± 0.46 | 5.92 ± 0.49a |
| EPA/% | 0.75 ± 0.02 | 1.00 ± 0.02a |
| C16:0/% | 49.54 ± 0.89 | 57.40 ± 0.72a |
Table 2 Fatty acid composition of the wild strain and B-sh-ER strain in fed-batch fermentation
| Lipid profile | Wild strain | B-sh-ER strain |
|---|---|---|
| total lipid/(g/L) | 56.90 ± 1.36 | 73.24 ± 1.09b |
| PUFAs/% | 42.34 ± 0.76 | 32.02 ± 1.21a |
| DHA/% | 33.22 ± 0.38 | 25.10 ± 0.68a |
| DPA/% | 8.37 ± 0.46 | 5.92 ± 0.49a |
| EPA/% | 0.75 ± 0.02 | 1.00 ± 0.02a |
| C16:0/% | 49.54 ± 0.89 | 57.40 ± 0.72a |
| Strains | DCW/(g/L) | EPA yield/(mg/L) | EPA proportion/% |
|---|---|---|---|
| Schizochytrium limacinum SR21 | 159.7 | 732.4 | 1.0 |
| Thraustochytrid Aurantiochytrium[ | 144.4 | 2700.0 | 3.1 |
| Schizochytrium sp. MYA1381[ | 182 | 1650.0 | 1.45 |
| Nitzschia laevis[ | 22.1 | 695.2 | 3.3 |
| Nitzschia laevis[ | — | 1112.0 | 2.4 |
| Phaeodactylum tricornutum[ | 16.2 | 356.4 | 2.2 |
| Mortierella alpine ATCC 32222[ | 20.1 | 588.5 | — |
| Mortierella alpina ST1358[ | 10 | 1800.0 | 26.4 |
Table 3 EPA yield and proportion of related strains
| Strains | DCW/(g/L) | EPA yield/(mg/L) | EPA proportion/% |
|---|---|---|---|
| Schizochytrium limacinum SR21 | 159.7 | 732.4 | 1.0 |
| Thraustochytrid Aurantiochytrium[ | 144.4 | 2700.0 | 3.1 |
| Schizochytrium sp. MYA1381[ | 182 | 1650.0 | 1.45 |
| Nitzschia laevis[ | 22.1 | 695.2 | 3.3 |
| Nitzschia laevis[ | — | 1112.0 | 2.4 |
| Phaeodactylum tricornutum[ | 16.2 | 356.4 | 2.2 |
| Mortierella alpine ATCC 32222[ | 20.1 | 588.5 | — |
| Mortierella alpina ST1358[ | 10 | 1800.0 | 26.4 |
| 引物名称 | 引物序列 |
|---|---|
| ORFB-ER-F | GTTGAAGCCTCCGCCTTTATG |
| ORFB-ER-R | CCTGCTCTTGGGTGATCTCGC |
| ORFC-ER-F | CAGCCTGCTCCTTGGTAATCT |
| ORFC-ER-R | CATCAAGAACCGCATCATCG |
| AT-F | TGCTACACGGTGCTGCTCTCTGATG |
| AT-R | ACGTACATTAGCGCTAGGCTGGGC |
| sh-ER-F | TCGCTCAAACCGCTGACATCGTA |
| sh-ER-R | CGGCGTAGTAGGCGTACTTCACG |
Table A1 Primers for RT-qPCR
| 引物名称 | 引物序列 |
|---|---|
| ORFB-ER-F | GTTGAAGCCTCCGCCTTTATG |
| ORFB-ER-R | CCTGCTCTTGGGTGATCTCGC |
| ORFC-ER-F | CAGCCTGCTCCTTGGTAATCT |
| ORFC-ER-R | CATCAAGAACCGCATCATCG |
| AT-F | TGCTACACGGTGCTGCTCTCTGATG |
| AT-R | ACGTACATTAGCGCTAGGCTGGGC |
| sh-ER-F | TCGCTCAAACCGCTGACATCGTA |
| sh-ER-R | CGGCGTAGTAGGCGTACTTCACG |
| 1 | Gong Y M, Wan X, Jiang M L, et al. Metabolic engineering of microorganisms to produce omega-3 very long-chain polyunsaturated fatty acids[J]. Progress in Lipid Research, 2014, 56: 19-35. |
| 2 | Peet M. Nutrition and schizophrenia: beyond omega-3 fatty acids[J]. Prostaglandins, Leukotrienes and Essential Fatty Acids, 2004, 70(4): 417-422. |
| 3 | Oppedisano F, Macrì R, Gliozzi M, et al. The anti-inflammatory and antioxidant properties of n-3 PUFAs: their role in cardiovascular protection[J]. Biomedicines, 2020, 8(9): 306. |
| 4 | Amigó N, Akinkuolie A O, Chiuve S E, et al. Habitual fish consumption, n-3 fatty acids, and nuclear magnetic resonance lipoprotein subfractions in women[J]. Journal of the American Heart Association, 2020, 9(5): e014963. |
| 5 | 夏尧干, 王宇, 邹雯燕, 等. 鱼油脂肪酸EPA和DHA的分离影响研究[J]. 淮阴工学院学报, 2020, 29(5): 49-52. |
| Xia Y G, Wang Y, Zou W Y, et al. Study on the separation effect of EPA and DHA from fish oil fatty acids[J]. Journal of Huaiyin Institute of Technology, 2020, 29(5): 49-52. | |
| 6 | Yazawa K. Production of eicosapentaenoic acid from marine bacteria[J]. Lipids, 1996, 31(1): S297-S300. |
| 7 | El Razak A A, Ward A C, Glassey J. Screening of marine bacterial producers of polyunsaturated fatty acids and optimisation of production[J]. Microbial Ecology, 2014, 67(2): 454-464. |
| 8 | Zhang J W, Burgess J G. Enhanced eicosapentaenoic acid production by a new deep-sea marine bacterium Shewanella electrodiphila MAR441T[J]. PLoS One, 2017, 12(11): e0188081. |
| 9 | Wen Z Y, Chen F. Heterotrophic production of eicosapentaenoic acid by microalgae[J]. Biotechnology Advances, 2003, 21(4): 273-294. |
| 10 | Wang S, Lan C Z, Wang Z J, et al. Optimizing eicosapentaenoic acid production by grafting a heterologous polyketide synthase pathway in the Thraustochytrid aurantiochytrium[J]. Journal of Agricultural and Food Chemistry, 2020, 68(40): 11253-11260. |
| 11 | Sayanova O, Napier J A. Metabolic engineering of microalgae for sustainable production of omega-3 long chain polyunsaturated fatty acids[J]. Current Biotechnology, 2016, 5(3): 198-212. |
| 12 | Hayashi S, Satoh Y, Ogasawara Y, et al. Control mechanism for cis double-bond formation by polyunsaturated fatty-acid synthases[J]. Angewandte Chemie International Edition, 2019, 58(8): 2326-2330. |
| 13 | Ren L J, Chen S L, Geng L J, et al. Exploring the function of acyltransferase and domain replacement in order to change the polyunsaturated fatty acid profile of Schizochytrium sp[J]. Algal Research, 2018, 29: 193-201. |
| 14 | Matsuda T, Sakaguchi K, Hamaguchi R, et al. Analysis of Δ12-fatty acid desaturase function revealed that two distinct pathways are active for the synthesis of PUFAs in T. aureum ATCC 34304[J]. Journal of Lipid Research, 2012, 53(6): 1210-1222. |
| 15 | Aasen I M, Ertesvåg H, Heggeset T M B, et al. Thraustochytrids as production organisms for docosahexaenoic acid (DHA), squalene, and carotenoids[J]. Applied Microbiology and Biotechnology, 2016, 100(10): 4309-4321. |
| 16 | Yaguchi T, Tanaka S, Yokochi T, et al. Production of high yields of docosahexaenoic acid by Schizochytrium sp. strain SR21[J]. Journal of the American Oil Chemists' Society, 1997, 74(11): 1431-1434. |
| 17 | Wallis J G, Watts J L, Browse J. Polyunsaturated fatty acid synthesis: what will they think of next?[J]. Trends in Biochemical Sciences, 2002, 27(9): 467-473. |
| 18 | Marrakchi H, Dewolf W E, Quinn C, et al. Characterization of Streptococcuspneumoniae enoyl-(acyl-carrier protein) reductase (FabK)[J]. The Biochemical Journal, 2003, 370(Pt 3): 1055-1062. |
| 19 | Heath R J, Rock C O. Enoyl-acyl carrier protein reductase (fabI) plays a determinant role in completing cycles of fatty acid elongation in Escherichia coli[J]. Journal of Biological Chemistry, 1995, 270(44): 26538-26542. |
| 20 | Ling X P, Zhou H, Yang Q H, et al. Functions of enyolreductase (ER) domains of PKS cluster in lipid synthesis and enhancement of PUFAs accumulation in Schizochytrium limacinum SR21 using triclosan as a regulator of ER[J]. Microorganisms, 2020, 8(2): 300. |
| 21 | Lee S J, Seo P S, Kim C H, et al. Isolation and characterization of the eicosapentaenoic acid biosynthesis gene cluster from Shewanella sp. BR-2[J]. Journal of Microbiology and Biotechnology, 2009, 19(9): 881-887. |
| 22 | Metz J G, Roessler P, Facciotti D, et al. Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes[J]. Science, 2001, 293(5528): 290-293. |
| 23 | Peng Y F, Chen W C, Xiao K, et al. DHA production in Escherichia coli by expressing reconstituted key genes of polyketide synthase pathway from marine bacteria[J]. PLoS One, 2016, 11(9): e0162861. |
| 24 | Lee S J, Kim C H, Seo P S, et al. Enhancement of heterologous production of eicosapentaenoic acid in Escherichia coli by substitution of promoter sequences within the biosynthesis gene cluster[J]. Biotechnology Letters, 2008, 30(12): 2139-2142. |
| 25 | Gemperlein K, Dietrich D, Kohlstedt M, et al. Polyunsaturated fatty acid production by Yarrowia lipolytica employing designed myxobacterial PUFA synthases[J]. Nature Communications, 2019, 10(1): 4055. |
| 26 | Xue Z X, Sharpe P L, Hong S P, et al. Production of omega-3 eicosapentaenoic acid by metabolic engineering of Yarrowia lipolytica[J]. Nature Biotechnology, 2013, 31(8): 734-740. |
| 27 | Takeyama H, Takeda D, Yazawa K, et al. Expression of the eicosapentaenoic acid synthesis gene cluster from Shewanella sp. in a transgenic marine cyanobacterium, Synechococcus sp[J]. Microbiology, 1997, 143 ( Pt 8): 2725-2731. |
| 28 | Ling X P, Guo J, Liu X T, et al. Impact of carbon and nitrogen feeding strategy on high production of biomass and docosahexaenoic acid (DHA) by Schizochytrium sp. LU310[J]. Bioresource Technology, 2015, 184: 139-147. |
| 29 | Li Z P, Meng T, Ling X P, et al. Overexpression of malonyl-CoA: ACP transacylase in Schizochytrium sp. to improve polyunsaturated fatty acid production[J]. Journal of Agricultural and Food Chemistry, 2018, 66(21): 5382-5391. |
| 30 | Li Z, Ling X, Zhou H, et al. Screening chemical modulators of benzoic acid derivatives to improve lipid accumulation in Schizochytrium limacinum SR21 with metabolomics analysis[J]. Biotechnology for Biofuels, 2019, 12: 209. |
| 31 | Pan X S, Wang B B, Duan R, et al. Enhancing astaxanthin accumulation in Xanthophyllomyces dendrorhous by a phytohormone: metabolomic and gene expression profiles[J]. Microbial Biotechnology, 2020, 13(5): 1446-1460. |
| 32 | Li J P, Wei Y X, Li J C, et al. A novel duplex SYBR Green real-time PCR with melting curve analysis method for beef adulteration detection[J]. Food Chemistry, 2021, 338: 127932. |
| 33 | Ren L J, Zhuang X Y, Chen S L, et al. Introduction of ω-3 desaturase obviously changed the fatty acid profile and sterol content of Schizochytrium sp[J]. Journal of Agricultural and Food Chemistry, 2015, 63(44): 9770-9776. |
| 34 | Ren L J, Huang H, Xiao A H, et al. Enhanced docosahexaenoic acid production by reinforcing acetyl-CoA and NADPH supply in Schizochytrium sp. HX-308[J]. Bioprocess and Biosystems Engineering, 2009, 32(6): 837-843. |
| 35 | Snoep J L, Yomano L P, Westerhoff H V, et al. Protein burden in Zymomonas mobilis: negative flux and growth control due to overproduction of glycolytic enzymes[J]. Microbiology, 1995, 141(9): 2329-2337. |
| 36 | Nishida I, Murata N. CHILLING SENSITIVITY IN PLANTS AND CYANOBACTERIA: the crucial contribution of membrane lipids[J]. Annual Review of Plant Physiology and Plant Molecular Biology, 1996, 47: 541-568. |
| 37 | Venkateswaran K, Moser D P, Dollhopf M E, et al. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov[J]. International Journal of Systematic Bacteriology, 1999, 49Pt 2: 705-724. |
| 38 | Lippmeier J C, Crawford K S, Owen C B, et al. Characterization of both polyunsaturated fatty acid biosynthetic pathways in Schizochytrium sp. [J]. Lipids, 2009, 44(7): 621-630. |
| 39 | Hauvermale A, Kuner J, Rosenzweig B, et al. Fatty acid production in Schizochytrium sp.: involvement of a polyunsaturated fatty acid synthase and a type I fatty acid synthase[J]. Lipids, 2006, 41(8): 739-747. |
| 40 | Cerón Garcí M C, Fernández Sevilla J M, Acién Fernández F G, et al. Mixotrophic growth of Phaeodactylum tricornutum on glycerol: growth rate and fatty acid profile[J]. Journal of Applied Phycology, 2000, 12(3/4/5): 239-248. |
| 41 | Shi H S, Chen H Q, Gu Z N, et al. Application of a delta-6 desaturase with α-linolenic acid preference on eicosapentaenoic acid production in Mortierella alpina[J]. Microbial Cell Factories, 2016, 15(1): 117. |
| 42 | Okuda T, Ando A, Negoro H, et al. Eicosapentaenoic acid (EPA) production by an oleaginous fungus Mortierella alpina expressing heterologous the Δ17-desaturase gene under ordinary temperature[J]. European Journal of Lipid Science and Technology, 2015, 117(12): 1919-1927. |
| 43 | Cui G Z, Ma Z X, Liu Y J, et al. Overexpression of glucose-6-phosphate dehydrogenase enhanced the polyunsaturated fatty acid composition of Aurantiochytrium sp. SD116[J]. Algal Research, 2016, 19: 138-145. |
| 44 | Khozin-Goldberg I, Yu H Z, Adlerstein D, et al. Triacylglycerols of the red microalga Porphyridium cruentum can contribute to the biosynthesis of eukaryotic galactolipids[J]. Lipids, 2000, 35(8): 881-889. |
| 45 | Fernández F G A, Pérez J A S, Sevilla J M F, et al. Modeling of eicosapentaenoic acid (EPA) production from Phaeodactylum tricornutum cultures in tubular photobioreactors. Effects of dilution rate, tube diameter, and solar irradiance[J]. Biotechnology and Bioengineering, 2000, 68(2): 173-183. |
| 46 | Huerlimann R, de Nys R, Growth Heimann K., content lipid, productivity, and fatty acid composition of tropical microalgae for scale-up production[J]. Biotechnology and Bioengineering, 2010, 107(2): 245-257. |
| [1] | Qi WANG, Bin ZHANG, Xiaoxin ZHANG, Hujian WU, Haitao ZHAN, Tao WANG. Synthesis of isoxepac and 2-ethylanthraquinone catalyzed by chloroaluminate-triethylamine ionic liquid/P2O5 [J]. CIESC Journal, 2023, 74(S1): 245-249. |
| [2] | Yaxin ZHAO, Xueqin ZHANG, Rongzhu WANG, Guo SUN, Shanjing YAO, Dongqiang LIN. Removal of monoclonal antibody aggregates with ion exchange chromatography by flow-through mode [J]. CIESC Journal, 2023, 74(9): 3879-3887. |
| [3] | Jiayi ZHANG, Jiali HE, Jiangpeng XIE, Jian WANG, Yu ZHAO, Dongqiang ZHANG. Research progress of pervaporation technology for N-methylpyrrolidone recovery in lithium battery production [J]. CIESC Journal, 2023, 74(8): 3203-3215. |
| [4] | Ruihang ZHANG, Pan CAO, Feng YANG, Kun LI, Peng XIAO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Analysis of key parameters affecting product purity of natural gas ethane recovery process via ZIF-8 nanofluid [J]. CIESC Journal, 2023, 74(8): 3386-3393. |
| [5] | Shuang LIU, Linzhou ZHANG, Zhiming XU, Suoqi ZHAO. Study on molecular level composition correlation of viscosity of residual oil and its components [J]. CIESC Journal, 2023, 74(8): 3226-3241. |
| [6] | Lei XING, Chunyu MIAO, Minghu JIANG, Lixin ZHAO, Xinya LI. Optimal design and performance analysis of downhole micro gas-liquid hydrocyclone [J]. CIESC Journal, 2023, 74(8): 3394-3406. |
| [7] | Wentao WU, Liangyong CHU, Lingjie ZHANG, Weimin TAN, Liming SHEN, Ningzhong BAO. High-efficient preparation of cardanol-based self-healing microcapsules [J]. CIESC Journal, 2023, 74(7): 3103-3115. |
| [8] | Yuanliang ZHANG, Xinqi LUAN, Weige SU, Changhao LI, Zhongxing ZHAO, Liqin ZHOU, Jianmin CHEN, Yan HUANG, Zhenxia ZHAO. Study on selective extraction of nicotine by ionic liquids composite extractant and DFT calculation [J]. CIESC Journal, 2023, 74(7): 2947-2956. |
| [9] | Jinming GAO, Yujiao GUO, Chenglin E, Chunxi LU. Study on the separation characteristics of a downstream gas-liquid vortex separator in a closed hood [J]. CIESC Journal, 2023, 74(7): 2957-2966. |
| [10] | Zhilong WANG, Ye YANG, Zhenzhen ZHAO, Tao TIAN, Tong ZHAO, Yahui CUI. Influence of mixing time and sequence on the dispersion properties of the cathode slurry of lithium-ion battery [J]. CIESC Journal, 2023, 74(7): 3127-3138. |
| [11] | Zhaolun WEN, Peirui LI, Zhonglin ZHANG, Xiao DU, Qiwang HOU, Yegang LIU, Xiaogang HAO, Guoqing GUAN. Design and optimization of cryogenic air separation process with dividing wall column based on self-heat regeneration [J]. CIESC Journal, 2023, 74(7): 2988-2998. |
| [12] | Kuikui HAN, Xianglong TAN, Jinzhi LI, Ting YANG, Chun ZHANG, Yongfen ZHANG, Hongquan LIU, Zhongwei YU, Xuehong GU. Four-channel hollow fiber MFI zeolite membrane for the separation of xylene isomers [J]. CIESC Journal, 2023, 74(6): 2468-2476. |
| [13] | Feng ZHU, Kailin CHEN, Xiaofeng HUANG, Yinzhu BAO, Wenbin LI, Jiaxin LIU, Weiqiang WU, Wangwei GAO. Performance study of KOH modified carbide slag for removal of carbonyl sulfide [J]. CIESC Journal, 2023, 74(6): 2668-2679. |
| [14] | Xingchi ZHU, Zhiyuan GUO, Zhiyong JI, Jing WANG, Panpan ZHANG, Jie LIU, Yingying ZHAO, Junsheng YUAN. Simulation and optimization of selective electrodialysis magnesium and lithium separation process [J]. CIESC Journal, 2023, 74(6): 2477-2485. |
| [15] | Xuejin GAO, Yuzhuo YAO, Huayun HAN, Yongsheng QI. Fault monitoring of fermentation process based on attention dynamic convolutional autoencoder [J]. CIESC Journal, 2023, 74(6): 2503-2521. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||