CIESC Journal ›› 2021, Vol. 72 ›› Issue (9): 4698-4707.DOI: 10.11949/0438-1157.20210060
• Catalysis, kinetics and reactors • Previous Articles Next Articles
Received:2021-01-11
Revised:2021-05-25
Online:2021-09-05
Published:2021-09-05
Contact:
Xing FAN
通讯作者:
樊星
作者简介:李泽严(1995—),男,硕士研究生,基金资助:CLC Number:
Zeyan LI, Xing FAN, Jian LI. Non-thermal plasma enhanced hydrolysis of urea decomposition by-products over TiO2[J]. CIESC Journal, 2021, 72(9): 4698-4707.
李泽严, 樊星, 李坚. 非热等离子体强化TiO2催化尿素分解副产物水解性能的研究[J]. 化工学报, 2021, 72(9): 4698-4707.
Add to citation manager EndNote|Ris|BibTeX

Fig.2 Hydrolysis curves of biuret by catalysis and plasma-enhanced catalysis in N2+H2O and 5%O2+N2+H2O (average discharge power: 5.6 W; discharge period: 0—85 min)
| 平均放电功率/W | 载气气氛 | CO2开始出现的温度/℃ | CO2达到峰值浓度时的温度/℃ | CO2开始消失的温度/℃ | NH3产率/% | CO2产率/% |
|---|---|---|---|---|---|---|
| 0 | N2+H2O | 43 | 94/161/200 | 261 | 82.1 | 71.0 |
| 0 | 5%O2+N2+H2O | 47 | 77/165/202 | 255 | 79.7 | 62.4 |
| 5.6 | N2+H2O | 36 | 61/136/176 | 221 | 70.3 | 59.8 |
| 5.6 | 5%O2+N2+H2O | 33 | 54/142/183 | 223 | 47.4 | 56.3 |
Table 1 Results of biuret hydrolysis by catalysis and plasma-enhanced catalysis
| 平均放电功率/W | 载气气氛 | CO2开始出现的温度/℃ | CO2达到峰值浓度时的温度/℃ | CO2开始消失的温度/℃ | NH3产率/% | CO2产率/% |
|---|---|---|---|---|---|---|
| 0 | N2+H2O | 43 | 94/161/200 | 261 | 82.1 | 71.0 |
| 0 | 5%O2+N2+H2O | 47 | 77/165/202 | 255 | 79.7 | 62.4 |
| 5.6 | N2+H2O | 36 | 61/136/176 | 221 | 70.3 | 59.8 |
| 5.6 | 5%O2+N2+H2O | 33 | 54/142/183 | 223 | 47.4 | 56.3 |
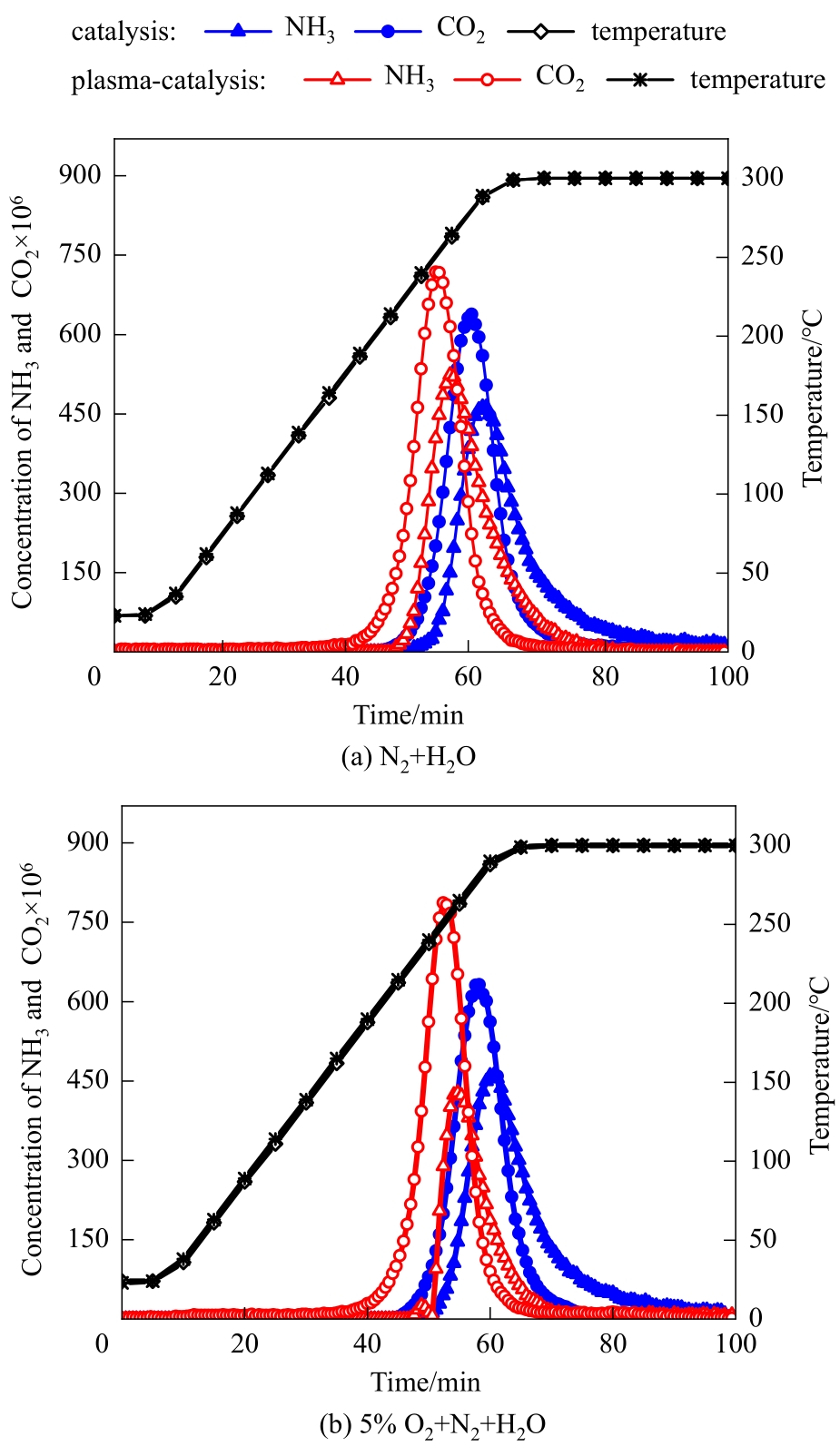
Fig.3 Hydrolysis curves of cyanuric acid by catalysis and plasma-enhanced catalysis in N2+H2O and 5%O2+N2+H2O (average discharge power: 6.2 W and 5.9 W without and with O2, respectively; discharge period: 0—85 min)
| 平均放电功率/W | 载气气氛 | CO2开始出现的 温度/℃ | CO2达到峰值浓度时的温度/℃ | CO2开始消失的 温度/℃ | NH3产率/% | CO2产率/% |
|---|---|---|---|---|---|---|
| 0 | N2+H2O | 217 | 282 | 300 | 54.5 | 57.3 |
| 0 | 5%O2+N2+H2O | 218 | 282 | 300 | 54.8 | 57.3 |
| 6.2 | N2+H2O | 162 | 253 | 300 | 52.6 | 64.0 |
| 5.9 | 5%O2+N2+H2O | 160 | 253 | 300 | 34.1 | 66.8 |
Table 2 Results of cyanuric acid hydrolysis by catalysis and plasma-enhanced catalysis
| 平均放电功率/W | 载气气氛 | CO2开始出现的 温度/℃ | CO2达到峰值浓度时的温度/℃ | CO2开始消失的 温度/℃ | NH3产率/% | CO2产率/% |
|---|---|---|---|---|---|---|
| 0 | N2+H2O | 217 | 282 | 300 | 54.5 | 57.3 |
| 0 | 5%O2+N2+H2O | 218 | 282 | 300 | 54.8 | 57.3 |
| 6.2 | N2+H2O | 162 | 253 | 300 | 52.6 | 64.0 |
| 5.9 | 5%O2+N2+H2O | 160 | 253 | 300 | 34.1 | 66.8 |
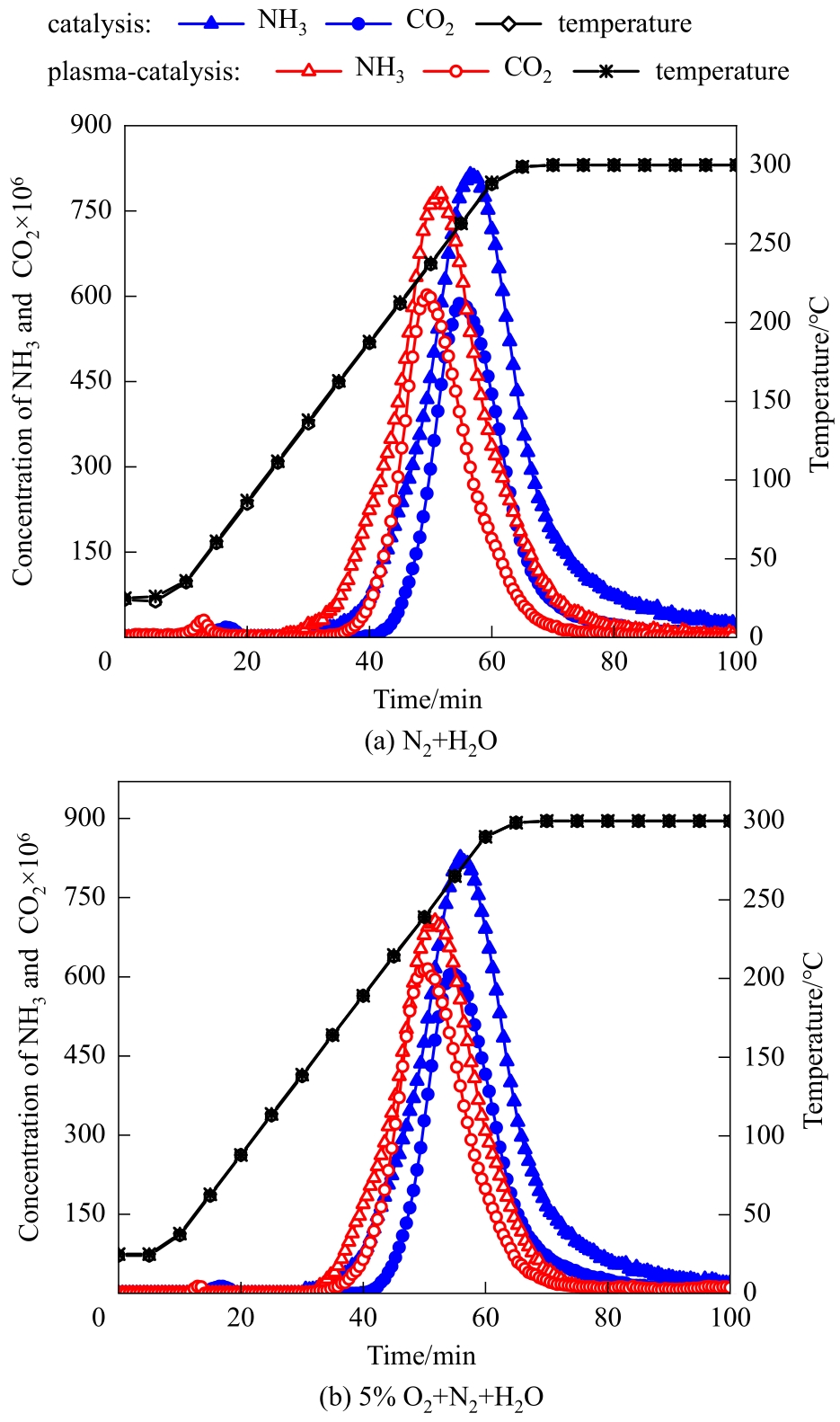
Fig.4 Hydrolysis curves of melamine by catalysis and plasma-enhanced catalysis in N2+H2O and 5%O2+N2+H2O (average discharge power: 5.5 W and 5.3 W without and with O2, respectively; discharge period: 0—85 min)
| 平均放电功率/W | 载气气氛 | CO2开始出现的 温度/℃ | CO2达到峰值浓度时的温度/℃ | CO2开始消失的 温度/℃ | NH3产率/% | CO2产率/% |
|---|---|---|---|---|---|---|
| 0 | N2+H2O | 199 | 263 | 300 | 68.1 | 73.0 |
| 0 | 5%O2+N2+H2O | 201 | 262 | 300 | 66.7 | 74.9 |
| 5.5 | N2+H2O | 171 | 235 | 300 | 60.7 | 71.5 |
| 5.3 | 5%O2+N2+H2O | 166 | 242 | 300 | 50.9 | 77.1 |
Table 3 Results of melamine hydrolysis by catalysis and plasma-enhanced catalysis
| 平均放电功率/W | 载气气氛 | CO2开始出现的 温度/℃ | CO2达到峰值浓度时的温度/℃ | CO2开始消失的 温度/℃ | NH3产率/% | CO2产率/% |
|---|---|---|---|---|---|---|
| 0 | N2+H2O | 199 | 263 | 300 | 68.1 | 73.0 |
| 0 | 5%O2+N2+H2O | 201 | 262 | 300 | 66.7 | 74.9 |
| 5.5 | N2+H2O | 171 | 235 | 300 | 60.7 | 71.5 |
| 5.3 | 5%O2+N2+H2O | 166 | 242 | 300 | 50.9 | 77.1 |

Fig.6 Concentration curves of N2O, NO and NO2 generated during discharge with TiO2 alone and during plasma-enhanced catalytic hydrolysis of biuret, cyanuric acid and melamine (discharge period: 0—85 min)
| 1 | Goldbach M, Roppertz A, Langenfeld P, et al. Urea decomposition in selective catalytic reduction on V2O5/WO3/TiO2 catalyst in diesel exhaust[J]. Chemical Engineering & Technology, 2017, 40(11): 2035-2043. |
| 2 | Reşitoğlu İ A, Altinişik K, Keskin A. The pollutant emissions from diesel-engine vehicles and exhaust after treatment systems[J]. Clean Technologies and Environmental Policy, 2015, 17(1): 15-27. |
| 3 | Pronobis M. Reduction of nitrogen oxide emissions[M]//Environmentally Oriented Modernization of Power Boilers. Amsterdam: Elsevier, 2020: 79-133. |
| 4 | Burr M, Gregory C. Vehicular exhausts[M]//Encyclopedia of Environmental Health. 2nd ed. Amsterdam: Elsevier, 2011: 335-343. |
| 5 | Lambert C K. Perspective on SCR NOx control for diesel vehicles[J]. Reaction Chemistry & Engineering, 2019, 4(6): 969-974. |
| 6 | Biswas S, Verma V, Schauer J J, et al. Chemical speciation of PM emissions from heavy-duty diesel vehicles equipped with diesel particulate filter (DPF) and selective catalytic reduction (SCR) retrofits[J]. Atmospheric Environment, 2009, 43(11): 1917-1925. |
| 7 | Yuan X M, Liu H Q, Gao Y. Diesel engine SCR control: current development and future challenges[J]. Emission Control Science and Technology, 2015, 1(2): 121-133. |
| 8 | Bernhard A M, Peitz D, Elsener M, et al. Catalytic urea hydrolysis in the selective catalytic reduction of NOx: catalyst screening and kinetics on anatase TiO2 and ZrO2[J]. Catalysis Science & Technology, 2013, 3(4): 942-951. |
| 9 | Ma Y, Wu X D, Zhang J Y, et al. Urea-related reactions and their active sites over Cu-SAPO-34: formation of NH3 and conversion of HNCO[J]. Applied Catalysis B: Environmental, 2018, 227: 198-208. |
| 10 | Todorova T, Peitz D, Kröcher O, et al. Guanidinium formate decomposition on the (101) TiO2-anatase surface: combined minimum energy reaction pathway calculations and temperature-programmed decomposition experiments[J]. The Journal of Physical Chemistry C, 2011, 115(4): 1195-1203. |
| 11 | Fan X, Kang S J, Li J. Plasma-enhanced hydrolysis of urea and SCR of NOx over V2O5-MoO3/TiO2: decrease of reaction temperature and increase of NOx conversion[J]. Fuel, 2020, 277: 118155. |
| 12 | Bernhard A M, Czekaj I, Elsener M, et al. Adsorption and catalytic thermolysis of gaseous urea on anatase TiO2 studied by HPLC analysis, DRIFT spectroscopy and DFT calculations[J]. Applied Catalysis B: Environmental, 2013, 134/135: 316-323. |
| 13 | Koebel M, Strutz E O. Thermal and hydrolytic decomposition of urea for automotive selective catalytic reduction systems: thermochemical and practical aspects[J]. Industrial & Engineering Chemistry Research, 2003, 42(10): 2093-2100. |
| 14 | Strots V O, Santhanam S, Adelman B J, et al. Deposit formation in urea-SCR systems[J]. SAE International Journal of Fuels and Lubricants, 2010, 2(2): 283-289. |
| 15 | Brack W, Heine B, Birkhold F, et al. Formation of urea-based deposits in an exhaust system: numerical predictions and experimental observations on a hot gas test bench[J]. Emission Control Science and Technology, 2016, 2(3): 115-123. |
| 16 | Ebrahimian V, Nicolle A, Habchi C. Detailed modeling of the evaporation and thermal decomposition of urea-water solution in SCR systems[J]. AIChE Journal, 2012, 58(7): 1998-2009. |
| 17 | Schaber P M, Colson J, Higgins S, et al. Study of the urea thermal decomposition (pyrolysis) reaction and importance to cyanuric acid production[J]. American Laboratory, 1999, 31(16): 13-21. |
| 18 | Schaber P M, Colson J, Higgins S, et al. Thermal decomposition (pyrolysis) of urea in an open reaction vessel[J]. Thermochimica Acta, 2004, 424(1/2): 131-142. |
| 19 | Bann B, Miller S A. Melamine and derivatives of melamine[J]. Chemical Reviews, 1958, 58(1): 131-172. |
| 20 | Eichelbaum M, Siemer A B, Farrauto R J, et al. The impact of urea on the performance of metal-exchanged zeolites for the selective catalytic reduction of NOx(Part Ⅱ): Catalytic, FTIR, and NMR studies[J]. Applied Catalysis B: Environmental, 2010, 97(1/2): 98-107. |
| 21 | Koebel M, Elsener M. Determination of urea and its thermal decomposition products by high-performance liquid chromatography[J]. Journal of Chromatography A, 1995, 689(1): 164-169. |
| 22 | Kröcher O, Elsener M. Materials for thermohydrolysis of urea in a fluidized bed[J]. Chemical Engineering Journal, 2009, 152(1): 167-176. |
| 23 | Xu L F, Watkins W, Snow R, et al. Laboratory and engine study of urea-related deposits in diesel urea-SCR after-treatment systems[R]. SAETechnical Paper Series. Warrendale, PA, United States: SAE International, 2007: 2007-01-1582. |
| 24 | 余俊波, 莫春兰, 黄文君, 等. 柴油机SCR系统尿素沉积物详细反应路径[J]. 内燃机学报, 2020, 38(2): 169-177. |
| Yu J B, Mo C L, Huang W J, et al. Detailed reaction pathways of urea deposit in diesel engine with selective catalytic reduction system[J]. Transactions of CSICE, 2020, 38(2): 169-177. | |
| 25 | Fang H L, DaCosta H F M. Urea thermolysis and NOx reduction with and without SCR catalysts[J]. Applied Catalysis B: Environmental, 2003, 46(1): 17-34. |
| 26 | Zhan Z Q, Müllner M, Lercher J A. Catalytic hydrolysis of s-triazine compounds over Al2O3[J]. Catalysis Today, 1996, 27(1/2): 167-173. |
| 27 | Bernhard A M, Peitz D, Elsener M, et al. Hydrolysis and thermolysis of urea and its decomposition byproducts biuret, cyanuric acid and melamine over anatase TiO2[J]. Applied Catalysis B: Environmental, 2012, 115/116: 129-137. |
| 28 | Mizuno A. Generation of non-thermal plasma combined with catalysts and their application in environmental technology[J]. Catalysis Today, 2013, 211: 2-8. |
| 29 | Chang J S. Physics and chemistry of plasma pollution control technology[J]. Plasma Sources Science and Technology, 2008, 17(4): 045004. |
| 30 | Kim H H, Ogata A. Nonthermal plasma activates catalyst: from current understanding and future prospects[J]. The European Physical Journal Applied Physics, 2011, 55(1): 13806. |
| 31 | Wang H, Cao Y Y, Chen Z W, et al. High-efficiency removal of NOxover natural mordenite using an enhanced plasma-catalytic process at ambient temperature[J]. Fuel, 2018, 224: 323-330. |
| 32 | Wang Y L, Craven M, Yu X T, et al. Plasma-enhanced catalytic synthesis of ammonia over a Ni/Al2O3 catalyst at near-room temperature: insights into the importance of the catalyst surface on the reaction mechanism[J]. ACS Catalysis, 2019, 9(12): 10780-10793. |
| [1] | Baomin DAI, Qilong WANG, Shengchun LIU, Jianing ZHANG, Xinhai LI, Fandi ZONG. Thermodynamic performance analysis of combined cooling and heating system based on combination of CO2 with the zeotropic refrigerant assisted subcooled [J]. CIESC Journal, 2023, 74(S1): 64-73. |
| [2] | Yepin CHENG, Daqing HU, Yisha XU, Huayan LIU, Hanfeng LU, Guokai CUI. Application of ionic liquid-based deep eutectic solvents for CO2 conversion [J]. CIESC Journal, 2023, 74(9): 3640-3653. |
| [3] | Yihao ZHANG, Zhenlei WANG. Fault detection using grouped support vector data description based on maximum information coefficient [J]. CIESC Journal, 2023, 74(9): 3865-3878. |
| [4] | Manzheng ZHANG, Meng XIAO, Peiwei YAN, Zheng MIAO, Jinliang XU, Xianbing JI. Working fluid screening and thermodynamic optimization of hazardous waste incineration coupled organic Rankine cycle system [J]. CIESC Journal, 2023, 74(8): 3502-3512. |
| [5] | Yaxin CHEN, Hang YUAN, Guanzhang LIU, Lei MAO, Chun YANG, Ruifang ZHANG, Guangya ZHANG. Advances in enzyme self-immobilization mediated by protein nanocages [J]. CIESC Journal, 2023, 74(7): 2773-2782. |
| [6] | Xiaoling TANG, Jiarui WANG, Xuanye ZHU, Renchao ZHENG. Biosynthesis of chiral epichlorohydrin by halohydrin dehalogenase based on Pickering emulsion system [J]. CIESC Journal, 2023, 74(7): 2926-2934. |
| [7] | Tan ZHANG, Guang LIU, Jinping LI, Yuhan SUN. Performance regulation strategies of Ru-based nitrogen reduction electrocatalysts [J]. CIESC Journal, 2023, 74(6): 2264-2280. |
| [8] | Maolin DONG, Lidong CHEN, Liulian HUANG, Weibing WU, Hongqi DAI, Huiyang BIAN. Research progress in preparation of lignonanocellulose by acid hydrotropes and their functional applications [J]. CIESC Journal, 2023, 74(6): 2281-2295. |
| [9] | Lei MAO, Guanzhang LIU, Hang YUAN, Guangya ZHANG. Efficient preparation of carbon anhydrase nanoparticles capable of capturing CO2 and their characteristics [J]. CIESC Journal, 2023, 74(6): 2589-2598. |
| [10] | Zijian WANG, Ming KE, Jiahan LI, Shuting LI, Jinru SUN, Yanbing TONG, Zhiping ZHAO, Jiaying LIU, Lu REN. Progress in preparation and application of short b-axis ZSM-5 molecular sieve [J]. CIESC Journal, 2023, 74(4): 1457-1473. |
| [11] | Xiaoyong GAO, Fuyu HUANG, Wanpeng ZHENG, Diao PENG, Yixu YANG, Dexian HUANG. Scheduling optimization of refinery and chemical production process considering the safety and stability of scheduling operation [J]. CIESC Journal, 2023, 74(4): 1619-1629. |
| [12] | Tianhao BAI, Xiaowen WANG, Mengzi YANG, Xinwei DUAN, Jie MI, Mengmeng WU. Study on release and inhibition behavior of COS during high-temperature gas desulfurization process using Zn-based oxide derived from hydrotalcite [J]. CIESC Journal, 2023, 74(4): 1772-1780. |
| [13] | Dingping LIU, Aihua CHEN, Xiangyang ZHANG, Wenhao HE, Hai WANG. Study on semi dry hydrolytic denitrification of aluminum ash [J]. CIESC Journal, 2023, 74(3): 1294-1302. |
| [14] | Yin XU, Jie CAI, Lu CHEN, Yu PENG, Fuzhen LIU, Hui ZHANG. Advances in heterogeneous visible light photocatalysis coupled with persulfate activation for water pollution control [J]. CIESC Journal, 2023, 74(3): 995-1009. |
| [15] | Runzhu LIU, Tiantian CHU, Xiaoa ZHANG, Chengzhong WANG, Junying ZHANG. Synthesis and properties of phenylene-containing α,ω-hydroxy-terminated fluorosilicone polymers [J]. CIESC Journal, 2023, 74(3): 1360-1369. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
