CIESC Journal ›› 2022, Vol. 73 ›› Issue (1): 322-331.DOI: 10.11949/0438-1157.20211441
• Separation engineering • Previous Articles Next Articles
Zixuan HUANG1( ),Huan CHEN1(
),Huan CHEN1( ),Hai LI1,2(
),Hai LI1,2( ),Minglong WANG1,Guangjin CHEN1,Bei LIU1
),Minglong WANG1,Guangjin CHEN1,Bei LIU1
Received:2021-10-09
Revised:2021-11-27
Online:2022-01-18
Published:2022-01-05
Contact:
Hai LI
黄子轩1( ),陈欢1(
),陈欢1( ),李海1,2(
),李海1,2( ),王明龙1,陈光进1,刘蓓1
),王明龙1,陈光进1,刘蓓1
通讯作者:
李海
作者简介:黄子轩(1996—),男,博士研究生,基金资助:CLC Number:
Zixuan HUANG, Huan CHEN, Hai LI, Minglong WANG, Guangjin CHEN, Bei LIU. Process simulation and energy consumption analysis of CO2/N2 pilot-scale separation using ZIF-8 slurry[J]. CIESC Journal, 2022, 73(1): 322-331.
黄子轩, 陈欢, 李海, 王明龙, 陈光进, 刘蓓. ZIF-8浆液中试分离CO2/N2过程模拟及能耗分析[J]. 化工学报, 2022, 73(1): 322-331.
Add to citation manager EndNote|Ris|BibTeX
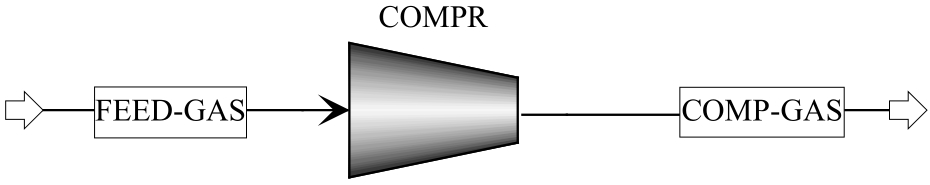
Fig.4 Computation module of Wcompr: gas compression module in the Aspen Plus (COMPR); gas stream before compression (FEED-GAS); gas stream after compression (COMP-GAS)

Fig.5 Computation module of Wmet: metering pump module in the Aspen Plus (MET-PUMP); lean slurry under desorption pressure (SLU-IN); lean slurry under sorption pressure (SLU-OUT)
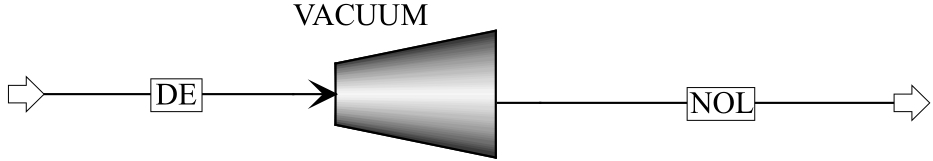
Fig.6 Computation module of Wvac: vacuum pump module in the Aspen Plus (VACUUM); gas stream under normal pressure (DE); gas stream under desorption pressure (NOL)

Fig.7 Computation module of Qheat: heat exchanger module in the Aspen Plus (HEATX); lean slurry from desorption tower to heat exchanger (LEAN-IN); lean slurry from heat exchanger to sorption tower (LEAN-OUT); rich slurry from the sorption tower to heat exchanger (RICH-IN); rich slurry from heat exchanger to heater (RICH-OUT); rich slurry from heater to desorption tower (RICH)

Fig.8 Sorption isotherms of CO2 at 303.15 K in fresh ZIF-8 slurry (a), the ZIF-8 slurry obtained from the desorption packed tower(desorption condition: the desorption temperature, pressure, and air-purge flow rate were fixed at 333.15 K, 0.08 MPa, and 200 L/h)(b); Sorption isotherm of N2 at 303.15 K in fresh ZIF-8 slurry (c)[33]
| ZIF-8浆液 | kCO2=(mT+n)p+MT+N | |||
|---|---|---|---|---|
| m | n | M | N | |
| 新鲜ZIF-8浆液 | -0.01148 | 4.20695 | 0.00029 | -0.54962 |
| 从中试解吸塔底获得的ZIF-8浆液① | -0.01776 | 5.82127 | 0.00156 | -0.88403 |
Table 1 The relationship between temperature, pressure, and the binary interaction parameter kCO2 of CO2 and ZIF-8 slurry
| ZIF-8浆液 | kCO2=(mT+n)p+MT+N | |||
|---|---|---|---|---|
| m | n | M | N | |
| 新鲜ZIF-8浆液 | -0.01148 | 4.20695 | 0.00029 | -0.54962 |
| 从中试解吸塔底获得的ZIF-8浆液① | -0.01776 | 5.82127 | 0.00156 | -0.88403 |
| T/K | kCO2 | pE/MPa | xcal | xexp | AADx/% |
|---|---|---|---|---|---|
| 293.15 | -0.4596 | 0.007 | 0.0067 | 0.0078 | 13.40 |
| 293.15 | -0.4503 | 0.018 | 0.0145 | 0.0137 | 5.24 |
| 293.15 | -0.4352 | 0.036 | 0.0223 | 0.0203 | 9.66 |
| 293.15 | -0.4032 | 0.074 | 0.0281 | 0.0265 | 6.15 |
| 303.15 | -0.4575 | 0.007 | 0.0046 | 0.0054 | 13.52 |
| 303.15 | -0.4495 | 0.018 | 0.0104 | 0.0103 | 0.55 |
| 303.15 | -0.4292 | 0.046 | 0.0191 | 0.0179 | 6.82 |
| 303.15 | -0.4045 | 0.08 | 0.0233 | 0.0230 | 1.30 |
| 303.15 | -0.3870 | 0.104 | 0.0237 | 0.0262 | 9.40 |
| 313.15 | -0.4530 | 0.011 | 0.0050 | 0.0050 | 1.15 |
| 313.15 | -0.4444 | 0.025 | 0.0098 | 0.0098 | 0.18 |
| 313.15 | -0.4334 | 0.043 | 0.0143 | 0.0144 | 0.75 |
| 313.15 | -0.4114 | 0.079 | 0.0193 | 0.0187 | 3.01 |
| 313.15 | -0.3906 | 0.113 | 0.0209 | 0.0215 | 3.03 |
| 总AADx | 5.30 |
Table 2 Experimental and simulated phase equilibrium data of CO2 absorption by fresh ZIF-8 slurry
| T/K | kCO2 | pE/MPa | xcal | xexp | AADx/% |
|---|---|---|---|---|---|
| 293.15 | -0.4596 | 0.007 | 0.0067 | 0.0078 | 13.40 |
| 293.15 | -0.4503 | 0.018 | 0.0145 | 0.0137 | 5.24 |
| 293.15 | -0.4352 | 0.036 | 0.0223 | 0.0203 | 9.66 |
| 293.15 | -0.4032 | 0.074 | 0.0281 | 0.0265 | 6.15 |
| 303.15 | -0.4575 | 0.007 | 0.0046 | 0.0054 | 13.52 |
| 303.15 | -0.4495 | 0.018 | 0.0104 | 0.0103 | 0.55 |
| 303.15 | -0.4292 | 0.046 | 0.0191 | 0.0179 | 6.82 |
| 303.15 | -0.4045 | 0.08 | 0.0233 | 0.0230 | 1.30 |
| 303.15 | -0.3870 | 0.104 | 0.0237 | 0.0262 | 9.40 |
| 313.15 | -0.4530 | 0.011 | 0.0050 | 0.0050 | 1.15 |
| 313.15 | -0.4444 | 0.025 | 0.0098 | 0.0098 | 0.18 |
| 313.15 | -0.4334 | 0.043 | 0.0143 | 0.0144 | 0.75 |
| 313.15 | -0.4114 | 0.079 | 0.0193 | 0.0187 | 3.01 |
| 313.15 | -0.3906 | 0.113 | 0.0209 | 0.0215 | 3.03 |
| 总AADx | 5.30 |
| T/K | kCO2 | pE/MPa | xcal | xexp | AADx/% |
|---|---|---|---|---|---|
| 293.15 | -0.4224 | 0.007 | 0.0039 | 0.0044 | 12.75 |
| 293.15 | -0.4113 | 0.025 | 0.0113 | 0.0096 | 17.46 |
| 293.15 | -0.3990 | 0.045 | 0.0166 | 0.0154 | 8.32 |
| 293.15 | -0.3751 | 0.084 | 0.0215 | 0.0207 | 3.65 |
| 293.15 | -0.3615 | 0.106 | 0.0222 | 0.0227 | 2.38 |
| 303.15 | -0.4067 | 0.01 | 0.0032 | 0.0035 | 7.90 |
| 303.15 | -0.3997 | 0.026 | 0.0074 | 0.0072 | 1.95 |
| 303.15 | -0.3932 | 0.041 | 0.0105 | 0.0112 | 5.88 |
| 303.15 | -0.3809 | 0.069 | 0.0147 | 0.0157 | 6.37 |
| 303.15 | -0.3648 | 0.106 | 0.0179 | 0.0190 | 5.77 |
| 313.15 | -0.3911 | 0.017 | 0.0033 | 0.0033 | 1.10 |
| 313.15 | -0.3875 | 0.031 | 0.0057 | 0.0054 | 4.66 |
| 313.15 | -0.3838 | 0.045 | 0.0078 | 0.0076 | 2.91 |
| 313.15 | -0.3797 | 0.061 | 0.0099 | 0.0098 | 1.65 |
| 313.15 | -0.3724 | 0.089 | 0.0131 | 0.0127 | 3.19 |
| 313.15 | -0.3690 | 0.102 | 0.0143 | 0.0141 | 1.56 |
| 总AADx | 5.47 |
Table 3 Experimental and simulated phase equilibrium data of CO2 absorption by ZIF-8 slurry obtained from the desorption packed tower
| T/K | kCO2 | pE/MPa | xcal | xexp | AADx/% |
|---|---|---|---|---|---|
| 293.15 | -0.4224 | 0.007 | 0.0039 | 0.0044 | 12.75 |
| 293.15 | -0.4113 | 0.025 | 0.0113 | 0.0096 | 17.46 |
| 293.15 | -0.3990 | 0.045 | 0.0166 | 0.0154 | 8.32 |
| 293.15 | -0.3751 | 0.084 | 0.0215 | 0.0207 | 3.65 |
| 293.15 | -0.3615 | 0.106 | 0.0222 | 0.0227 | 2.38 |
| 303.15 | -0.4067 | 0.01 | 0.0032 | 0.0035 | 7.90 |
| 303.15 | -0.3997 | 0.026 | 0.0074 | 0.0072 | 1.95 |
| 303.15 | -0.3932 | 0.041 | 0.0105 | 0.0112 | 5.88 |
| 303.15 | -0.3809 | 0.069 | 0.0147 | 0.0157 | 6.37 |
| 303.15 | -0.3648 | 0.106 | 0.0179 | 0.0190 | 5.77 |
| 313.15 | -0.3911 | 0.017 | 0.0033 | 0.0033 | 1.10 |
| 313.15 | -0.3875 | 0.031 | 0.0057 | 0.0054 | 4.66 |
| 313.15 | -0.3838 | 0.045 | 0.0078 | 0.0076 | 2.91 |
| 313.15 | -0.3797 | 0.061 | 0.0099 | 0.0098 | 1.65 |
| 313.15 | -0.3724 | 0.089 | 0.0131 | 0.0127 | 3.19 |
| 313.15 | -0.3690 | 0.102 | 0.0143 | 0.0141 | 1.56 |
| 总AADx | 5.47 |

Fig.10 Sorption heat of ZIF-8 slurry in fresh ZIF-8 slurry (a) and the ZIF-8 slurry obtained from the desorption packed tower (the desorption temperature, pressure, and air-purge flow rate were fixed at 333.15 K, 0.08 MPa, and 200 L/h) (b)
| 理论塔板级数 | 气相中CO2浓度,y/% | 液相中CO2浓度,x/% | 气相中CO2分压,p/MPa | 平衡级温度,T/K | CO2-浆液二元交互作用参数,kCO2 |
|---|---|---|---|---|---|
| 塔顶(净化气/贫液) | 0 | 0 | 0 | 303.15 | -0.4626 |
| 2 | 0.0001 | 0 | 0 | 303.15 | -0.4626 |
| 3 | 0.0005 | 0 | 0 | 303.15 | -0.4626 |
| 4 | 0.0034 | 0.0002 | 0 | 303.15 | -0.4626 |
| 5 | 0.0235 | 0.0015 | 0.0001 | 303.16 | -0.4625 |
| 6 | 0.1612 | 0.0105 | 0.001 | 303.22 | -0.4619 |
| 7 | 1.078 | 0.0706 | 0.0065 | 303.6 | -0.4578 |
| 8 | 6.2328 | 0.4297 | 0.0374 | 305.9 | -0.4358 |
| 塔底(原料气/富液) | 20 | 1.5859 | 0.12 | 313.3 | -0.3865 |
Table 4 The simulation results of multi-stage CO2 absorption by fresh ZIF-8 slurry
| 理论塔板级数 | 气相中CO2浓度,y/% | 液相中CO2浓度,x/% | 气相中CO2分压,p/MPa | 平衡级温度,T/K | CO2-浆液二元交互作用参数,kCO2 |
|---|---|---|---|---|---|
| 塔顶(净化气/贫液) | 0 | 0 | 0 | 303.15 | -0.4626 |
| 2 | 0.0001 | 0 | 0 | 303.15 | -0.4626 |
| 3 | 0.0005 | 0 | 0 | 303.15 | -0.4626 |
| 4 | 0.0034 | 0.0002 | 0 | 303.15 | -0.4626 |
| 5 | 0.0235 | 0.0015 | 0.0001 | 303.16 | -0.4625 |
| 6 | 0.1612 | 0.0105 | 0.001 | 303.22 | -0.4619 |
| 7 | 1.078 | 0.0706 | 0.0065 | 303.6 | -0.4578 |
| 8 | 6.2328 | 0.4297 | 0.0374 | 305.9 | -0.4358 |
| 塔底(原料气/富液) | 20 | 1.5859 | 0.12 | 313.3 | -0.3865 |
| 理论塔板级数 | 气相中CO2浓度,y/% | 液相中CO2浓度,x/% | 气相中CO2分压,p/MPa | 平衡级温度,T/K | CO2-浆液二元交互作用参数,kCO2 |
|---|---|---|---|---|---|
| 塔顶(净化气/贫液) | 0.0429 | 0 | 0.0003 | 303.15 | -0.411 |
| 2 | 0.1794 | 0.0089 | 0.0011 | 303.2 | -0.4106 |
| 3 | 0.6083 | 0.0369 | 0.0036 | 303.38 | -0.4092 |
| 4 | 1.894 | 0.1223 | 0.0114 | 303.93 | -0.4051 |
| 5 | 5.2582 | 0.3561 | 0.0315 | 305.42 | -0.395 |
| 6 | 11.503 | 0.8351 | 0.069 | 308.49 | -0.3791 |
| 7 | 17.4369 | 1.3552 | 0.1046 | 311.81 | -0.3679 |
| 8 | 19.5741 | 1.5612 | 0.1174 | 313.13 | -0.365 |
| 塔底(原料气/富液) | 20 | 1.5832 | 0.12 | 313.27 | -0.3644 |
Table 5 The simulation results of multi-stage CO2 absorption by ZIF-8 slurry obtained from the desorption packed tower
| 理论塔板级数 | 气相中CO2浓度,y/% | 液相中CO2浓度,x/% | 气相中CO2分压,p/MPa | 平衡级温度,T/K | CO2-浆液二元交互作用参数,kCO2 |
|---|---|---|---|---|---|
| 塔顶(净化气/贫液) | 0.0429 | 0 | 0.0003 | 303.15 | -0.411 |
| 2 | 0.1794 | 0.0089 | 0.0011 | 303.2 | -0.4106 |
| 3 | 0.6083 | 0.0369 | 0.0036 | 303.38 | -0.4092 |
| 4 | 1.894 | 0.1223 | 0.0114 | 303.93 | -0.4051 |
| 5 | 5.2582 | 0.3561 | 0.0315 | 305.42 | -0.395 |
| 6 | 11.503 | 0.8351 | 0.069 | 308.49 | -0.3791 |
| 7 | 17.4369 | 1.3552 | 0.1046 | 311.81 | -0.3679 |
| 8 | 19.5741 | 1.5612 | 0.1174 | 313.13 | -0.365 |
| 塔底(原料气/富液) | 20 | 1.5832 | 0.12 | 313.27 | -0.3644 |
| Test No. | Tde/K | pde/MPa | Vde-air/(L/h) | pab/MPa | Vin-mixgas/(L/h) | φ | Cout-CO2/% (mol) | ΔSV/(mol/L) | ηCO2/% | wtotal/(GJ/t CO2) |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 298 | 0.08 | 200 | 0.6 | 300 | 18 | 3.07 | 0.12 | 84.6 | 0.680 |
| S2 | 298 | 0.08 | 600 | 0.6 | 720 | 42 | 5.82 | 0.26 | 69.1 | 0.609 |
| S3 | 313 | 0.08 | 600 | 0.6 | 720 | 42 | 3.08 | 0.32 | 84.1 | 0.649 |
| S4 | 333 | 0.08 | 200 | 0.6 | 720 | 90 | 1.42 | 0.75 | 93.0 | 0.507 |
| S5 | 333 | 0.08 | 200 | 0.6 | 720 | 80 | 0.92 | 0.68 | 95.0 | 0.509 |
| S6 | 333 | 0.08 | 200 | 0.5 | 720 | 80 | 2.02 | 0.64 | 89.9 | 0.474 |
| SMEA | 333 | 0.08 | 200 | 0.6 | 720 | 90 | 3.39 | 0.66 | 82.5 | 0.957 |
Table 6 Effect of different operating conditions of ZIF-8 slurry on the CO2 capture equivalent work in the packed tower
| Test No. | Tde/K | pde/MPa | Vde-air/(L/h) | pab/MPa | Vin-mixgas/(L/h) | φ | Cout-CO2/% (mol) | ΔSV/(mol/L) | ηCO2/% | wtotal/(GJ/t CO2) |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 298 | 0.08 | 200 | 0.6 | 300 | 18 | 3.07 | 0.12 | 84.6 | 0.680 |
| S2 | 298 | 0.08 | 600 | 0.6 | 720 | 42 | 5.82 | 0.26 | 69.1 | 0.609 |
| S3 | 313 | 0.08 | 600 | 0.6 | 720 | 42 | 3.08 | 0.32 | 84.1 | 0.649 |
| S4 | 333 | 0.08 | 200 | 0.6 | 720 | 90 | 1.42 | 0.75 | 93.0 | 0.507 |
| S5 | 333 | 0.08 | 200 | 0.6 | 720 | 80 | 0.92 | 0.68 | 95.0 | 0.509 |
| S6 | 333 | 0.08 | 200 | 0.5 | 720 | 80 | 2.02 | 0.64 | 89.9 | 0.474 |
| SMEA | 333 | 0.08 | 200 | 0.6 | 720 | 90 | 3.39 | 0.66 | 82.5 | 0.957 |
| Test No. | wcompr/(GJ/t CO2) | wheat/(GJ/t CO2) | wpump/(GJ/t CO2) | wtotal/(GJ/t CO2) |
|---|---|---|---|---|
| S1 | 0.477 | 0.000 | 0.203 | 0.680 |
| S2 | 0.478 | 0.000 | 0.131 | 0.609 |
| S3 | 0.412 | 0.119 | 0.117 | 0.649 |
| S4 | 0.382 | 0.081 | 0.045 | 0.507 |
| S5 | 0.374 | 0.084 | 0.051 | 0.509 |
| S6 | 0.343 | 0.086 | 0.045 | 0.474 |
| SMEA | 0.418 | 0.177 | 0.052 | 0.957 |
Table 7 The composition of CO2 capture equivalent work in different operating conditions
| Test No. | wcompr/(GJ/t CO2) | wheat/(GJ/t CO2) | wpump/(GJ/t CO2) | wtotal/(GJ/t CO2) |
|---|---|---|---|---|
| S1 | 0.477 | 0.000 | 0.203 | 0.680 |
| S2 | 0.478 | 0.000 | 0.131 | 0.609 |
| S3 | 0.412 | 0.119 | 0.117 | 0.649 |
| S4 | 0.382 | 0.081 | 0.045 | 0.507 |
| S5 | 0.374 | 0.084 | 0.051 | 0.509 |
| S6 | 0.343 | 0.086 | 0.045 | 0.474 |
| SMEA | 0.418 | 0.177 | 0.052 | 0.957 |
| 1 | Shafiee S, Topal E. When will fossil fuel reserves be diminished? [J]. Energy Policy, 2009, 37(1): 181-189. |
| 2 | Raupach M R, Marland G, Ciais P, et al. Global and regional drivers of accelerating CO2 emissions[J]. PNAS, 2007, 104(24): 10288-10293. |
| 3 | Renfrew S E, Starr D E, Strasser P. Electrochemical approaches toward CO2 capture and concentration[J]. ACS Catalysis, 2020, 10(21): 13058-13074. |
| 4 |
Chen Y, Liu C, Guo S, et al. CO2 capture and conversion to value-added products promoted by MXene-based materials[J]. Green Energy & Environment, 2020. doi: 10.1016/j.gee.2020.11.008.
DOI |
| 5 | Nourouzi-Lavasani S, Larachi F, Benali M. Energy and hydrogen coproduction from (athabasca bitumen) coke gasification with CO2 capture[J]. Industrial & Engineering Chemistry Research, 2008, 47(18): 7118-7129. |
| 6 | Gui X, Tang Z G, Fei W Y. CO2 capture with physical solvent dimethyl carbonate at high pressures[J]. Journal of Chemical & Engineering Data, 2010, 55(9): 3736-3741. |
| 7 | Rayer A V, Henni A, Tontiwachwuthikul P. High pressure physical solubility of carbon dioxide (CO2) in mixed polyethylene glycol dimethyl ethers (Genosorb 1753)[J]. The Canadian Journal of Chemical Engineering, 2012, 90(3): 576-583. |
| 8 | Sun L, Smith R. Rectisol wash process simulation and analysis[J]. Journal of Cleaner Production, 2013, 39: 321-328. |
| 9 | Fauth D J, Frommell E A, Hoffman J S, et al. Eutectic salt promoted lithium zirconate: novel high temperature sorbent for CO2 capture[J]. Fuel Processing Technology, 2005, 86(14/15): 1503-1521. |
| 10 | Yeh J T, Resnik K P, Rygle K, et al. Semi-batch absorption and regeneration studies for CO2 capture by aqueous ammonia[J]. Fuel Processing Technology, 2005, 86(14/15): 1533-1546. |
| 11 | Oyevaar M H, Morssinkhof R W J, Westerterp K R. The kinetics of the reaction between CO2 and diethanolamine in aqueous ethyleneglycol at 298 K: a viscous gas-liquid reaction system for the determination of interfacial areas in gas-liquid contactors[J]. Chemical Engineering Science, 1990, 45(11): 3283-3298. |
| 12 | Chowdhury F A, Yamada H, Higashii T, et al. CO2 capture by tertiary amine absorbents: a performance comparison study[J]. Industrial & Engineering Chemistry Research, 2013, 52(24): 8323-8331. |
| 13 | Clausse M, Merel J, Meunier F. Numerical parametric study on CO2 capture by indirect thermal swing adsorption[J]. International Journal of Greenhouse Gas Control, 2011, 5(5): 1206-1213. |
| 14 | Wang L, Liu Z, Li P, et al. Experimental and modeling investigation on post-combustion carbon dioxide capture using zeolite 13X-APG by hybrid VTSA process[J]. Chemical Engineering Journal, 2012, 197: 151-161. |
| 15 | Das M, Koros W J. Performance of 6FDA-6FpDA polyimide for propylene/propane separations[J]. Journal of Membrane Science, 2010, 365(1/2): 399-408. |
| 16 | Liu Q, Wang N Y, Caro J, et al. Bio-inspired polydopamine: a versatile and powerful platform for covalent synthesis of molecular sieve membranes[J]. Journal of the American Chemical Society, 2013, 135(47): 17679-17682. |
| 17 | Kwon H T, Jeong H K, Lee A S, et al. Heteroepitaxially grown zeolitic imidazolate framework membranes with unprecedented propylene/propane separation performances[J]. Journal of the American Chemical Society, 2015, 137(38): 12304-12311. |
| 18 | Hart A, Gnanendran N. Cryogenic CO2 capture in natural gas[J]. Energy Procedia, 2009, 1(1): 697-706. |
| 19 | Liu H, Wang J, Chen G J, et al. High-efficiency separation of a CO2/H2 mixture via hydrate formation in W/O emulsions in the presence of cyclopentane and TBAB[J]. International Journal of Hydrogen Energy, 2014, 39(15): 7910-7918. |
| 20 | O'Reilly N, Giri N, James S. Porous liquids[J]. Chemistry-A European Journal, 2007, 13(11): 3020-3025. |
| 21 | Zhang J S, Chai S H, Qiao Z A, et al. Porous liquids: a promising class of media for gas separation[J]. Angewandte Chemie, 2015, 54(3): 932-936. |
| 22 | Knebel A, Bavykina A, Datta S J, et al. Solution processable metal-organic frameworks for mixed matrix membranes using porous liquids[J]. Nature Materials, 2020, 19(12): 1346-1353. |
| 23 | Banerjee R, Phan A, Wang B, et al. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture[J]. Science, 2008, 319(5865): 939-943. |
| 24 | Wang B, Côté A P, Furukawa H, et al. Colossal cages in zeolitic imidazolate frameworks as selective carbon dioxide reservoirs[J]. Nature, 2008, 453(7192): 207-211. |
| 25 | Li J R, Sculley J, Zhou H C. Metal-organic frameworks for separations[J]. Chemical Reviews, 2012, 112(2): 869-932. |
| 26 | Lei Z G, Dai C N, Song W J. Adsorptive absorption: a preliminary experimental and modeling study on CO2 solubility[J]. Chemical Engineering Science, 2015, 127: 260-268. |
| 27 | Liu H, Liu B, Lin L C, et al. A hybrid absorption-adsorption method to efficiently capture carbon[J]. Nat. Commun., 2014, 5: 5147. |
| 28 | Pan Y, Li H, Zhang X X, et al. Large-scale synthesis of ZIF-67 and highly efficient carbon capture using a ZIF-67/glycol-2-methylimidazole slurry[J]. Chemical Engineering Science, 2015, 137: 504-514. |
| 29 | Yang M K, Han Y, Zou E B, et al. Separation of IGCC syngas by using ZIF-8/dimethylacetamide slurry with high CO2 sorption capacity and sorption speed but low sorption heat[J]. Energy, 2020, 201: 117605. |
| 30 | Chen W, Zou E B, Zuo J Y, et al. Separation of ethane from natural gas using porous ZIF-8/water-glycol slurry[J]. Industrial & Engineering Chemistry Research, 2019, 58(23): 9997-10006. |
| 31 | Li H, Gao X T, Jia C Z, et al. Enrichment of hydrogen from a hydrogen/propylene gas mixture using ZIF-8/water-glycol slurry[J]. Energies, 2018, 11(7): 1890. |
| 32 | Chen W, Guo X N, Zou E B, et al. A continuous and high-efficiency process to separate coal bed methane with porous ZIF-8 slurry: experimental study and mathematical modelling[J]. Green Energy & Environment, 2020, 5(3): 347-363. |
| 33 | Li H, Liu B, Yang M K, et al. CO2 separation performance of zeolitic imidazolate framework-8 porous slurry in a pilot-scale packed tower[J]. Industrial & Engineering Chemistry Research, 2020, 59(13): 6154-6163. |
| 34 | Peng D Y, Robinson D B. A new two-constant equation of state[J]. Industrial & Engineering Chemistry Fundamentals, 1976, 15(1): 59-64. |
| 35 | Wang Z Q, Tanabe K, Cohen S. Tuning hydrogen sorption properties of metal-organic frameworks by postsynthetic covalent modification[J]. Chemistry-A European Journal, 2010, 16(1): 212-217. |
| 36 | House K Z, Harvey C F, Aziz M J, et al. The energy penalty of post-combustion CO2 capture & storage and its implications for retrofitting the US installed base[J]. Energy & Environmental Science, 2009, 2(2): 193. |
| [1] | Ruihang ZHANG, Pan CAO, Feng YANG, Kun LI, Peng XIAO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Analysis of key parameters affecting product purity of natural gas ethane recovery process via ZIF-8 nanofluid [J]. CIESC Journal, 2023, 74(8): 3386-3393. |
| [2] | Lei MAO, Guanzhang LIU, Hang YUAN, Guangya ZHANG. Efficient preparation of carbon anhydrase nanoparticles capable of capturing CO2 and their characteristics [J]. CIESC Journal, 2023, 74(6): 2589-2598. |
| [3] | Hao WANG, Siyang TANG, Shan ZHONG, Bin LIANG. An investigation of the enhancing effect of solid particle surface on the CO2 desorption behavior in chemical sorption process with MEA solution [J]. CIESC Journal, 2023, 74(4): 1539-1548. |
| [4] | Xuerong GU, Shuoshi LIU, Siyu YANG. Research on multi-parameter optimization method based on parallel EGO and surrogate-assisted model [J]. CIESC Journal, 2023, 74(3): 1205-1215. |
| [5] | Xiaowan PENG, Xiaonan GUO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Modeling and simulation of CH4/N2 separation process with two absorption-adsorption columns using ZIF-8 slurry [J]. CIESC Journal, 2023, 74(2): 784-795. |
| [6] | Xuqing WANG, Shenglin YAN, Litao ZHU, Xibao ZHANG, Zhenghong LUO. Research progress on the mass transfer process of CO2 absorption by amines in a packed column [J]. CIESC Journal, 2023, 74(1): 237-256. |
| [7] | Yingxi DANG, Peng TAN, Xiaoqin LIU, Linbing SUN. Temperature swing for CO2 capture driven by radiative cooling and solar heating [J]. CIESC Journal, 2023, 74(1): 469-478. |
| [8] | Mai ZHANG, Yao TIAN, Zhiqi GUO, Ye WANG, Guangjin DOU, Hao SONG. Design and optimization of photocatalysis-biological hybrid system for green synthesis of fuels and chemicals [J]. CIESC Journal, 2022, 73(7): 2774-2789. |
| [9] | Miao LI, Hong ZHAO, Biao JIANG, Siyuan CHEN, Long YAN. Thermodynamic analysis on synthesis of key intermediate BaC2 in coal to acetylene [J]. CIESC Journal, 2022, 73(5): 1908-1919. |
| [10] | Senshan CAO, Feng XU, Xionglin LUO. Process simulation of stream circulation system based on stability: [J]. CIESC Journal, 2022, 73(3): 1256-1269. |
| [11] | Qian LIU, Xianglan ZHANG, Zhiping LI, Zhuoqi LI, Hong YU. Multiscale screening of ionic liquids as extractive solvents for oil-hydroxybenzene separation [J]. CIESC Journal, 2022, 73(11): 5011-5024. |
| [12] | Li LIU, Peng JIANG, Wei WANG, Tonghuan ZHANG, Liwen MU, Xiaohua LU, Jiahua ZHU. Coupling process simulation and random forest model for analyzing and predicting biomass-to-hydrogen conversion [J]. CIESC Journal, 2022, 73(11): 5230-5239. |
| [13] | Guixian LI, Ke WANG, Jian WANG, Wenliang MENG, Jingwei LI, Yong YANG, Zongliang FAN, Dongliang WANG, Huairong ZHOU. Optimal design of membrane separation process for capturing CO2 from flue gas of coal-fired power plant [J]. CIESC Journal, 2022, 73(11): 5065-5077. |
| [14] | Xianhui ZHU, Fu WANG, Jiecheng XIA, Jinliang YUAN. Density functional theory investigation on the NH3 and CO2 absorption by functional ionic liquids [J]. CIESC Journal, 2022, 73(10): 4324-4334. |
| [15] | Dong JI, Jian WANG, Ke WANG, Jingwei LI, Wenliang MENG, Yong YANG, Guixian LI, Dongliang WANG, Huairong ZHOU. Process research of methanol production by CO2 coupled green hydrogen with different CO2 capture technologies [J]. CIESC Journal, 2022, 73(10): 4565-4575. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||