CIESC Journal ›› 2022, Vol. 73 ›› Issue (11): 5011-5024.DOI: 10.11949/0438-1157.20221062
• Separation engineering • Previous Articles Next Articles
Qian LIU( ), Xianglan ZHANG(
), Xianglan ZHANG( ), Zhiping LI, Zhuoqi LI, Hong YU
), Zhiping LI, Zhuoqi LI, Hong YU
Received:2022-07-28
Revised:2022-08-25
Online:2022-12-06
Published:2022-11-05
Contact:
Xianglan ZHANG
通讯作者:
张香兰
作者简介:刘潜(1995—),男,博士研究生,460905289@qq.com
基金资助:CLC Number:
Qian LIU, Xianglan ZHANG, Zhiping LI, Zhuoqi LI, Hong YU. Multiscale screening of ionic liquids as extractive solvents for oil-hydroxybenzene separation[J]. CIESC Journal, 2022, 73(11): 5011-5024.
刘潜, 张香兰, 李志平, 栗卓琦, 喻红. 油酚分离过程离子液体萃取溶剂的多尺度筛选[J]. 化工学报, 2022, 73(11): 5011-5024.
Add to citation manager EndNote|Ris|BibTeX
| Cations | Names | Abbreviations |
|---|---|---|
| [C01] | 1-ethyl-3-methyl-imidazolium | [C2mim]+ |
| [C02] | 1-propyl-3-methyl-imidazolium | [C3mim]+ |
| [C03] | 1-butyl-3-methyl-imidazolium | [C4mim]+ |
| [C04] | 1-pentyl-3-methyl-imidazolium | [C5mim]+ |
| [C05] | 1-hexyl-3-methyl-imidazolium | [C6mim]+ |
| [C06] | 1-ethyl-pyridinium | [C2py]+ |
| [C07] | 1-propyl-pyridinium | [C3py]+ |
| [C08] | 1-butyl-pyridinium | [C4py]+ |
| [C09] | 1-pentyl-pyridinium | [C5py]+ |
| [C10] | 1-hexyl-pyridinium | [C6py]+ |
| [C11] | 1-ethyl-1-methyl-pyrrolidinium | [C2mpyr]+ |
| [C12] | 1-propyl-1-methyl-pyrrolidinium | [C3mpyr]+ |
| [C13] | 1-butyl-1-methyl-pyrrolidinium | [C4mpyr]+ |
| [C14] | 1-pentyl-1-methyl-pyrrolidinium | [C5mpyr]+ |
| [C15] | 1-hexyl-1-methyl-pyrrolidinium | [C6mpyr]+ |
| [C16] | 1-ethyl-1-methyl-piperidinium | [C2mpip]+ |
| [C17] | 1-propyl-1-methyl-piperidinium | [C3mpip]+ |
| [C18] | 1-butyl-1-methyl-piperidinium | [C4mpip]+ |
| [C19] | 1-pentyl-1-methyl-piperidinium | [C5mpip]+ |
| [C20] | 1-hexyl-1-methyl-piperidinium | [C6mpip]+ |
| [C21] | 4-ethyl-4-methyl-morpholinium | [C2mmor]+ |
| [C22] | 4-propyl-4-methyl-morpholinium | [C3mmor]+ |
| [C23] | 4-butyl-4-methyl-morpholinium | [C4mmor]+ |
| [C24] | 4-pentyl-4-methyl-morpholinium | [C5mmor]+ |
| [C25] | 4-hexyl-4-methyl-morpholinium | [C6mmor]+ |
| [C26] | ethyl-trimethyl-ammonium | [N2111]+ |
| [C27] | propyl-trimethyl-ammonium | [N3111]+ |
| [C28] | butyl-trimethyl-ammonium | [N4111]+ |
| [C29] | pentyl-trimethyl-ammonium | [N5111]+ |
| [C30] | hexyl-trimethyl-ammonium | [N6111]+ |
Table 1 Names and abbreviations of cations
| Cations | Names | Abbreviations |
|---|---|---|
| [C01] | 1-ethyl-3-methyl-imidazolium | [C2mim]+ |
| [C02] | 1-propyl-3-methyl-imidazolium | [C3mim]+ |
| [C03] | 1-butyl-3-methyl-imidazolium | [C4mim]+ |
| [C04] | 1-pentyl-3-methyl-imidazolium | [C5mim]+ |
| [C05] | 1-hexyl-3-methyl-imidazolium | [C6mim]+ |
| [C06] | 1-ethyl-pyridinium | [C2py]+ |
| [C07] | 1-propyl-pyridinium | [C3py]+ |
| [C08] | 1-butyl-pyridinium | [C4py]+ |
| [C09] | 1-pentyl-pyridinium | [C5py]+ |
| [C10] | 1-hexyl-pyridinium | [C6py]+ |
| [C11] | 1-ethyl-1-methyl-pyrrolidinium | [C2mpyr]+ |
| [C12] | 1-propyl-1-methyl-pyrrolidinium | [C3mpyr]+ |
| [C13] | 1-butyl-1-methyl-pyrrolidinium | [C4mpyr]+ |
| [C14] | 1-pentyl-1-methyl-pyrrolidinium | [C5mpyr]+ |
| [C15] | 1-hexyl-1-methyl-pyrrolidinium | [C6mpyr]+ |
| [C16] | 1-ethyl-1-methyl-piperidinium | [C2mpip]+ |
| [C17] | 1-propyl-1-methyl-piperidinium | [C3mpip]+ |
| [C18] | 1-butyl-1-methyl-piperidinium | [C4mpip]+ |
| [C19] | 1-pentyl-1-methyl-piperidinium | [C5mpip]+ |
| [C20] | 1-hexyl-1-methyl-piperidinium | [C6mpip]+ |
| [C21] | 4-ethyl-4-methyl-morpholinium | [C2mmor]+ |
| [C22] | 4-propyl-4-methyl-morpholinium | [C3mmor]+ |
| [C23] | 4-butyl-4-methyl-morpholinium | [C4mmor]+ |
| [C24] | 4-pentyl-4-methyl-morpholinium | [C5mmor]+ |
| [C25] | 4-hexyl-4-methyl-morpholinium | [C6mmor]+ |
| [C26] | ethyl-trimethyl-ammonium | [N2111]+ |
| [C27] | propyl-trimethyl-ammonium | [N3111]+ |
| [C28] | butyl-trimethyl-ammonium | [N4111]+ |
| [C29] | pentyl-trimethyl-ammonium | [N5111]+ |
| [C30] | hexyl-trimethyl-ammonium | [N6111]+ |
| Anions | Names | Abbreviations |
|---|---|---|
| [A01] | acetate | [Ac]- |
| [A02] | chloride | [Cl]- |
| [A03] | bromide | [Br]- |
| [A04] | hydrogen sulfate | [HSO4]- |
| [A05] | methyl sulfate | [MeSO4]- |
| [A06] | ethyl sulfate | [EtSO4]- |
| [A07] | dicyanamide | [DCA]- |
| [A08] | thiocyanate | [SCN]- |
| [A09] | tricyanomethane | [C(CN)3]- |
| [A10] | nitrate | [NO3]- |
| [A11] | tetrafluoroborate | [BF4]- |
| [A12] | hexafluorophosphate | [PF6]- |
Table 2 Names and abbreviations of anions
| Anions | Names | Abbreviations |
|---|---|---|
| [A01] | acetate | [Ac]- |
| [A02] | chloride | [Cl]- |
| [A03] | bromide | [Br]- |
| [A04] | hydrogen sulfate | [HSO4]- |
| [A05] | methyl sulfate | [MeSO4]- |
| [A06] | ethyl sulfate | [EtSO4]- |
| [A07] | dicyanamide | [DCA]- |
| [A08] | thiocyanate | [SCN]- |
| [A09] | tricyanomethane | [C(CN)3]- |
| [A10] | nitrate | [NO3]- |
| [A11] | tetrafluoroborate | [BF4]- |
| [A12] | hexafluorophosphate | [PF6]- |
| IL | Tm/K | η/cP | ||||
|---|---|---|---|---|---|---|
| [C2mim][DCA] | 19.87 | 728.37 | 0.0281 | 7.66×10-4 | 275.58 | 19.91 |
| [C3mim][DCA] | 19.99 | 584.74 | 0.0375 | 1.32×10-3 | 271.82 | 23.23 |
| [C2py][DCA] | 14.53 | 825.36 | 0.0181 | 5.44×10-4 | 263.55 | 20.47 |
| [C3py][DCA] | 16.46 | 628.81 | 0.0284 | 1.07×10-3 | 259.79 | 23.88 |
| [C3mim][SCN] | 21.66 | 515.33 | 0.0429 | 1.33×10-3 | 297.27 | 52.05 |
| [C2py][SCN] | 21.08 | 686.24 | 0.0285 | 5.57×10-4 | 288.99 | 45.85 |
| [C3py][SCN] | 18.53 | 567.55 | 0.0334 | 1.09×10-3 | 285.24 | 53.51 |
| Glycol | 3.35 | 80.66 | 0.0508 | 1.44×10-3 | 260.25 | 17.33 |
Table 3 7 ionic liquids screened after the first two steps by infinite dilution thermodynamic properties and physical property constraints (with glycol as the benchmark)
| IL | Tm/K | η/cP | ||||
|---|---|---|---|---|---|---|
| [C2mim][DCA] | 19.87 | 728.37 | 0.0281 | 7.66×10-4 | 275.58 | 19.91 |
| [C3mim][DCA] | 19.99 | 584.74 | 0.0375 | 1.32×10-3 | 271.82 | 23.23 |
| [C2py][DCA] | 14.53 | 825.36 | 0.0181 | 5.44×10-4 | 263.55 | 20.47 |
| [C3py][DCA] | 16.46 | 628.81 | 0.0284 | 1.07×10-3 | 259.79 | 23.88 |
| [C3mim][SCN] | 21.66 | 515.33 | 0.0429 | 1.33×10-3 | 297.27 | 52.05 |
| [C2py][SCN] | 21.08 | 686.24 | 0.0285 | 5.57×10-4 | 288.99 | 45.85 |
| [C3py][SCN] | 18.53 | 567.55 | 0.0334 | 1.09×10-3 | 285.24 | 53.51 |
| Glycol | 3.35 | 80.66 | 0.0508 | 1.44×10-3 | 260.25 | 17.33 |
| IL | MW/(g·mol-1) | Tb/K | Tc/K | Pc/bar | Vc/(cm3·mol-1) | ω | ρ/(g·cm-3) |
|---|---|---|---|---|---|---|---|
| [C2mim][DCA] | 177.2 | 670.64 | 998.48 | 35.1 | 562.0 | 0.3548 | 1.0750 |
| [C2py][DCA] | 174.2 | 736.28 | 1123.34 | 32.6 | 571.1 | 0.2336 | 1.0243 |
| [C2py][SCN] | 166.2 | 676.19 | 926.58 | 29.0 | 554.7 | 0.6922 | 1.0970 |
Table 4 Molecular weights, normal boiling points, critical properties, acentric factors, and densities of 3 ionic liquids
| IL | MW/(g·mol-1) | Tb/K | Tc/K | Pc/bar | Vc/(cm3·mol-1) | ω | ρ/(g·cm-3) |
|---|---|---|---|---|---|---|---|
| [C2mim][DCA] | 177.2 | 670.64 | 998.48 | 35.1 | 562.0 | 0.3548 | 1.0750 |
| [C2py][DCA] | 174.2 | 736.28 | 1123.34 | 32.6 | 571.1 | 0.2336 | 1.0243 |
| [C2py][SCN] | 166.2 | 676.19 | 926.58 | 29.0 | 554.7 | 0.6922 | 1.0970 |
| IL | A0/(J·mol-1·K-1) | A1/(J·mol-1·K-2) | A2/(J·mol-1·K-3) | A3/(J·mol-1·K-4) |
|---|---|---|---|---|
| [C2mim][DCA] | 13.371 | 0.76460 | -3.728×10-4 | -3.68×10-8 |
| [C2py][DCA] | 20.691 | 0.66048 | -2.091×10-4 | -4.43×10-8 |
| [C2py][SCN] | -22.939 | 0.56668 | -7.310×10-5 | -8.73×10-8 |
Table 5 Polynomial coefficients of ideal gas heat capacity of 3 ionic liquids
| IL | A0/(J·mol-1·K-1) | A1/(J·mol-1·K-2) | A2/(J·mol-1·K-3) | A3/(J·mol-1·K-4) |
|---|---|---|---|---|
| [C2mim][DCA] | 13.371 | 0.76460 | -3.728×10-4 | -3.68×10-8 |
| [C2py][DCA] | 20.691 | 0.66048 | -2.091×10-4 | -4.43×10-8 |
| [C2py][SCN] | -22.939 | 0.56668 | -7.310×10-5 | -8.73×10-8 |
| Component | NRTL parameters/K | RMSD | ||||||
|---|---|---|---|---|---|---|---|---|
| i-j | aij | aji | bij | bji | cij | |||
| {[C2mim][DCA] (1) + m-cresol (2) + cumene (3)} | ||||||||
| 1-2 | 3.57 | -1.77 | 684.03 | -299.00 | 0.3 | 0.0139 | ||
| 1-3 | 2.48 | -3.42 | -108.54 | 2630.06 | 0.2 | |||
| 2-3 | 28.46 | -7.23 | 5067.29 | 2902.47 | 0.3 | |||
| {[C2py][DCA] (1) + m-cresol (2) + cumene (3)} | ||||||||
| 1-2 | 0.65 | -1.64 | 1459.52 | -351.76 | 0.3 | 0.0157 | ||
| 1-3 | 2.38 | -2.47 | 39.90 | 2356.43 | 0.2 | |||
| 2-3 | 37.04 | -6.78 | 5271.13 | 2685.83 | 0.3 | |||
| {[C2py][SCN] (1) + m-cresol (2) + cumene (3)} | ||||||||
| 1-2 | 4.18 | -0.87 | 974.17 | -637.33 | 0.3 | 0.0178 | ||
| 1-3 | 4.46 | -1.63 | -513.91 | 2097.21 | 0.2 | |||
| 2-3 | 15.66 | -5.45 | 5657.96 | 2101.41 | 0.3 | |||
Table 6 NRTL parameters and RMSD values of three {ionic liquid + m-cresol + cumene} ternary systems
| Component | NRTL parameters/K | RMSD | ||||||
|---|---|---|---|---|---|---|---|---|
| i-j | aij | aji | bij | bji | cij | |||
| {[C2mim][DCA] (1) + m-cresol (2) + cumene (3)} | ||||||||
| 1-2 | 3.57 | -1.77 | 684.03 | -299.00 | 0.3 | 0.0139 | ||
| 1-3 | 2.48 | -3.42 | -108.54 | 2630.06 | 0.2 | |||
| 2-3 | 28.46 | -7.23 | 5067.29 | 2902.47 | 0.3 | |||
| {[C2py][DCA] (1) + m-cresol (2) + cumene (3)} | ||||||||
| 1-2 | 0.65 | -1.64 | 1459.52 | -351.76 | 0.3 | 0.0157 | ||
| 1-3 | 2.38 | -2.47 | 39.90 | 2356.43 | 0.2 | |||
| 2-3 | 37.04 | -6.78 | 5271.13 | 2685.83 | 0.3 | |||
| {[C2py][SCN] (1) + m-cresol (2) + cumene (3)} | ||||||||
| 1-2 | 4.18 | -0.87 | 974.17 | -637.33 | 0.3 | 0.0178 | ||
| 1-3 | 4.46 | -1.63 | -513.91 | 2097.21 | 0.2 | |||
| 2-3 | 15.66 | -5.45 | 5657.96 | 2101.41 | 0.3 | |||
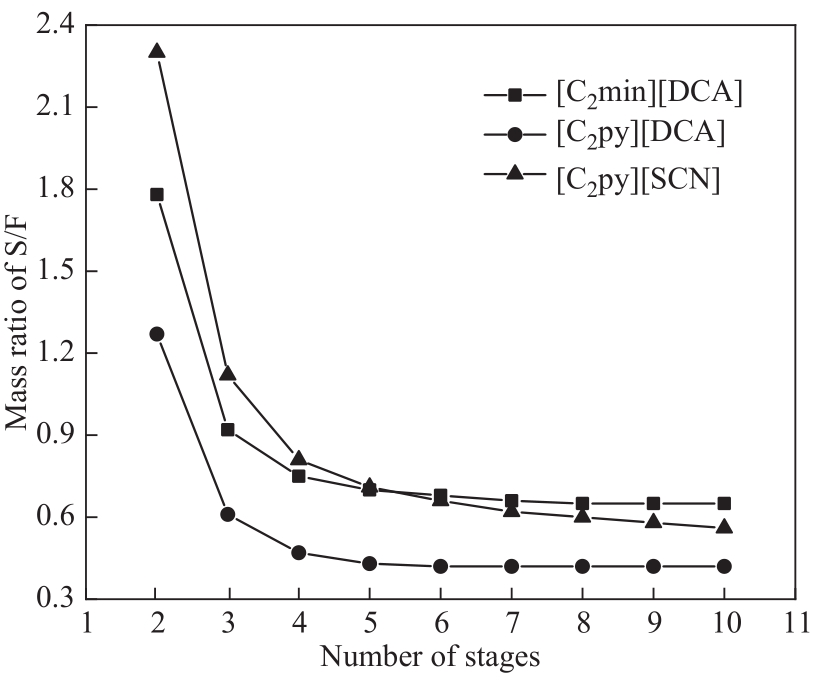
Fig.11 Mass ratio of solvent-to-feed (S/F) as a function of the number of stages in the extraction column to meet the separation requirements of m-cresol and cumene
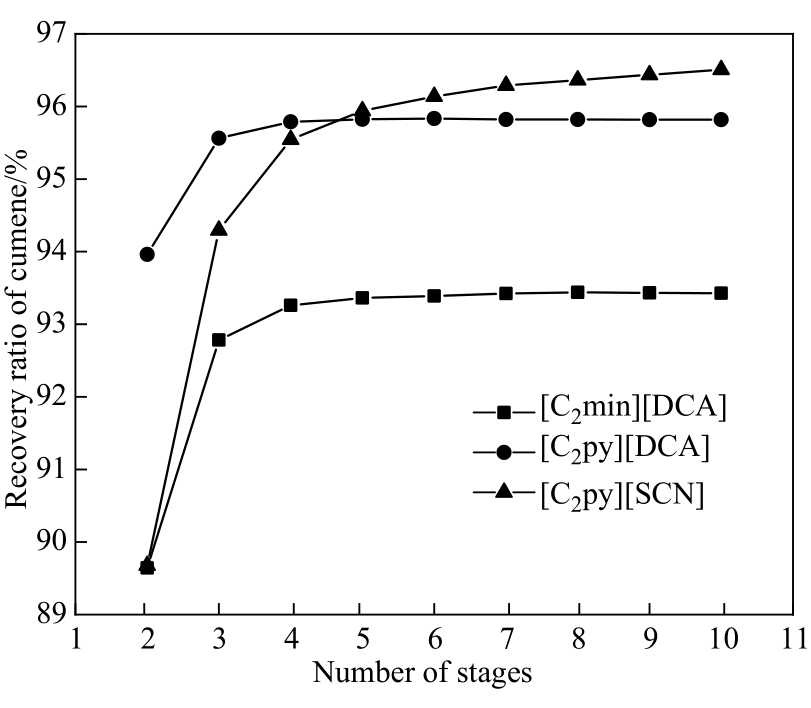
Fig.12 Recovery ratio of cumene as a function of the number of stages in the extraction column when meeting the separation requirements of m-cresol and cumene
Extraction column (298.15 K, 1 ×105 Pa, 8 stages) | Distillation column (0.005 ×105 Pa) | Cumene product | m-Cresol product | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL | IL makeup/ (kg·h-1) | IL in recycle/ (kg·h-1) | Operating conditions | Heat duty/ kW | Recovery ratio/ % | Mass purity/ % | Recovery ratio/ % | Mass purity/ % | ||||
| D∶F | NS | FS | RR | |||||||||
| [C2mim][DCA] | 1.63 | 6498.37 | 0.3479 | 8 | 3 | 0.42 | 1215.23 ① | 93.43 | 99.99 | 99.99 | 86.69 | |
| 0.9981 | 7 | 3 | 0.18 | 769.94 ② | ||||||||
| [C2py][DCA] | 1.17 | 4197.59 | 0.4403 | 8 | 3 | 0.45 | 1126.48 ① | 95.84 | 99.99 | 99.99 | 91.15 | |
| 0.9981 | 6 | 3 | 0.11 | 698.65 ② | ||||||||
| [C2py][SCN] | 0.73 | 5999.27 | 0.3522 | 6 | 3 | 0.25 | 1027.74 ① | 96.37 | 99.99 | 99.99 | 92.16 | |
| 0.9981 | 7 | 3 | 0.17 | 727.01 ② | ||||||||
Table 7 Main results of process simulation for the separation of m-cresol and cumene using 3 ionic liquids
Extraction column (298.15 K, 1 ×105 Pa, 8 stages) | Distillation column (0.005 ×105 Pa) | Cumene product | m-Cresol product | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL | IL makeup/ (kg·h-1) | IL in recycle/ (kg·h-1) | Operating conditions | Heat duty/ kW | Recovery ratio/ % | Mass purity/ % | Recovery ratio/ % | Mass purity/ % | ||||
| D∶F | NS | FS | RR | |||||||||
| [C2mim][DCA] | 1.63 | 6498.37 | 0.3479 | 8 | 3 | 0.42 | 1215.23 ① | 93.43 | 99.99 | 99.99 | 86.69 | |
| 0.9981 | 7 | 3 | 0.18 | 769.94 ② | ||||||||
| [C2py][DCA] | 1.17 | 4197.59 | 0.4403 | 8 | 3 | 0.45 | 1126.48 ① | 95.84 | 99.99 | 99.99 | 91.15 | |
| 0.9981 | 6 | 3 | 0.11 | 698.65 ② | ||||||||
| [C2py][SCN] | 0.73 | 5999.27 | 0.3522 | 6 | 3 | 0.25 | 1027.74 ① | 96.37 | 99.99 | 99.99 | 92.16 | |
| 0.9981 | 7 | 3 | 0.17 | 727.01 ② | ||||||||
| 1 | Ji Y A, Hou Y C, Ren S H, et al. Separation of phenolic compounds from oil mixtures using environmentally benign biological reagents based on Brønsted acid-Lewis base interaction [J]. Fuel, 2019, 239: 926-934. |
| 2 | Yao C F, Hou Y C, Ren S H, et al. Efficient separation of phenol from model oils using environmentally benign quaternary ammonium-based zwitterions via forming deep eutectic solvents [J]. Chemical Engineering Journal, 2017, 326: 620-626. |
| 3 | Yi L, Feng J, Li W Y, et al. High-performance separation of phenolic compounds from coal-based liquid oil by deep eutectic solvents[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(8): 7777-7783. |
| 4 | Jiao T T, Qin X Z, Zhang H W, et al. Separation of phenol and pyridine from coal tar via liquid-liquid extraction using deep eutectic solvents [J]. Chemical Engineering Research and Design, 2019, 145: 112-121. |
| 5 | Gai H J, Qiao L, Zhong C Y, et al. A solvent based separation method for phenolic compounds from low-temperature coal tar [J]. Journal of Cleaner Production, 2019, 223: 1-11. |
| 6 | 侯玉翠, 彭威, 杨春梅, 等. 咪唑基离子液体萃取分离模拟油酚混合物 [J]. 化工学报, 2013, 64(S1): 118-123. |
| Hou Y C, Peng W, Yang C M, et al. Extraction of phenolic compounds from simulated oil with imidazolium based ionic liquids [J]. CIESC Journal, 2013, 64(S1): 118-123. | |
| 7 | Hou Y C, Ren Y H, Peng W, et al. Separation of phenols from oil using imidazolium-based ionic liquids [J]. Industrial & Engineering Chemistry Research, 2013, 52(50): 18071-18075. |
| 8 | Ji Y A, Hou Y C, Ren S H, et al. Highly efficient extraction of phenolic compounds from oil mixtures by trimethylamine-based dicationic ionic liquids via forming deep eutectic solvents [J]. Fuel Processing Technology, 2018, 171: 183-191. |
| 9 | Ji Y A, Hou Y C, Ren S H, et al. Highly efficient separation of phenolic compounds from oil mixtures by imidazolium-based dicationic ionic liquids via forming deep eutectic solvents [J]. Energy & Fuels, 2017, 31(9): 10274-10282. |
| 10 | Yao C F, Hou Y C, Ren S H, et al. Efficient separation of phenolic compounds from model oils by dual-functionalized ionic liquids [J]. Chemical Engineering and Processing - Process Intensification, 2018, 128: 216-222. |
| 11 | Zhu C, Li F F, Zhang J, et al. Performance of functionalized ionic liquid with double chemical sites for separating phenolic compounds: mechanism and liquid-liquid behavior studies [J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106790. |
| 12 | Xu D M, Zhong P, Peng L J, et al. Multiscale evaluation of the efficiently separation of phenols using a designed cationic functionalized ionic liquid based on Brønsted/Lewis coordination [J]. Journal of Molecular Liquids, 2022, 345: 117901. |
| 13 | Xu D M, Wang S X, Zhang T, et al. Extraction and interaction insights for enhanced separation of phenolic compounds from model coal tar using a hydroxyl-functionalized ionic liquid [J]. Chemical Engineering Research and Design, 2022, 178: 567-574. |
| 14 | Song Z, Zhou T, Zhang J N, et al. Screening of ionic liquids for solvent-sensitive extraction -with deep desulfurization as an example [J]. Chemical Engineering Science, 2015, 129: 69-77. |
| 15 | 董一春. 离子液体预测型热力学模型及其在萃取精馏分离甲缩醛和甲醇中的应用[D]. 北京: 北京化工大学, 2020. |
| Dong Y C. Predictive thermodynamics models for ionic liquids and their application in the separation of methylal and methanol mixture by extractive distillation [D]. Beijing: Beijing University of Chemical Technology, 2020. | |
| 16 | Anantharaj R, Banerjee T. Quantum chemical studies on the simultaneous interaction of thiophene and pyridine with ionic liquid [J]. AIChE Journal, 2011, 57(3): 749-764. |
| 17 | Song Z, Zhang C Y, Qi Z W, et al. Computer-aided design of ionic liquids as solvents for extractive desulfurization [J]. AIChE Journal, 2018, 64(3): 1013-1025. |
| 18 | Song Z, Li X X, Chao H, et al. Computer-aided ionic liquid design for alkane/cycloalkane extractive distillation process[J]. Green Energy & Environment, 2019, 4(2): 154-165. |
| 19 | Diedenhofen M, Klamt A. COSMO-RS as a tool for property prediction of IL mixtures— a review [J]. Fluid Phase Equilibria, 2010, 294(1-2): 31-38. |
| 20 | Qin H, Wang Z H, Zhou T, et al. Comprehensive evaluation of COSMO-RS for predicting ternary and binary ionic liquid-containing vapor-liquid equilibria [J]. Industrial & Engineering Chemistry Research, 2021, 60(48): 17761-17777. |
| 21 | Lyu Z X, Zhou T, Chen L F, et al. Simulation based ionic liquid screening for benzene-cyclohexane extractive separation [J]. Chemical Engineering Science, 2014, 113: 45-53. |
| 22 | Gao S R, Chen X C, Abro R, et al. Desulfurization of fuel oil: conductor-like screening model for real solvents study on capacity of ionic liquids for thiophene and dibenzothiophene [J]. Industrial & Engineering Chemistry Research, 2015, 54(38): 9421-9430. |
| 23 | Song Z, Zhang J J, Zeng Q, et al. Effect of cation alkyl chain length on liquid-liquid equilibria of {ionic liquids + thiophene + heptane}: COSMO-RS prediction and experimental verification [J]. Fluid Phase Equilibria, 2016, 425: 244-251. |
| 24 | 张志刚, 张德彪, 张亲亲, 等. 基于COSMO-RS方法筛选离子液体分离乙酸乙酯-乙腈共沸物 [J]. 化工学报, 2019, 70(1): 146-153. |
| Zhang Z G, Zhang D B, Zhang Q Q, et al. Screening of ionic liquids for separation of ethyl acetate-acetonitrile azeotrope based on COSMO-RS [J]. CIESC Journal, 2019, 70(1): 146-153. | |
| 25 | Liu Q, Zhang X L, Li W. Separation of m-cresol from aromatic hydrocarbon and alkane using ionic liquids via hydrogen bond interaction [J]. Chinese Journal of Chemical Engineering, 2019, 27(11): 2675-2686. |
| 26 | 刘潜, 张香兰, 李巍. 基于COSMO-RS模型的分离油酚混合物的离子液体萃取剂筛选 [J]. 化工学报, 2018, 69(12): 5100-5111. |
| Liu Q, Zhang X L, Li W. Screening ionic liquids solvent for separation of oil and hydroxybenzene mixtures based on COSMO-RS model [J]. CIESC Journal, 2018, 69(12): 5100-5111. | |
| 27 | Liu Q, Bi J, Zhang X L. Effect of water on phenol separation from model oil with ionic liquids based on COSMO-RS calculation and experimental study [J]. ACS Omega, 2021, 6(41): 27368-27378. |
| 28 | Liu Q, Zhang X L. Highly efficient separation of phenolic compounds from low-temperature coal tar by composite extractants with low viscosity [J]. Journal of Molecular Liquids, 2022, 360: 119417. |
| 29 | Song Z, Zhou T, Qi Z W, et al. Systematic method for screening ionic liquids as extraction solvents exemplified by an extractive desulfurization process [J]. ACS Sustainable Chemistry & Engineering, 2017, 5(4): 3382-3389. |
| 30 | Kulajanpeng K, Suriyapraphadilok U, Gani R. Systematic screening methodology and energy efficient design of ionic liquid-based separation processes [J]. Journal of Cleaner Production, 2016, 111: 93-107. |
| 31 | Qin Z X, Cheng H Y, Song Z, et al. Selection of deep eutectic solvents for extractive deterpenation of lemon essential oil [J]. Journal of Molecular Liquids, 2022, 350: 118524. |
| 32 | Qin H, Cheng J, Yu H T, et al. Hierarchical ionic liquid screening integrating COSMO-RS and Aspen Plus for selective recovery of hydrofluorocarbons and hydrofluoroolefins from a refrigerant blend [J]. Industrial & Engineering Chemistry Research, 2022, 61(11): 4083-4094. |
| 33 | Jiang C H, Cheng H Y, Qin Z X, et al. COSMO-RS prediction and experimental verification of 1,5-pentanediamine extraction from aqueous solution by ionic liquids [J]. Green Energy & Environment, 2021, 6(3): 422-431. |
| 34 | 吴明尧. 基于季铵盐低共熔溶剂的柑橘精油脱萜过程研究[D]. 上海: 华东理工大学, 2021. |
| Wu M Y. Extractive deterpenation of citrus essential oils using quaternary ammonium-based deep eutectic solvents[D]. Shanghai: East China University of Science and Technology, 2021. | |
| 35 | Song Z, Hu X T, Zhou Y G, et al. Rational design of double salt ionic liquids as extraction solvents: separation of thiophene/n-octane as example [J]. AIChE Journal, 2019, 65(8): e16625. |
| 36 | Lazzús J A. A group contribution method to predict the melting point of ionic liquids [J]. Fluid Phase Equilibria, 2012, 313: 1-6. |
| 37 | Lazzús J A, Pulgar-Villarroel G. A group contribution method to estimate the viscosity of ionic liquids at different temperatures [J]. Journal of Molecular Liquids, 2015, 209: 161-168. |
| 38 | Huang Y, Dong H F, Zhang X P, et al. A new fragment contribution-corresponding states method for physicochemical properties prediction of ionic liquids [J]. AIChE Journal, 2013, 59(4): 1348-1359. |
| 39 | Nancarrow P, Lewis M, AbouChacra L. Group contribution methods for estimation of ionic liquid heat capacities: critical evaluation and extension [J]. Chemical Engineering & Technology, 2015, 38(4): 632-644. |
| 40 | Liu X K, Zhang X L. Solvent screening and liquid-liquid measurement for extraction of phenols from aromatic hydrocarbon mixtures [J]. The Journal of Chemical Thermodynamics, 2019, 129: 12-21. |
| 41 | Li A, Zhu C, Zhang L Z, et al. Efficient extraction and theoretical insights for separating o-, m-, and p-cresol from model coal tar by an ionic liquid [Emim][DCA] [J]. Canadian Journal of Chemical Engineering, 2022, 100: S205-S212. |
| 42 | Li A, Xu X, Zhang L Z, et al. Separation of cresol from coal tar by imidazolium-based ionic liquid [Emim][SCN]: interaction exploration and extraction experiment [J]. Fuel, 2020, 264: 116908. |
| 43 | Sun C G, Tang S K. Guanidinium dicyanamide-based nitrogen-rich energetic salts as additives of hypergolic ionic liquids [J]. Energy & Fuels, 2020, 34(11): 15068-15071. |
| 44 | Liu Q, Zhang X L. Systematic method of screening deep eutectic solvents as extractive solvents for m-cresol/cumene separation [J]. Separation and Purification Technology, 2022, 291: 120853. |
| 45 | Ma X X, Wei J, Guan W, et al. Ionic parachor and its application to pyridinium-based ionic liquids of {[C n py][DCA] (n = 2, 3, 4, 5, 6)} [J]. The Journal of Chemical Thermodynamics, 2015, 89: 51-59. |
| [1] | Qi WANG, Bin ZHANG, Xiaoxin ZHANG, Hujian WU, Haitao ZHAN, Tao WANG. Synthesis of isoxepac and 2-ethylanthraquinone catalyzed by chloroaluminate-triethylamine ionic liquid/P2O5 [J]. CIESC Journal, 2023, 74(S1): 245-249. |
| [2] | Ruimin CHE, Wenqiu ZHENG, Xiaoyu WANG, Xin LI, Feng XU. Research progress on homogeneous processing of cellulose in ionic liquids [J]. CIESC Journal, 2023, 74(9): 3615-3627. |
| [3] | Yaxin ZHAO, Xueqin ZHANG, Rongzhu WANG, Guo SUN, Shanjing YAO, Dongqiang LIN. Removal of monoclonal antibody aggregates with ion exchange chromatography by flow-through mode [J]. CIESC Journal, 2023, 74(9): 3879-3887. |
| [4] | Meisi CHEN, Weida CHEN, Xinyao LI, Shangyu LI, Youting WU, Feng ZHANG, Zhibing ZHANG. Advances in silicon-based ionic liquid microparticle enhanced gas capture and conversion [J]. CIESC Journal, 2023, 74(9): 3628-3639. |
| [5] | Lizhi WANG, Qiancheng HANG, Yeling ZHENG, Yan DING, Jiaji CHEN, Qing YE, Jinlong LI. Separation of methyl propionate + methanol azeotrope using ionic liquid entrainers [J]. CIESC Journal, 2023, 74(9): 3731-3741. |
| [6] | Jie CHEN, Yongsheng LIN, Kai XIAO, Chen YANG, Ting QIU. Study on catalytic synthesis of sec-butanol by tunable choline-based basic ionic liquids [J]. CIESC Journal, 2023, 74(9): 3716-3730. |
| [7] | Minghao SONG, Fei ZHAO, Shuqing LIU, Guoxuan LI, Sheng YANG, Zhigang LEI. Multi-scale simulation and study of volatile phenols removal from simulated oil by ionic liquids [J]. CIESC Journal, 2023, 74(9): 3654-3664. |
| [8] | Shaoqi YANG, Shuheng ZHAO, Lungang CHEN, Chenguang WANG, Jianjun HU, Qing ZHOU, Longlong MA. Hydrodeoxygenation of lignin-derived compounds to alkanes in Raney Ni-protic ionic liquid system [J]. CIESC Journal, 2023, 74(9): 3697-3707. |
| [9] | Zehao MI, Er HUA. DFT and COSMO-RS theoretical analysis of SO2 absorption by polyamines type ionic liquids [J]. CIESC Journal, 2023, 74(9): 3681-3696. |
| [10] | Xudong YU, Qi LI, Niancu CHEN, Li DU, Siying REN, Ying ZENG. Phase equilibria and calculation of aqueous ternary system KCl + CaCl2 + H2O at 298.2, 323.2, and 348.2 K [J]. CIESC Journal, 2023, 74(8): 3256-3265. |
| [11] | Shuang LIU, Linzhou ZHANG, Zhiming XU, Suoqi ZHAO. Study on molecular level composition correlation of viscosity of residual oil and its components [J]. CIESC Journal, 2023, 74(8): 3226-3241. |
| [12] | Lei XING, Chunyu MIAO, Minghu JIANG, Lixin ZHAO, Xinya LI. Optimal design and performance analysis of downhole micro gas-liquid hydrocyclone [J]. CIESC Journal, 2023, 74(8): 3394-3406. |
| [13] | Jiayi ZHANG, Jiali HE, Jiangpeng XIE, Jian WANG, Yu ZHAO, Dongqiang ZHANG. Research progress of pervaporation technology for N-methylpyrrolidone recovery in lithium battery production [J]. CIESC Journal, 2023, 74(8): 3203-3215. |
| [14] | Ruihang ZHANG, Pan CAO, Feng YANG, Kun LI, Peng XIAO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Analysis of key parameters affecting product purity of natural gas ethane recovery process via ZIF-8 nanofluid [J]. CIESC Journal, 2023, 74(8): 3386-3393. |
| [15] | Zhaolun WEN, Peirui LI, Zhonglin ZHANG, Xiao DU, Qiwang HOU, Yegang LIU, Xiaogang HAO, Guoqing GUAN. Design and optimization of cryogenic air separation process with dividing wall column based on self-heat regeneration [J]. CIESC Journal, 2023, 74(7): 2988-2998. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||