CIESC Journal ›› 2022, Vol. 73 ›› Issue (7): 3240-3250.DOI: 10.11949/0438-1157.20220316
• Energy and environmental engineering • Previous Articles Next Articles
Wentao LI( ),Huijuan LIN,Hai ZHONG(
),Huijuan LIN,Hai ZHONG( )
)
Received:2022-03-02
Revised:2022-04-01
Online:2022-08-01
Published:2022-07-05
Contact:
Hai ZHONG
通讯作者:
钟海
作者简介:李文涛(1996—),男,硕士研究生,基金资助:CLC Number:
Wentao LI, Huijuan LIN, Hai ZHONG. LiF-rich SEI generated by in-situ gel polymer electrolyte process for lithium metal rechargeable batteries[J]. CIESC Journal, 2022, 73(7): 3240-3250.
李文涛, 林慧娟, 钟海. 原位构建富氟SEI的凝胶电解质用于金属锂二次电池[J]. 化工学报, 2022, 73(7): 3240-3250.
Add to citation manager EndNote|Ris|BibTeX
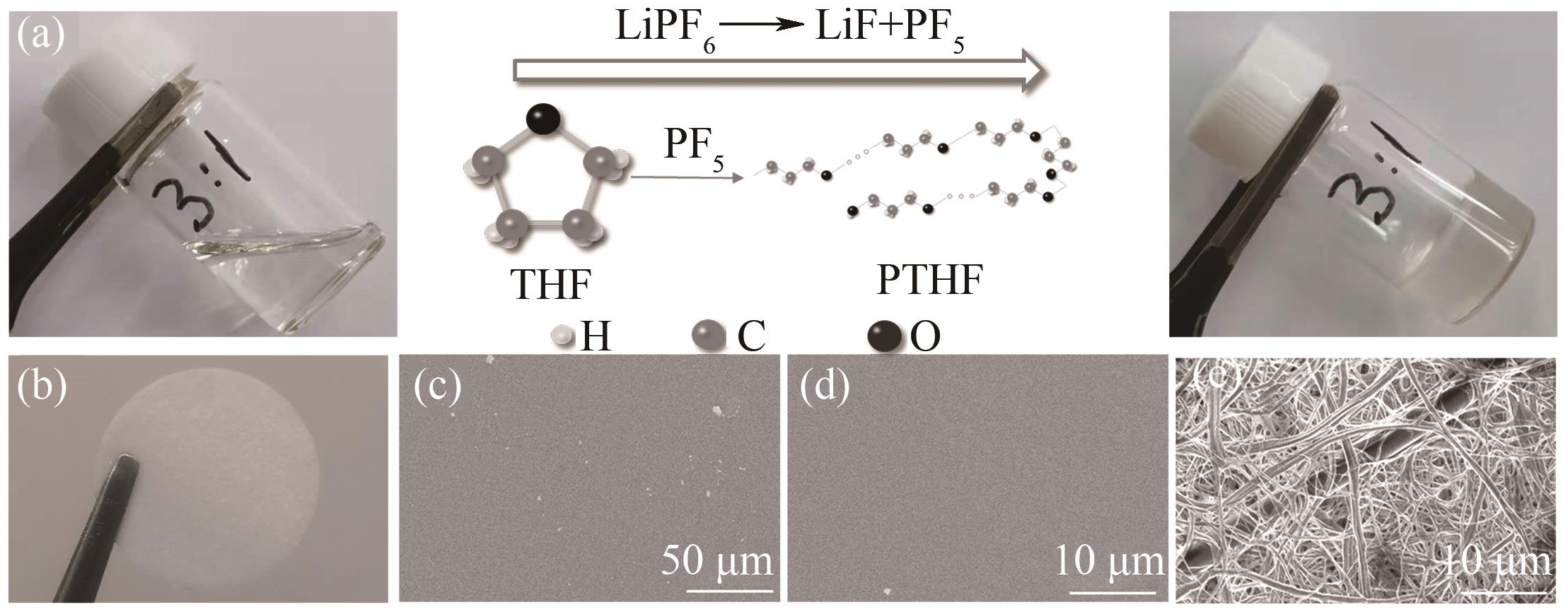
Fig.1 (a) Illustration of polymerization mechanism; (b) Photograph of GPE with cellulose film; (c), (d) SEM images of GPE under different magnifications; (e) SEM image of cellulose film
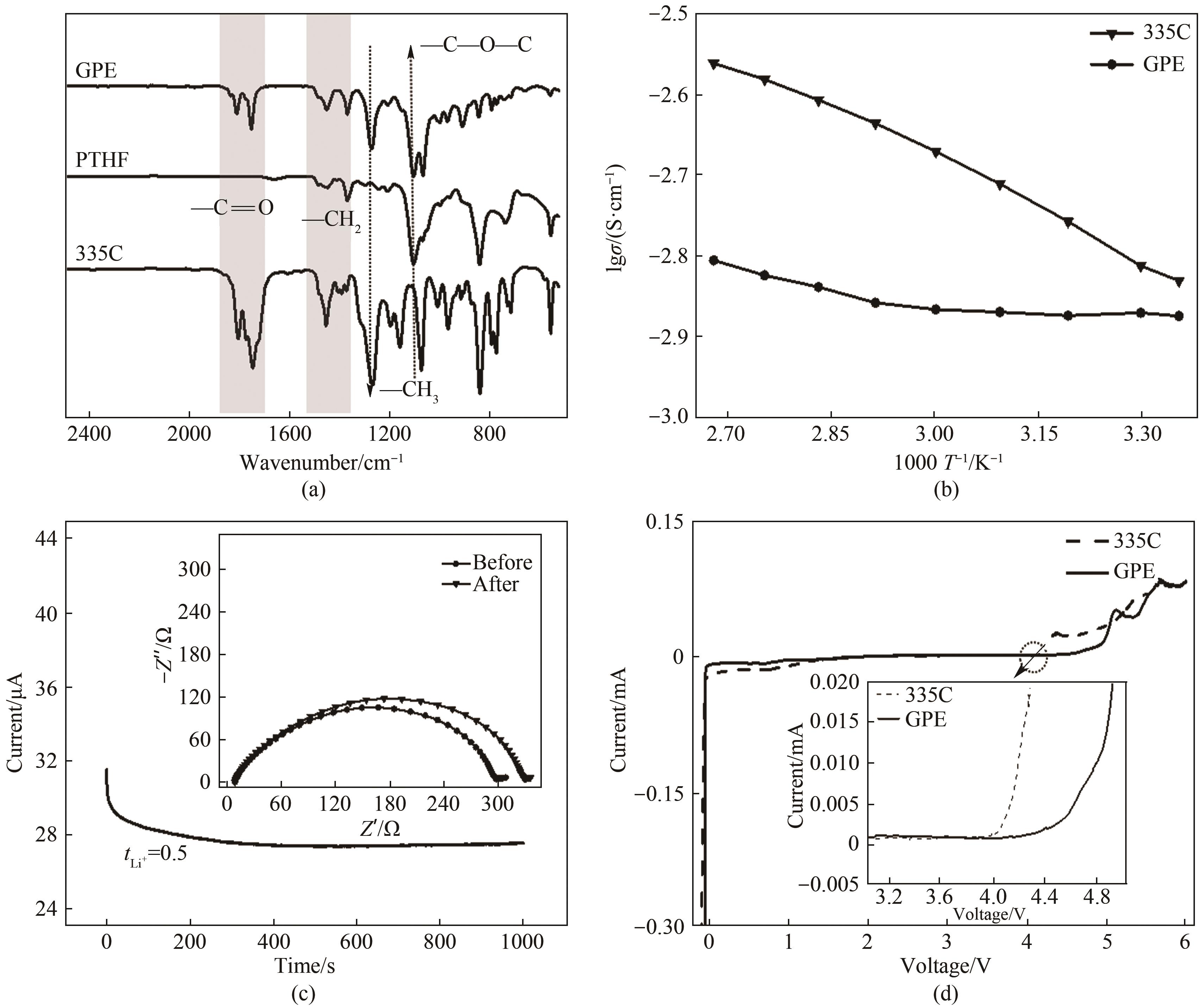
Fig.2 (a) FTIR spectra of GPE, PTHF and 335C electrolyte; (b) Temperature dependent ionic conductivity of GPE and 335C electrolyte; (c) The chronoamperometry profile of Li|GPE|Li symmetric cell(the inset displays the impedance spectra before and after chronoamperometry); (d) Linear sweep curves of GPE and 335C electrolyte

Fig.3 (a) AC impedance spectra of Li|GPE|Li symmetrical cells for different time at room temperature; (b) Cyclic performance of Li symmetric cells with GPE, 335C electrolyte at a current density of 0.5 mA·cm-2 with area capacity of 0.5 mAh·cm-2; Typical SEM images of the Li anode with (c) 335Celectrolyte, and (d) GPE after 20 cycles
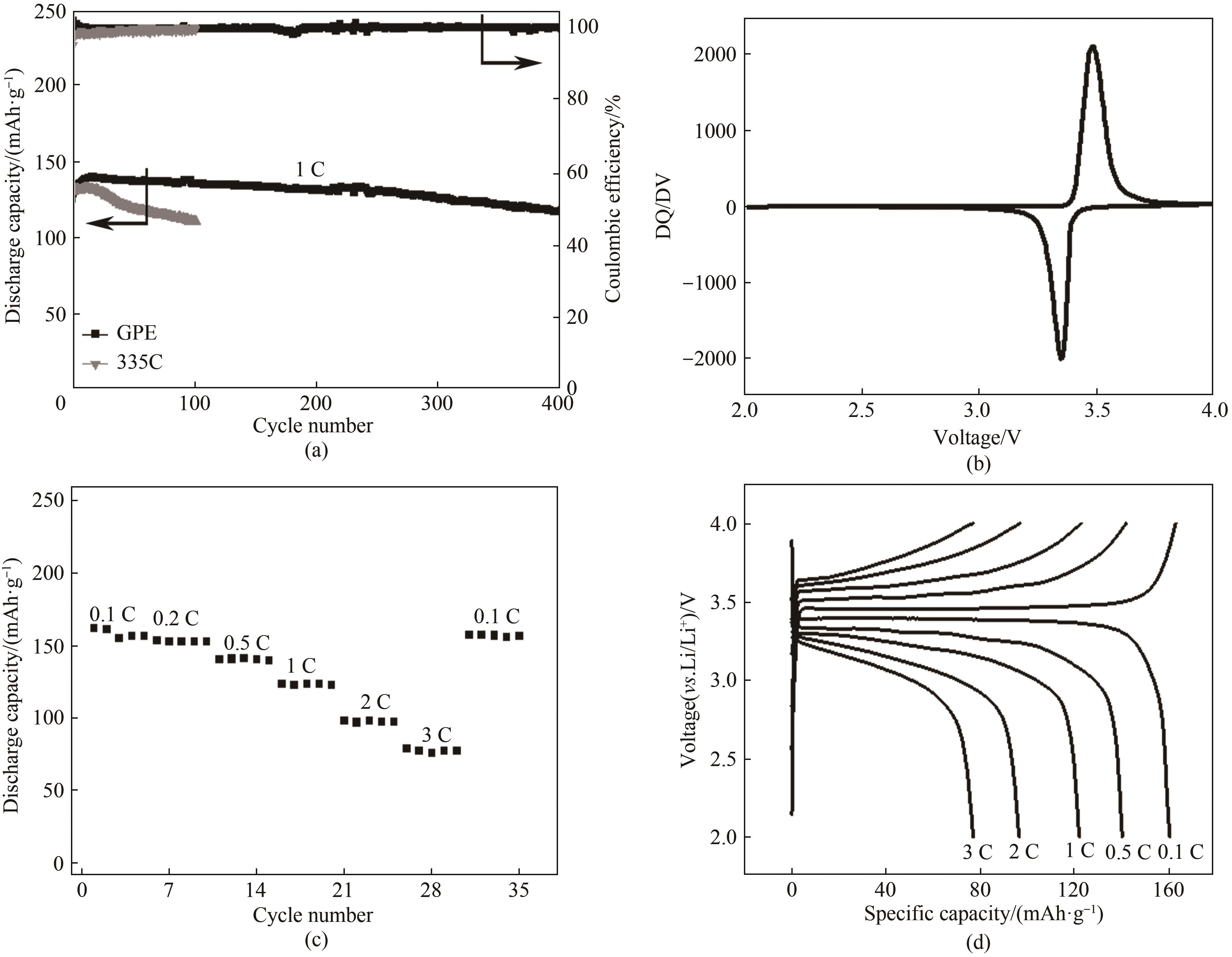
Fig.4 (a) Long-term cycling performance of Li|GPE|LiFePO4 cells(the cells were charged and discharged between 2.0—4.0 V at a current rate of 1 C); (b) DQ/DV diagram corresponding to charge and discharge curves of LMBs; (c) Rate performance of Li|GPE|LiFePO4 at various current rate; (d) Corresponding voltage profiles at various current rates

Fig.5 XPS spectra of Li metal (a) F 1s spectra, (b) Li 1s spectra in GPE and 335C electrolyte after 50 cycle;(c) Schematic diagram of LiF produce reaction
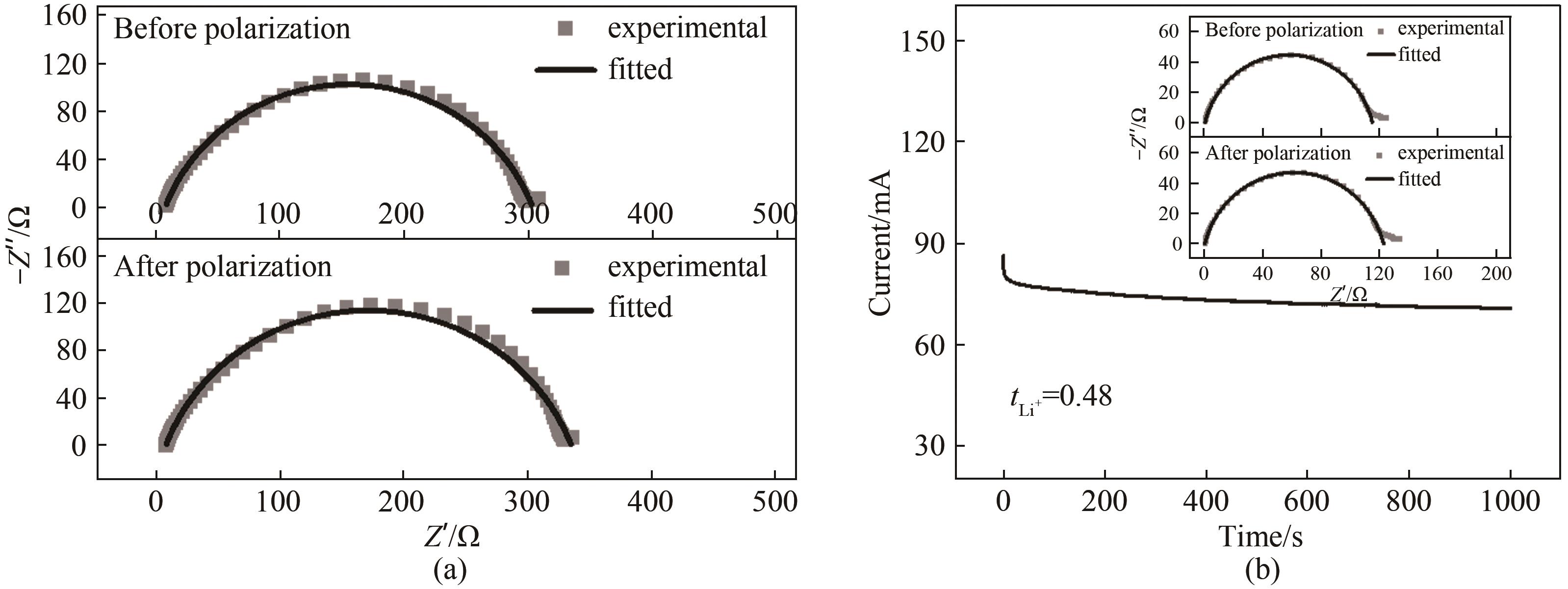
Fig.A2 (a) The impedance spectra of Li|GPE|Li symmetric cell before and after chronoamperometry. (b) The chronoamperometry profile of Li|335C|Li symmetric cell(The inset displays the impedance spectra before and after chronoamperometry)

Fig.A4 (a) AC impedance spectra of Li|335C|Li symmetrical cell for different time at room temperature; (b) Typical SEM images at the surface of fresh Li anode; (c),(d) Typical SEM images of the Li anode with GPE after 20th cycles under different scales
| 1 | Liu F Q, T, Li T, Yang Y J, et al. Investigation on the copolymer electrolyte of poly(1, 3-dioxolane-co-formaldehyde)[J]. Macromolecular Rapid Communications, 2020, 41(9): 2000047. |
| 2 | Bai M H, Xie K Y, Hong B, et al. An artificial Li3PO4 solid electrolyte interphase layer to achieve petal-shaped deposition of lithium[J]. Solid State Ionics, 2019, 333: 101-104. |
| 3 | Zhang Q K, Zhang X Q, Yuan H, et al. Thermally stable and nonflammable electrolytes for lithium metal batteries: progress and perspectives[J]. Small Science, 2021, 1(10): 2100058. |
| 4 | Jin D Q, Hu K, Hou R, et al. Vertical nanoarrays with lithiophilic sites suppress the growth of lithium dendrites for ultrastable lithium metal batteries[J]. Chemical Engineering Journal, 2021, 405: 126808. |
| 5 | Golozar M, Paolella A, Demers H, et al. Direct observation of lithium metal dendrites with ceramic solid electrolyte [J]. Scientific Reports, 2020, 10: 18410. |
| 6 | Ke X Y, Wang Y, Dai L M, et al. Cell failures of all-solid-state lithium metal batteries with inorganic solid electrolytes: lithium dendrites[J]. Energy Storage Materials, 2020, 33: 309-328. |
| 7 | Chi S S, Liu Y C, Zhao N, et al. Solid polymer electrolyte soft interface layer with 3D lithium anode for all-solid-state lithium batteries[J]. Energy Storage Materials, 2019, 17: 309-316. |
| 8 | Lu Y, Zhao C Z, Yuan H, et al. Critical current density in solid-state lithium metal batteries: mechanism, influences, and strategies[J]. Advanced Functional Materials, 2021, 31(18): 2009925. |
| 9 | Chen L, Li W X, Fan L Z, et al. Intercalated electrolyte with high transference number for dendrite-free solid-state lithium batteries[J]. Advanced Functional Materials, 2019, 29(28): 1901047. |
| 10 | Tabani Z, Maghsoudi H, Fathollahi Zonouz A. High electrochemical stability of polyvinylidene fluoride (PVDF) porous membranes using phase inversion methods for lithium-ion batteries[J]. Journal of Solid State Electrochemistry, 2021, 25(2): 651-657. |
| 11 | Tarascon J M, Gozdz A S, Schmutz C, et al. Performance of Bellcore’s plastic rechargeable Li-ion batteries[J]. Solid State Ionics, 1996, 86/87/88: 49-54. |
| 12 | Zhou J Q, Ji H Q, Liu J, et al. A new high ionic conductive gel polymer electrolyte enables highly stable quasi-solid-state lithium sulfur battery[J]. Energy Storage Materials, 2019, 22: 256-264. |
| 13 | Chen F, Yang D J, Zha W P, et al. Solid polymer electrolytes incorporating cubic Li7La3Zr2O12 for all-solid-state lithium rechargeable batteries[J]. Electrochimica Acta, 2017, 258: 1106-1114. |
| 14 | Wang Q Y, Xu X Q, Hong B, et al. Molecular engineering of a gel polymer electrolyte via in situ polymerization for high performance lithium metal batteries[J]. Chemical Engineering Journal, 2022, 428: 131331. |
| 15 | Deng B, Jing M X, Li L X, et al. Nano-zirconia boosting the ionic conductivity and lithium dendrite inhibition ability of a poly(1, 3-dioxolane) solid electrolyte for high-voltage solid-state lithium batteries[J]. Sustainable Energy & Fuels, 2021, 5(21): 5461-5470. |
| 16 | Zhang X L, Zhao S Y, Fan W, et al. Long cycling, thermal stable, dendrites free gel polymer electrolyte for flexible lithium metal batteries[J]. Electrochimica Acta, 2019, 301: 304-311. |
| 17 | Wu H, Tang B, Du X F, et al. LiDFOB initiated in situ polymerization of novel eutectic solution enables room-temperature solid lithium metal batteries[J]. Advanced Science, 2020, 7(23): 2003370. |
| 18 | Shen Y Q, Zhu W, Papadaki M, et al. Thermal decomposition of solid benzoyl peroxide using advanced reactive system screening tool: effect of concentration, confinement and selected acids and bases[J]. Journal of Loss Prevention in the Process Industries, 2019, 60: 28-34. |
| 19 | Zhong H, Wang C, Xu Z, et al. A novel quasi-solid state electrolyte with highly effective polysulfide diffusion inhibition for lithium-sulfur batteries[J]. Scientific Reports, 2016, 6: 25484. |
| 20 | Xie M, Wu Y, Liu Y, et al. Pathway of in situ polymerization of 1, 3-dioxolane in LiPF6 electrolyte on Li metal anode[J]. Materials Today Energy, 2021, 21: 100730. |
| 21 | Cui Y Y, Liang X M, Chai J C, et al. High performance solid polymer electrolytes for rechargeable batteries: a self-catalyzed strategy toward facile synthesis[J]. Advanced Science, 2017, 4(11): 1700174. |
| 22 | Ma Q, Yue J P, Fan M, et al. Formulating the electrolyte towards high-energy and safe rechargeable lithium-metal batteries[J]. Angewandte Chemie International Edition, 2021, 60(30): 16554-16560. |
| 23 | Tominaga Y, Kato S, Nishimura N. Preparation and electrochemical characterization of magnesium gel electrolytes based on crosslinked poly(tetrahydrofuran)[J]. Polymer, 2021, 224: 123743. |
| 24 | Li T, Zhang X Q, Shi P, et al. Fluorinated solid-electrolyte interphase in high-voltage lithium metal batteries[J]. Joule, 2019, 3(11): 2647-2661. |
| 25 | Yuan Y X, Wu F, Bai Y, et al. Regulating Li deposition by constructing LiF-rich host for dendrite-free lithium metal anode[J]. Energy Storage Materials, 2019, 16: 411-418. |
| 26 | Feng Y Y, Zhang C F, Li B, et al. Low-volume-change, dendrite-free lithium metal anodes enabled by lithophilic 3D matrix with LiF-enriched surface[J]. Journal of Materials Chemistry A, 2019, 7(11): 6090-6098. |
| 27 | Hoene R, Reichert K H W. Zur aufklärung des initiierungsschrittes der kationischen polymerisation von tetrahydrofuran mit PF5 [J]. Die Makromolekulare Chemie, 1976, 177(12): 3545-3570. |
| 28 | Zhang C J, Hu L F, Wu H L, et al. Dual organocatalysts for highly active and selective synthesis of linear poly(γ-butyrolactone)s with high molecular weights[J]. Macromolecules, 2018, 51(21): 8705-8711. |
| 29 | Huang S Q, Cui Z L, Qiao L X, et al. An in situ polymerized solid polymer electrolyte enables excellent interfacial compatibility in lithium batteries[J]. Electrochimica Acta, 2019, 299: 820-827. |
| 30 | O'Connor C J, Cleverly D R. Fourier-transform infrared assay of bile salt-stimulated lipase activity in reversed micelles[J]. Journal of Chemical Technology & Biotechnology, 1994, 61(3): 209-214. |
| 31 | 赵航, 魏闯, 康鑫, 等. 锂离子电池三元正极材料的研究进展[J]. 中国陶瓷, 2020, 56(5): 10-15. |
| Zhao H, Wei C, Kang X, et al. Research progress of ternary cathode materials for lithium ion battery[J]. China Ceramics, 2020, 56(5): 10-15. | |
| 32 | 蓝兹炜, 张建茹, 李园园, 等. 基于锂离子电池正极材料的一元/二元复合正极材料研究进展[J]. 储能科学与技术, 2021, 10(1): 27-39. |
| Lan Z W, Zhang J R, Li Y Y, et al. Research progress of mono/binary composite cathode materials based on lithium-ion battery cathode materials[J]. Energy Storage Science and Technology, 2021, 10(1): 27-39. | |
| 33 | Xu C, Sun B, Gustafsson T, et al. Interface layer formation in solid polymer electrolyte lithium batteries: an XPS study[J]. Journal of Materials Chemistry A, 2014, 2(20): 7256-7264. |
| 34 | Che H Y, Yang X R, Wang H, et al. Long cycle life of sodium-ion pouch cell achieved by using multiple electrolyte additives[J]. Journal of Power Sources, 2018, 407: 173-179. |
| 35 | Winkler V, Hanemann T, Bruns M. Comparative surface analysis study of the solid electrolyte interphase formation on graphite anodes in lithium-ion batteries depending on the electrolyte composition[J]. Surface and Interface Analysis, 2017, 49(5): 361-369. |
| 36 | Fu J L, Ji X, Chen J, et al. Lithium nitrate regulated sulfone electrolytes for lithium metal batteries[J]. Angewandte Chemie International Edition, 2020, 59(49): 22194-22201. |
| 37 | Qiao Y, Wu J W, Zhao J, et al. Synergistic effect of bifunctional catalytic sites and defect engineering for high-performance Li-CO2 batteries[J]. Energy Storage Materials, 2020, 27: 133-139. |
| 38 | Wang L N, Liu J Y, Yuan S Y, et al. To mitigate self-discharge of lithium–sulfur batteries by optimizing ionic liquid electrolytes[J]. Energy & Environmental Science, 2016, 9(1): 224-231. |
| 39 | Krotkov D, Schneier D, Menkin D S, et al. Operando terahertz spectroscopy of solid electrolyte interphase evolution on silicon anodes[J]. Batteries & Supercaps, 2022, 5(1): e202100183. |
| 40 | Hamidah N L, Wang F M, Nugroho G. The understanding of solid electrolyte interface (SEI) formation and mechanism as the effect of flouro-o-phenylenedimaleimaide (F-MI) additive on lithium-ion battery[J]. Surface and Interface Analysis, 2019, 51(3): 345-352. |
| [1] | Hao WANG, Zhenlei WANG. Model simplification strategy of cracking furnace coking based on adaptive spectroscopy method [J]. CIESC Journal, 2023, 74(9): 3855-3864. |
| [2] | Huakun HU, Wendong XUE, Sida HUO, Yong LI, Peng JIANG. Review of SEI film forming additives for electrolyte of lithium ion battery [J]. CIESC Journal, 2022, 73(4): 1436-1454. |
| [3] | Maolin YE, Fenghua TAN, Yuping LI, Yuhe LIAO, Chenguang WANG, Longlong MA. Life cycle environmental impact assessment of mixed alcohol via gasification of agricultural and forestry residues and catalytic synthesis [J]. CIESC Journal, 2022, 73(3): 1369-1378. |
| [4] | Yongshuai LI, Yi ZHENG, Lan LI, Xinshuang LI, Xinyi ZHAO, Hui PAN, Hao LING. Influence of lift model on gas-liquid-solid flow field in polyethylene fluidized bed reactor [J]. CIESC Journal, 2022, 73(12): 5355-5366. |
| [5] | Bozheng LIU, Jingbo WANG, Tao ZENG, Yaxia YIN, Yuguo GUO. Safety differences of LiFePO4 batteries at the beginning of life and end of life [J]. CIESC Journal, 2022, 73(12): 5555-5563. |
| [6] | Pei ZHOU, Xiuping ZHANG, Jingchun TANG, Lei YANG, Bin YE, Ronghua HUANG. Experimental and theoretical study on bubble lift-off diameter in subcooled flow boiling [J]. CIESC Journal, 2022, 73(1): 194-203. |
| [7] | Fang WANG,Shengkun JIA,Huishu ZHANG,Xigang YUAN,Kuotsung YU. POD modal analysis of turbulent diffusion based on experimental data [J]. CIESC Journal, 2021, 72(9): 4531-4543. |
| [8] | XU Wenhua, LIU Dongfu, HE Lihua, LIU Xuheng, ZHAO Zhongwei. Kinetic study on electrochemical intercalation/deintercalation method for lithium extraction from brine [J]. CIESC Journal, 2021, 72(6): 3105-3115. |
| [9] | ZHANG Xi,ZHANG Lilong,LI Rui,WU Yulong. Life cycle assessment of straw fast pyrolysis based on energy integration [J]. CIESC Journal, 2021, 72(5): 2792-2800. |
| [10] | NIE Xuan, ZHOU Kuibin, WU Yueqiong, HUANG Mengyuan, JIANG Juncheng. Study on the flame shape of gas-solid jet diffusion [J]. CIESC Journal, 2021, 72(5): 2878-2886. |
| [11] | WU Yueqiong, ZHOU Kuibin, HUANG Mengyuan, ZHOU Mengya. Flame behavior of jet fire confined by the tank wall [J]. CIESC Journal, 2021, 72(5): 2896-2904. |
| [12] | LI Jingyi, KUANG Zhuoxian. Exploration and reference of PHSER method covering entire project lifecycle [J]. CIESC Journal, 2021, 72(3): 1634-1642. |
| [13] | CAO Jianing, GAO Xiang, LUO Yingwu, SU Rongxin. Study on preparation and performance of aqueous binder for lithium iron phosphate electrodes in lithium-ion battery [J]. CIESC Journal, 2021, 72(2): 1169-1180. |
| [14] | ZHANG Chao, LIU Youzhi, JIAO Weizhou, ZHANG Qiaoling. A computational mass transfer model for the simulation of biodegradation process of phenol waste water in an internal loop airlift reactor [J]. CIESC Journal, 2021, 72(2): 965-974. |
| [15] | Zhenzhen YE, Xinqi CHEN, Jian WANG, Bofan LI, Chaojie CUI, Gang ZHANG, Luming QIAN, Ying JIN, Weizhong QIAN. Evaluation of aging performance under high temperature of ionic liquid-based pouch supercapacitor [J]. CIESC Journal, 2021, 72(12): 6351-6360. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||