CIESC Journal ›› 2022, Vol. 73 ›› Issue (10): 4498-4506.DOI: 10.11949/0438-1157.20220826
• Catalysis, kinetics and reactors • Previous Articles Next Articles
Hongyun YOU( ), Jingjun LIN, Kaiyue HUANG, Riyang SHU(
), Jingjun LIN, Kaiyue HUANG, Riyang SHU( ), Zhipeng TIAN, Chao WANG, Ying CHEN
), Zhipeng TIAN, Chao WANG, Ying CHEN
Received:2022-06-13
Revised:2022-08-31
Online:2022-11-02
Published:2022-10-05
Contact:
Riyang SHU
尤红运( ), 林景骏, 黄凯越, 舒日洋(
), 林景骏, 黄凯越, 舒日洋( ), 田志鹏, 王超, 陈颖
), 田志鹏, 王超, 陈颖
通讯作者:
舒日洋
作者简介:尤红运(1996—),男,硕士研究生,2112002273@mail2.gdut.edu.cn
基金资助:CLC Number:
Hongyun YOU, Jingjun LIN, Kaiyue HUANG, Riyang SHU, Zhipeng TIAN, Chao WANG, Ying CHEN. Mechanism of solvent effect on hydrogenation of lignin-derived phenolic compounds[J]. CIESC Journal, 2022, 73(10): 4498-4506.
尤红运, 林景骏, 黄凯越, 舒日洋, 田志鹏, 王超, 陈颖. 溶剂效应对木质素酚类化合物加氢反应的影响机理[J]. 化工学报, 2022, 73(10): 4498-4506.
Add to citation manager EndNote|Ris|BibTeX
| 序号 | 催化剂 | 转化率/% | 选择性/% | |
|---|---|---|---|---|
| 环己醇 | 环己酮 | |||
| 1 | Ru/C | 14.6 | 95.9 | 4.1 |
| 2 | Ru/C + WO3 | 6.2 | 93.6 | 6.4 |
| 3 | Ru/C + Nb2O5 | 9.2 | 94.1 | 5.9 |
| 4 | Ru/C + ZrO2 | 13.7 | 96.1 | 3.9 |
| 5 | Ru/C + TiO2 | 23.2 | 97.5 | 2.5 |
| 6 | Ru/C + HPW | 2.1 | 84.7 | 15.3 |
| 7 | Ru/C + HSiW | 2.2 | 86.5 | 13.5 |
| 8 | Ru/C + Al2O3 | 100 | 100 | 0 |
| 9 | Ru/C + Al2O3① | 69.8 | 99.1 | 0.9 |
| 10 | Ru/C + Al2O3② | 30.4 | 98.0 | 2.0 |
| 11 | Al2O3 | 0 | — | — |
Table 1 The effect of different catalysts on the phenol hydrogenation
| 序号 | 催化剂 | 转化率/% | 选择性/% | |
|---|---|---|---|---|
| 环己醇 | 环己酮 | |||
| 1 | Ru/C | 14.6 | 95.9 | 4.1 |
| 2 | Ru/C + WO3 | 6.2 | 93.6 | 6.4 |
| 3 | Ru/C + Nb2O5 | 9.2 | 94.1 | 5.9 |
| 4 | Ru/C + ZrO2 | 13.7 | 96.1 | 3.9 |
| 5 | Ru/C + TiO2 | 23.2 | 97.5 | 2.5 |
| 6 | Ru/C + HPW | 2.1 | 84.7 | 15.3 |
| 7 | Ru/C + HSiW | 2.2 | 86.5 | 13.5 |
| 8 | Ru/C + Al2O3 | 100 | 100 | 0 |
| 9 | Ru/C + Al2O3① | 69.8 | 99.1 | 0.9 |
| 10 | Ru/C + Al2O3② | 30.4 | 98.0 | 2.0 |
| 11 | Al2O3 | 0 | — | — |
| 序号 | 溶剂 | 转化率/% | 选择性/% | |
|---|---|---|---|---|
| 环己醇 | 环己酮 | |||
| 1 | 正己烷 | 17.6 | 81.2 | 18.8 |
| 2 | 正辛烷 | 25.7 | 84.7 | 15.3 |
| 3 | 正十二烷 | 20.2 | 86.3 | 13.7 |
| 4 | 甲醇 | 24.6 | 97.9 | 2.1 |
| 5 | 乙醇 | 100 | 100 | 0 |
| 6 | 正丙醇 | 57.1 | 100 | 0 |
| 7 | 正丁醇 | 52.6 | 95.4 | 4.6 |
| 8 | 正己醇 | 31.0 | 97.2 | 2.8 |
| 9 | 正辛醇 | 14.4 | 91.3 | 8.7 |
Table 2 The phenol hydrogenation results of different solvents
| 序号 | 溶剂 | 转化率/% | 选择性/% | |
|---|---|---|---|---|
| 环己醇 | 环己酮 | |||
| 1 | 正己烷 | 17.6 | 81.2 | 18.8 |
| 2 | 正辛烷 | 25.7 | 84.7 | 15.3 |
| 3 | 正十二烷 | 20.2 | 86.3 | 13.7 |
| 4 | 甲醇 | 24.6 | 97.9 | 2.1 |
| 5 | 乙醇 | 100 | 100 | 0 |
| 6 | 正丙醇 | 57.1 | 100 | 0 |
| 7 | 正丁醇 | 52.6 | 95.4 | 4.6 |
| 8 | 正己醇 | 31.0 | 97.2 | 2.8 |
| 9 | 正辛醇 | 14.4 | 91.3 | 8.7 |
| 溶剂 | π* | α | β | 转化率/% | ||
|---|---|---|---|---|---|---|
| 甲醇 | 55.4 | 0.7647 | 0.60 | 0.98 | 0.66 | 24.6 |
| 乙醇 | 51.9 | 0.6563 | 0.54 | 0.86 | 0.75 | 100 |
| 正丙醇 | 50.7 | 0.6192 | 0.52 | 0.84 | 0.90 | 57.1 |
| 正丁醇 | 50.2 | 0.6037 | 0.47 | 0.84 | 0.84 | 52.6 |
| 正己醇 | 48.8 | 0.5604 | 0.40 | 0.80 | 0.84 | 31.0 |
| 正辛醇 | 48.3 | 0.5449 | 0.40 | 0.77 | 0.81 | 14.4 |
Table 3 The property parameters of some organic solvents and conversion of phenol hydrogenation
| 溶剂 | π* | α | β | 转化率/% | ||
|---|---|---|---|---|---|---|
| 甲醇 | 55.4 | 0.7647 | 0.60 | 0.98 | 0.66 | 24.6 |
| 乙醇 | 51.9 | 0.6563 | 0.54 | 0.86 | 0.75 | 100 |
| 正丙醇 | 50.7 | 0.6192 | 0.52 | 0.84 | 0.90 | 57.1 |
| 正丁醇 | 50.2 | 0.6037 | 0.47 | 0.84 | 0.84 | 52.6 |
| 正己醇 | 48.8 | 0.5604 | 0.40 | 0.80 | 0.84 | 31.0 |
| 正辛醇 | 48.3 | 0.5449 | 0.40 | 0.77 | 0.81 | 14.4 |
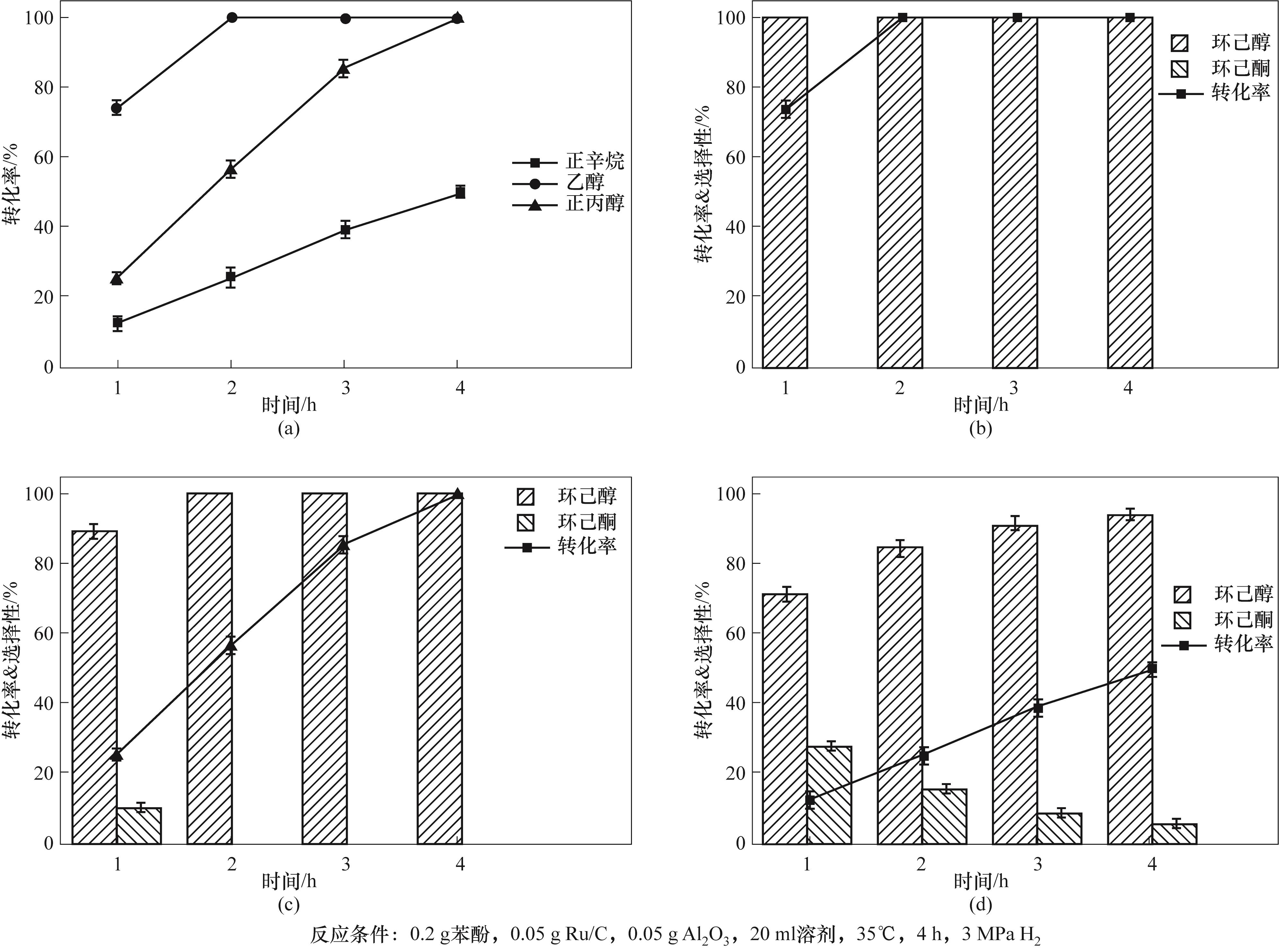
Fig.3 (a)Variation of phenol conversion with time in different solvents;(b)Product distribution of phenol hydrogenation in ethanol solvent;(c)Product distribution of phenol hydrogenation in n-propanol solvent;(d)Product distribution of phenol hydrogenation in octane solvent
| 底物 | 转化率/% | 选择性/% | ||
|---|---|---|---|---|
 | 100 |
7.7 |
92.3 | |
 | 100 |
7.8 |
92.2 | |
 | 100 |
2.7 |
94.9 |
2.4 |
 | 100 |
9.8 |
90.2 | |
 | 100 |
10.3 |
89.7 | |
 | 65.5 |
100 | ||
 | 39.4 |
100 | ||
Table 4 Catalytic hydrogenation of other phenolic model compounds
| 底物 | 转化率/% | 选择性/% | ||
|---|---|---|---|---|
 | 100 |
7.7 |
92.3 | |
 | 100 |
7.8 |
92.2 | |
 | 100 |
2.7 |
94.9 |
2.4 |
 | 100 |
9.8 |
90.2 | |
 | 100 |
10.3 |
89.7 | |
 | 65.5 |
100 | ||
 | 39.4 |
100 | ||
| 1 | Huber G W, Chheda J N, Barrett C J, et al. Production of liquid alkanes by aqueous-phase processing of biomass-derived carbohydrates[J]. Science, 2005, 308(5727): 1446-1450. |
| 2 | Yang Z, Luo B W, Shu R Y, et al. Efficient hydrodeoxygenation of phenolic compounds and raw lignin-oil under a temperature-controlled phase-transfer catalysis[J]. Fuel, 2021, 291: 120091. |
| 3 | Dou X M, Jiang X, Li W Z, et al. Highly efficient conversion of Kraft lignin into liquid fuels with a Co-Zn-beta zeolite catalyst[J]. Applied Catalysis B: Environmental, 2020, 268: 118429. |
| 4 | 王江丽, 薛敏, 赵承科, 等. 木质素分级对其应用性能的影响[J]. 化工学报, 2022, 73(5): 1894-1907. |
| Wang J L, Xue M, Zhao C K, et al. Influences of lignin fractionation on its utilization[J]. CIESC Journal, 2022, 73(5): 1894-1907. | |
| 5 | 王东玲, 王文锦, 彭梓芳, 等. 醇溶剂提取松木木质素及其结构表征[J]. 化工学报, 2020, 71(8): 3761-3769. |
| Wang D L, Wang W J, Peng Z F, et al. Structure characterization of pine lignin extracted by different alcohol solvents[J]. CIESC Journal, 2020, 71(8): 3761-3769. | |
| 6 | Li C Z, Zhao X C, Wang A Q, et al. Catalytic transformation of lignin for the production of chemicals and fuels[J]. Chemical Reviews, 2015, 115(21): 11559-11624. |
| 7 | Long J X, Xu Y, Wang T J, et al. Efficient base-catalyzed decomposition and in situ hydrogenolysis process for lignin depolymerization and char elimination[J]. Applied Energy, 2015, 141: 70-79. |
| 8 | Zhang X, Wang K G, Chen J H, et al. Mild hydrogenation of bio-oil and its derived phenolic monomers over Pt-Ni bimetal-based catalysts[J]. Applied Energy, 2020, 275: 115154. |
| 9 | 李雁斌, 徐莹, 马隆龙, 等. 邻甲酚液相原位加氢反应[J]. 高等学校化学学报, 2014, 35(12): 2654-2661. |
| Li Y B, Xu Y, Ma L L, et al. Research on in situ hydrogenation of o-cresol over Ni/CMK-3 catalysts[J]. Chemical Journal of Chinese Universities, 2014, 35(12): 2654-2661. | |
| 10 | Wen J L, Sun S L, Yuan T Q, et al. Understanding the chemical and structural transformations of lignin macromolecule during torrefaction[J]. Applied Energy, 2014, 121: 1-9. |
| 11 | Shu R Y, Zhong Z J, You H Y, et al. Hydrodeoxygenation of lignin-derived phenolic compounds over Ru/TiO2-CeO2 catalyst prepared by photochemical reduction method[J]. Journal of the Energy Institute, 2021, 99: 1-8. |
| 12 | Niu Z, Zhang S, Sun Y, et al. Controllable synthesis of N i / S i O ₂ hollow spheres and their excellent catalytic performance in 4-nitrophenol reduction[J]. Dalton Transactions, 2014, 43(44): 16911-16918. |
| 13 | Shu R Y, Zhang Q, Xu Y, et al. Hydrogenation of lignin-derived phenolic compounds over step by step precipitated Ni/SiO2 [J]. RSC Advances, 2016, 6(7): 5214-5222. |
| 14 | Mao C, Zheng J W, Matsagar B M, et al. Highly-efficient Ru/Al-SBA-15 catalysts with strong Lewis acid sites for the water-assisted hydrogenation of p-phthalic acid[J]. Catalysis Science & Technology, 2020, 10(8): 2443-2451. |
| 15 | Rao D M, Xue X G, Cui G Q, et al. Metal-acid site synergistic catalysis in Ru–ZrO2 toward selective hydrogenation of benzene to cyclohexene[J]. Catalysis Science & Technology, 2018, 8(1): 236-243. |
| 16 | You H Y, Yang Z, Lin J J, et al. Hydrogenation of lignin-derived phenolic compounds over Ru/C enhanced by Al2O3 catalyst at room temperature[J]. ChemistrySelect, 2022, 7(19): e202200709. |
| 17 | Li A Q, Shen K, Chen J Y, et al. Highly selective hydrogenation of phenol to cyclohexanol over MOF-derived non-noble Co-Ni@NC catalysts[J]. Chemical Engineering Science, 2017, 166: 66-76. |
| 18 | Li F, Cao B, Zhu W X, et al. Hydrogenation of phenol over Pt/CNTs: the effects of Pt loading and reaction solvents[J]. Catalysts, 2017, 7(5): 145. |
| 19 | Takagi H, Isoda T, Kusakabe K, et al. Effects of solvents on the hydrogenation of mono-aromatic compounds using noble-metal catalysts[J]. Energy & Fuels, 1999, 13(6): 1191-1196. |
| 20 | Shi Y K, Qu G F, Ning P, et al. Advances of application of ionic liquids in catalytic oxidation reactions[J]. Advanced Materials Research, 2011, 233/234/235: 499-506. |
| 21 | Neri G, Visco A M, Donato A, et al. Hydrogenation of phenol to cyclohexanone over palladium and alkali-doped palladium catalysts[J]. Applied Catalysis A: General, 1994, 110(1): 49-59. |
| 22 | Chen Y Z, Liaw C W, Lee L I. Selective hydrogenation of phenol to cyclohexanone over palladium supported on calcined Mg/Al hydrotalcite[J]. Applied Catalysis A: General, 1999, 177(1): 1-8. |
| 23 | Kimura Y, Haraguchi K. Clay-alcohol-water dispersions: anomalous viscosity changes due to network formation of clay nanosheets induced by alcohol clustering[J]. Langmuir, 2017, 33(19): 4758-4768. |
| 24 | 章胜男, 韩东梅, 任山, 等. 有机电极材料固定化策略[J]. 化学进展, 2020, 32(1): 103-118. |
| Zhang S N, Han D M, Ren S, et al. Immobilization strategies of organic electrode materials[J]. Progress in Chemistry, 2020, 32(1): 103-118. | |
| 25 | Wang X Y, Rinaldi R. Solvent effects on the hydrogenolysis of diphenyl ether with raney nickel and their implications for the conversion of lignin[J]. ChemSusChem, 2012, 5(8): 1455-1466. |
| 26 | Mazzieri V A, L'Argentiére P C, Fígoli N S. Influence of methanol addition during selective hydrogenation of benzene to cyclohexene[J]. Reaction Kinetics and Catalysis Letters, 2004, 81(1): 107-112. |
| 27 | Kim G J, Kim M S, Byun J Y, et al. Effects of Ru addition to Pd/Al2O3 catalysts on methanol steam reforming reaction: a mechanistic study[J]. Applied Catalysis A: General, 2019, 572: 115-123. |
| 28 | Strobel A B, Egert T, Langguth P. Predicting leachables solubilization in polysorbate 80 solutions by a linear solvation energy relationship (LSER)[J]. Pharmaceutical Research, 2021, 38(9): 1549-1561. |
| 29 | Marcus Y. The properties of organic liquids that are relevant to their use as solvating solvents[J]. ChemInform, 1994, 25(12): 409-460. |
| 30 | Liu H Z, Jiang T, Han B X, et al. Selective phenol hydrogenation to cyclohexanone over a dual supported Pd-Lewis acid catalyst[J]. Science, 2009, 326(5957): 1250-1252. |
| 31 | Laurent E, Delmon B. Influence of oxygen-, nitrogen-, and sulfur-containing compounds on the hydrodeoxygenation of phenols over sulfided cobalt-molybdenum/.gamma.-alumina and nickel-molybdenum/.gamma.-alumina catalysts[J]. Industrial & Engineering Chemistry Research, 1993, 32(11): 2516-2524. |
| 32 | Zhuang L, Li H X, Dai W L, et al. Liquid phase hydrogenation of phenol to cyclohexenone over a Pd-La-B amorphous catalyst[J]. Chemistry Letters, 2003, 32(11): 1072-1073. |
| 33 | Velu S, Kapoor M P, Inagaki S, et al. Vapor phase hydrogenation of phenol over palladium supported on mesoporous CeO2 and ZrO2 [J]. Applied Catalysis A: General, 2003, 245(2): 317-331. |
| [1] | Yepin CHENG, Daqing HU, Yisha XU, Huayan LIU, Hanfeng LU, Guokai CUI. Application of ionic liquid-based deep eutectic solvents for CO2 conversion [J]. CIESC Journal, 2023, 74(9): 3640-3653. |
| [2] | Jie CHEN, Yongsheng LIN, Kai XIAO, Chen YANG, Ting QIU. Study on catalytic synthesis of sec-butanol by tunable choline-based basic ionic liquids [J]. CIESC Journal, 2023, 74(9): 3716-3730. |
| [3] | Yitong LI, Hang GUO, Hao CHEN, Fang YE. Study on operating conditions of proton exchange membrane fuel cells with non-uniform catalyst distributions [J]. CIESC Journal, 2023, 74(9): 3831-3840. |
| [4] | Xuejin YANG, Jintao YANG, Ping NING, Fang WANG, Xiaoshuang SONG, Lijuan JIA, Jiayu FENG. Research progress in dry purification technology of highly toxic gas PH3 [J]. CIESC Journal, 2023, 74(9): 3742-3755. |
| [5] | Xin YANG, Xiao PENG, Kairu XUE, Mengwei SU, Yan WU. Preparation of molecularly imprinted-TiO2 and its properties of photoelectrocatalytic degradation of solubilized PHE [J]. CIESC Journal, 2023, 74(8): 3564-3571. |
| [6] | Feifei YANG, Shixi ZHAO, Wei ZHOU, Zhonghai NI. Sn doped In2O3 catalyst for selective hydrogenation of CO2 to methanol [J]. CIESC Journal, 2023, 74(8): 3366-3374. |
| [7] | Kaixuan LI, Wei TAN, Manyu ZHANG, Zhihao XU, Xuyu WANG, Hongbing JI. Design of cobalt-nitrogen-carbon/activated carbon rich in zero valent cobalt active site and application of catalytic oxidation of formaldehyde [J]. CIESC Journal, 2023, 74(8): 3342-3352. |
| [8] | Pan LI, Junyang MA, Zhihao CHEN, Li WANG, Yun GUO. Effect of the morphology of Ru/α-MnO2 on NH3-SCO performance [J]. CIESC Journal, 2023, 74(7): 2908-2918. |
| [9] | Yajie YU, Jingru LI, Shufeng ZHOU, Qingbiao LI, Guowu ZHAN. Construction of nanomaterial and integrated catalyst based on biological template: a review [J]. CIESC Journal, 2023, 74(7): 2735-2752. |
| [10] | Yuming TU, Gaoyan SHAO, Jianjie CHEN, Feng LIU, Shichao TIAN, Zhiyong ZHOU, Zhongqi REN. Advances in the design, synthesis and application of calcium-based catalysts [J]. CIESC Journal, 2023, 74(7): 2717-2734. |
| [11] | Qiyu ZHANG, Lijun GAO, Yuhang SU, Xiaobo MA, Yicheng WANG, Yating ZHANG, Chao HU. Recent advances in carbon-based catalysts for electrochemical reduction of carbon dioxide [J]. CIESC Journal, 2023, 74(7): 2753-2772. |
| [12] | Jipeng ZHOU, Wenjun HE, Tao LI. Reaction engineering calculation of deactivation kinetics for ethylene catalytic oxidation over irregular-shaped catalysts [J]. CIESC Journal, 2023, 74(6): 2416-2426. |
| [13] | Tan ZHANG, Guang LIU, Jinping LI, Yuhan SUN. Performance regulation strategies of Ru-based nitrogen reduction electrocatalysts [J]. CIESC Journal, 2023, 74(6): 2264-2280. |
| [14] | Chen WANG, Xiufeng SHI, Xianfeng WU, Fangjia WEI, Haohong ZHANG, Yin CHE, Xu WU. Preparation of Mn3O4 catalyst by redox method and study on its catalytic oxidation performance and mechanism of toluene [J]. CIESC Journal, 2023, 74(6): 2447-2457. |
| [15] | Yong LI, Jiaqi GAO, Chao DU, Yali ZHAO, Boqiong LI, Qianqian SHEN, Husheng JIA, Jinbo XUE. Construction of Ni@C@TiO2 core-shell dual-heterojunctions for advanced photo-thermal catalytic hydrogen generation [J]. CIESC Journal, 2023, 74(6): 2458-2467. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||