CIESC Journal ›› 2025, Vol. 76 ›› Issue (12): 6151-6162.DOI: 10.11949/0438-1157.20250416
• Reviews and monographs • Previous Articles Next Articles
Received:2025-04-20
Revised:2025-05-24
Online:2026-01-23
Published:2025-12-31
Contact:
Yanqin XU
通讯作者:
徐彦芹
作者简介:蔡文静(2001—),女,硕士研究生,384246686@qq.com
CLC Number:
Wenjing CAI, Yanqin XU. Interface regulation and research progress of sulfide electrolytes[J]. CIESC Journal, 2025, 76(12): 6151-6162.
蔡文静, 徐彦芹. 硫化物电解质的界面调控及研究进展[J]. 化工学报, 2025, 76(12): 6151-6162.
Add to citation manager EndNote|Ris|BibTeX
| 体系类别 | 电解质 | 离子电导率/ (S·cm-1) | 文献 |
|---|---|---|---|
| Li-P-S | Li7P3S11 | 3.2×10-3 | [ |
| Li7P3S11 | 1.7×10-2 | [ | |
| β-Li3PS4 | 1.6×10-4 | [ | |
| Thio-LISICON | Li4SnS4 | 7.0×10-5 | [ |
| Li3.25Ge0.25P0.75S3.25 | 2.2×10-3 | [ | |
| LGPS | Li10SiP2S12 | 2.3×10-3 | [ |
| Li10SnP2S12 | 4×10-3 | [ | |
| Li10GeP2S12 | 1.2×10-2 | [ | |
| Li9.54Si1.74P1.44S11.7Cl0.3 | 2.5×10-2 | [ | |
| Li9.54(Si0.6Ge0.4)1.74P1.44S11.1Br0.3O0.6 | 3.2×10-2 | [ | |
| Argyrodite | Li6PS5Cl | 4.9×10-3 | [ |
| Li6PS5Br | 2.6×10-3 | [ | |
| Li6PS5I | 10-7 | [ | |
| Li5.5PS4.5Cl1.5 | 9.4×10-3 | [ | |
| Li6.6Sb0.4Si0.6S5I | 1.48×10-2 | [ |
Table 1 Common sulfide electrolytes and their ionic conductivity
| 体系类别 | 电解质 | 离子电导率/ (S·cm-1) | 文献 |
|---|---|---|---|
| Li-P-S | Li7P3S11 | 3.2×10-3 | [ |
| Li7P3S11 | 1.7×10-2 | [ | |
| β-Li3PS4 | 1.6×10-4 | [ | |
| Thio-LISICON | Li4SnS4 | 7.0×10-5 | [ |
| Li3.25Ge0.25P0.75S3.25 | 2.2×10-3 | [ | |
| LGPS | Li10SiP2S12 | 2.3×10-3 | [ |
| Li10SnP2S12 | 4×10-3 | [ | |
| Li10GeP2S12 | 1.2×10-2 | [ | |
| Li9.54Si1.74P1.44S11.7Cl0.3 | 2.5×10-2 | [ | |
| Li9.54(Si0.6Ge0.4)1.74P1.44S11.1Br0.3O0.6 | 3.2×10-2 | [ | |
| Argyrodite | Li6PS5Cl | 4.9×10-3 | [ |
| Li6PS5Br | 2.6×10-3 | [ | |
| Li6PS5I | 10-7 | [ | |
| Li5.5PS4.5Cl1.5 | 9.4×10-3 | [ | |
| Li6.6Sb0.4Si0.6S5I | 1.48×10-2 | [ |
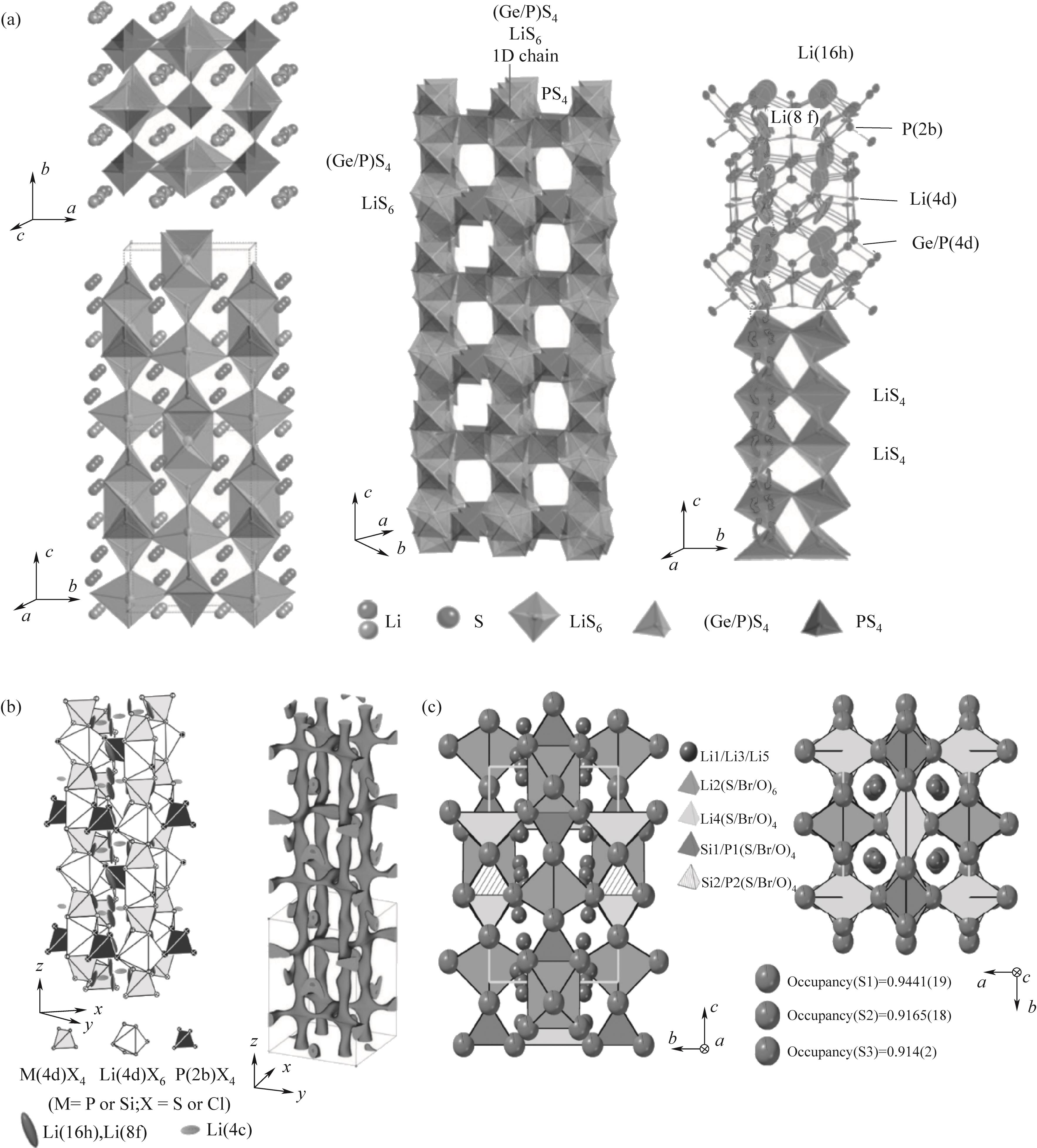
Fig.1 (a) The structure and conduction pathway of Li10GeP2S12[26]; (b) Li9.54Si1.74P1.44S11.7Cl0.3 structure[29]; (c) Li9.54(Si0.6Ge0.4)1.74P1.44S11.1Br0.3O0.6 structure(c)[5]
| [1] | Yan C, Xu R, Xiao Y, et al. Toward critical electrode/electrolyte interfaces in rechargeable batteries[J]. Advanced Functional Materials, 2020, 30(23): 1909887. |
| [2] | Lee S, Kim Y, Park C, et al. Interplay of athode-halide solid electrolyte in enhancing thermal stability of charged cathode material in all-solid-state batteries [J]. ACS Energy Letters, 2024, 9(4): 1369-1380. |
| [3] | Manthiram A, Yu X W, Wang S F. Lithium battery chemistries enabled by solid-state electrolytes[J]. Nature Reviews Materials, 2017, 2(4): 16103. |
| [4] | Wang Z Y, Xia J L, Ji X, et al. Lithium anode interlayer design for all-solid-state lithium-metal batteries[J]. Nature Energy, 2024, 9: 251-262. |
| [5] | Li Y X, Song S B, Kim H, et al. A lithium superionic conductor for millimeter-thick battery electrode[J]. Science, 2023, 381(6653): 50-53. |
| [6] | Bates A M, Preger Y, Torres-Castro L, et al. Are solid-state batteries safer than lithium-ion batteries?[J]. Joule, 2022, 6(4): 742-755. |
| [7] | Ma Y, Zhang R Z, Ma Y J, et al. Interface and electrode microstructure engineering for optimizing performance of the LiNiO2 cathode in all-solid-state batteries [J]. Chemistry of Materials, 2024, 36(5): 2588-2598. |
| [8] | Zhang X Y, Yang J F, Deng E L, et al. Argyrodite based all-solid-state-batteries: recent advances and perspective[J]. Energy Storage Materials, 2025, 79: 104339. |
| [9] | Famprikis T, Canepa P, Dawson J A, et al. Fundamentals of inorganic solid-state electrolytes for batteries[J]. Nature Materials, 2019, 18: 1278-1291. |
| [10] | Shah N J, Fang C, Osti N C, et al. Nanosecond solvation dynamics in a polymer electrolyte for lithium batteries[J]. Nature Materials, 2024, 23(5): 664-669. |
| [11] | Zhou S J, Liu K X, Wang Z Y, et al. An ultra-thin asymmetric solid polymer electrolyte for in situ integrated lithium-metal battery[J]. Chemical Engineering Journal, 2025, 504: 158548. |
| [12] | Kim K J, Balaish M, Wadaguchi M, et al. Solid-state Li-metal batteries: challenges and horizons of oxide and sulfide solid electrolytes and their interfaces[J]. Advanced Energy Materials, 2021, 11(1): 2002689. |
| [13] | Li J W, Li Y Y, Wang Y X, et al. Preparation, design and interfacial modification of sulfide solid electrolytes for all-solid-state lithium metal batteries[J]. Energy Storage Materials, 2025, 74: 103962. |
| [14] | Yao M X, Shi J T, Luo A H, et al. Advances in sulfide solid-state electrolytes for lithium batteries[J]. Energy Storage Materials, 2025, 75: 104018. |
| [15] | Zhang Y J, Sun J C, Li L S, et al. Advancements in the emerging rare-earth halide solid electrolytes for next-generation all-solid-state lithium batteries[J]. Coordination Chemistry Reviews, 2025, 528: 216432. |
| [16] | Chen S J, Xie D J, Liu G Z, et al. Sulfide solid electrolytes for all-solid-state lithium batteries: structure, conductivity, stability and application[J]. Energy Storage Materials, 2018, 14: 58-74. |
| [17] | Wu J H, Shen L, Zhang Z H, et al. All-solid-state lithium batteries with sulfide electrolytes and oxide cathodes[J]. Electrochemical Energy Reviews, 2021, 4(1): 101-135. |
| [18] | Wang C H, Adair K, Sun X L. All-solid-state lithium metal batteries with sulfide electrolytes: understanding interfacial ion and electron transport[J]. Accounts of Materials Research, 2022, 3(1): 21-32. |
| [19] | Yu T, Liu Y K, Li H Y, et al. Ductile inorganic solid electrolytes for all-solid-state lithium batteries[J]. Chemical Reviews, 2025, 125(6): 3595-3662. |
| [20] | Zhang Q, Cao D X, Ma Y, et al. Sulfide-based solid-state electrolytes: synthesis, stability, and potential for all-solid-state batteries[J]. Advanced Materials, 2019, 31(44): 1901131. |
| [21] | Ribes M, Barrau B, Souquet J L. Sulfide glasses: glass forming region, structure and ionic conduction of glasses in Na2S-XS2 (X=Si; Ge), Na2S-P2S5 and Li2S-GeS2 systems[J]. Journal of Non-Crystalline Solids, 1980, 38: 271-276. |
| [22] | Yamane H, Shibata M, Shimane Y, et al. Crystal structure of a superionic conductor, Li7P3S11 [J]. Solid State Ionics, 2007, 178(15/16/17/18): 1163-1167. |
| [23] | Seino Y, Ota T, Takada K, et al. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries[J]. Energy & Environmental Science, 2014, 7(2): 627-631. |
| [24] | Liu Z C, Fu W J, Andrew Payzant E, et al. Anomalous high ionic conductivity of nanoporous β-Li3PS4 [J]. Journal of the American Chemical Society, 2013, 135(3): 975-978. |
| [25] | Kaib T, Haddadpour S, Kapitein M, et al. New lithium chalcogenidotetrelates, LiChT: synthesis and characterization of the Li+-conducting tetralithium ortho-sulfidostannate Li4SnS4 [J]. Chemistry of Materials, 2012, 24(11): 2211-2219. |
| [26] | Kamaya N, Homma K, Yamakawa Y, et al. A lithium superionic conductor[J]. Nature Materials, 2011, 10: 682-686. |
| [27] | Whiteley J M, Woo J H, Hu E Y, et al. Empowering the lithium metal battery through a silicon-based superionic conductor[J]. Journal of the Electrochemical Society, 2014, 161(12): A1812-A1817. |
| [28] | Bron P, Johansson S, Zick K, et al. Li10SnP2S12: an affordable lithium superionic conductor[J]. Journal of the American Chemical Society, 2013, 135(42): 15694-15697. |
| [29] | Kato Y, Hori S, Saito T, et al. High-power all-solid-state batteries using sulfide superionic conductors[J]. Nature Energy, 2016, 1(4): 16030. |
| [30] | Yu C, Ganapathy S, Hageman J, et al. Facile synthesis toward the optimal structure-conductivity characteristics of the argyrodite Li6PS5Cl solid-state electrolyte[J]. ACS Applied Materials & Interfaces, 2018, 10(39): 33296-33306. |
| [31] | Yu C, Hageman J, Ganapathy S, et al. Tailoring Li6PS5Br ionic conductivity and understanding of its role in cathode mixtures for high performance all-solid-state Li-S batteries[J]. Journal of Materials Chemistry A, 2019, 7(17): 10412-10421. |
| [32] | Zhang J, Li L J, Zheng C, et al. Silicon-doped argyrodite solid electrolyte Li6PS5I with improved ionic conductivity and interfacial compatibility for high-performance all-solid-state lithium batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(37): 41538-41545. |
| [33] | Adeli P, Bazak J D, Park K H, et al. Boosting solid-state diffusivity and conductivity in lithium superionic argyrodites by halide substitution[J]. Angewandte Chemie International Edition, 2019, 58(26): 8681-8686. |
| [34] | Zhou L D, Assoud A, Zhang Q, et al. New family of argyrodite thioantimonate lithium superionic conductors[J]. Journal of the American Chemical Society, 2019, 141(48): 19002-19013. |
| [35] | Dietrich C, Weber D A, Culver S, et al. Synthesis, structural characterization, and lithium ion conductivity of the lithium thiophosphate Li2P2S6 [J]. Inorganic Chemistry, 2017, 56(11): 6681-6687. |
| [36] | Hayashi A, Hama S, Mizuno F, et al. Characterization of Li2S-P2S5 glass-ceramics as a solid electrolyte for lithium secondary batteries[J]. Solid State Ionics, 2004, 175(1/2/3/4): 683-686. |
| [37] | Murayama M, Kanno R, Kawamoto Y, et al. Structure of the thio-LISICON, Li4GeS4 [J]. Solid State Ionics, 2002, 154: 789-794. |
| [38] | Zhou P F, Wang J B, Cheng F Y, et al. A solid lithium superionic conductor Li11AlP2S12 with a thio-LISICON analogous structure[J]. Chemical Communications, 2016, 52(36): 6091-6094. |
| [39] | Sahu G, Lin Z, Li J C, et al. Air-stable, high-conduction solid electrolytes of arsenic-substituted Li4SnS4 [J]. Energy & Environmental Science, 2014, 7(3): 1053-1058. |
| [40] | Zhang P, Li L, Du P, et al. Li8.2SiP1.4S9.6: a novel sulfide solid electrolyte for lithium-ion battery[J]. Journal of Energy Storage, 2025, 123: 116789. |
| [41] | Deiseroth H J, Kong S T, Eckert H, et al. Li6PS5X: a class of crystalline Li-rich solids with an unusually high Li+ mobility[J]. Angewandte Chemie International Edition, 2008, 47(4): 755-758. |
| [42] | Kong S, Gün Ö, Koch B, et al. Structural characterisation of the Li argyrodites Li7PS6 and Li7PSe6 and their solid solutions: quantification of site preferences by MAS-NMR spectroscopy[J]. Chemistry – A European Journal, 2010, 16(17): 5138-5147. |
| [43] | Hanghofer I, Brinek M, Eisbacher S L, et al. Substitutional disorder: structure and ion dynamics of the argyrodites Li6PS5Cl, Li6PS5Br and Li6PS5I[J]. Physical Chemistry Chemical Physics, 2019, 21(16): 8489-8507. |
| [44] | Adeli P, Bazak J D, Huq A, et al. Influence of aliovalent cation substitution and mechanical compression on Li-ion conductivity and diffusivity in argyrodite solid electrolytes[J]. Chemistry of Materials, 2021, 33(1): 146-157. |
| [45] | Zhang Z R, Zhang J X, Jia H H, et al. Enhancing ionic conductivity of solid electrolyte by lithium substitution in halogenated Li-Argyrodite[J]. Journal of Power Sources, 2020, 450: 227601. |
| [46] | Bai X T, Duan Y, Zhuang W D, et al. Research progress in Li-argyrodite-based solid-state electrolytes[J]. Journal of Materials Chemistry A, 2020, 8(48): 25663-25686. |
| [47] | Pearson R G. Hard and soft acids and bases[J]. Journal of the American Chemical Society, 1963, 85(22): 3533-3539. |
| [48] | Lu P S, Wu D X, Chen L Q, et al. Air stability of solid-state sulfide batteries and electrolytes[J]. Electrochemical Energy Reviews, 2022, 5(3): 3. |
| [49] | Xu H J, Cao G Q, Shen Y L, et al. Enabling argyrodite sulfides as superb solid-state electrolyte with remarkable interfacial stability against electrodes[J]. Energy & Environmental Materials, 2022, 5(3): 852-864. |
| [50] | Liu H, Zhu Q S, Liang Y H, et al. Versatility of Sb-doping enabling argyrodite electrolyte with superior moisture stability and Li metal compatibility towards practical all-solid-state Li metal batteries[J]. Chemical Engineering Journal, 2023, 462: 142183. |
| [51] | Li G Y, Wu S P, Zheng H P, et al. Sn-O dual-substituted chlorine-rich argyrodite electrolyte with enhanced moisture and electrochemical stability[J]. Advanced Functional Materials, 2023, 33(11): 2211805. |
| [52] | Zhang C J. Tuning the composition[J]. Nature Energy, 2023, 8(8): 772. |
| [53] | Tan D H S, Banerjee A, Chen Z, et al. Author correction: from nanoscale interface characterization to sustainable energy storage using all-solid-state batteries[J]. Nature Nanotechnology, 2021, 16: 479. |
| [54] | Liang Y H, Liu H, Wang G X, et al. Challenges, interface engineering, and processing strategies toward practical sulfide-based all-solid-state lithium batteries[J]. InfoMat, 2022, 4(5): e12292. |
| [55] | Wagner C. The electrical conductivity of semi-conductors involving inclusions of another phase[J]. Journal of Physics and Chemistry of Solids, 1972, 33(5): 1051-1059. |
| [56] | Maier J. Ionic conduction in space charge regions[J]. Progress in Solid State Chemistry, 1995, 23(3): 171-263. |
| [57] | Liang C C. Conduction characteristics of the lithium iodide-aluminum oxide solid electrolytes[J]. Journal of the Electrochemical Society, 1973, 120(10): 1289. |
| [58] | Haruyama J, Sodeyama K, Han L Y, et al. Space-charge layer effect at interface between oxide cathode and sulfide electrolyte in all-solid-state lithium-ion battery[J]. Chemistry of Materials, 2014, 26(14): 4248-4255. |
| [59] | Xiao Y H, Miara L J, Wang Y, et al. Computational screening of cathode coatings for solid-state batteries[J]. Joule, 2019, 3(5): 1252-1275. |
| [60] | Strauss F, Teo J H, Maibach J, et al. Li2ZrO3-coated NCM622 for application in inorganic solid-state batteries: role of surface carbonates in the cycling performance[J]. ACS Applied Materials & Interfaces, 2020, 12(51): 57146-57154. |
| [61] | Luo Q Y, Yu C, Wei C C, et al. Enabling superior electrochemical performances of Li10SnP2S12-based all-solid-state batteries using lithium halide electrolytes[J]. Ceramics International, 2023, 49(7): 11485-11493. |
| [62] | Sakuda A, Hayashi A, Tatsumisago M. Interfacial observation between LiCoO2 electrode and Li2S-P2S5 solid electrolytes of all-solid-state lithium secondary batteries using transmission electron microscopy[J]. Chemistry of Materials, 2010, 22(3): 949-956. |
| [63] | Kim K H, Iriyama Y, Yamamoto K, et al. Characterization of the interface between LiCoO2 and Li7La3Zr2O12 in an all-solid-state rechargeable lithium battery[J]. Journal of Power Sources, 2011, 196(2): 764-767. |
| [64] | Zhu Y Z, He X F, Mo Y F. Origin of outstanding stability in the lithium solid electrolyte materials: insights from thermodynamic analyses based on first-principles calculations[J]. ACS Applied Materials & Interfaces, 2015, 7(42): 23685-23693. |
| [65] | Han F D, Zhu Y Z, He X F, et al. Electrochemical stability of Li10GeP2S12 and Li7La3Zr2O12 solid electrolytes[J]. Advanced Energy Materials, 2016, 6(8): 1501590. |
| [66] | Dong Z L, Gan Y, Martins V, et al. Novel sulfide-chloride solid-state electrolytes with tunable anion ratio for highly stable solid-state sodium-ion batteries[J]. Advanced Materials, 2025, n/a(n/a): 2503107. |
| [67] | Auvergniot J, Cassel A, Ledeuil J B, et al. Interface stability of argyrodite Li6PS5Cl toward LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 in bulk all-solid-state batteries[J]. Chemistry of Materials, 2017, 29(9): 3883-3890. |
| [68] | Li Y X, Daikuhara S, Hori S, et al. Oxygen substitution for Li-Si-P-S-Cl solid electrolytes toward purified Li10GeP2S12-type phase with enhanced electrochemical stabilities for all-solid-state batteries[J]. Chemistry of Materials, 2020, 32(20): 8860-8867. |
| [69] | Oh P P, Yun D J, Choi D J H, et al. Development of high-energy anodes for all-solid-state lithium batteries based on sulfide electrolytes[J]. Angewandte Chemie International Edition, 2022, 61(25): e202201249. |
| [70] | Jing S H, Wang K, Li S J, et al. An all-in-one approach for sulfide solid electrolyte with bidirectional stabilization shells enabling 4.6 V all-solid-state lithium batteries[J]. Energy Storage Materials, 2025, 76: 104131. |
| [71] | Hu X, Zhang Z J, Zhang X, et al. External-pressure–electrochemistry coupling in solid-state lithium metal batteries[J]. Nature Reviews Materials, 2024, 9(5): 305-320. |
| [72] | Lu Y, Zhao C Z, Yuan H, et al. Critical current density in solid-state lithium metal batteries: mechanism, influences, and strategies[J]. Advanced Functional Materials, 2021, 31(18): 2009925. |
| [73] | Liu J, Yuan H, Liu H, et al. Unlocking the failure mechanism of solid state lithium metal batteries[J]. Advanced Energy Materials, 2022, 12(4): 2100748. |
| [74] | Porz L, Swamy T, Sheldon B W, et al. Mechanism of lithium metal penetration through inorganic solid electrolytes[J]. Advanced Energy Materials, 2017, 7(20): 1701003. |
| [75] | Kasemchainan J, Zekoll S, Spencer Jolly D, et al. Critical stripping current leads to dendrite formation on plating in lithium anode solid electrolyte cells[J]. Nature Materials, 2019, 18(10): 1105-1111. |
| [76] | Yang D X, Gao D X, Jiang D M, et al. Grain boundary electronic insulation for high-performance all-solid-state lithium batteries[J]. Angewandte Chemie International Edition, 2023, 62(5): e202215680. |
| [77] | Pang M C, Yang K, Brugge R, et al. Interactions are important: Linking multi-physics mechanisms to the performance and degradation of solid-state batteries[J]. Materials Today, 2021, 49: 145-183. |
| [78] | Mangani L R, Villevieille C. Mechanical vs. chemical stability of sulphide-based solid-state batteries. Which one is the biggest challenge to tackle? Overview of solid-state batteries and hybrid solid state batteries[J]. Journal of Materials Chemistry A, 2020, 8(20): 10150-10167. |
| [79] | Kato A, Yamamoto M, Sakuda A, et al. Mechanical properties of Li2S-P2S5 glasses with lithium halides and application in all-solid-state batteries[J]. ACS Applied Energy Materials, 2018, 1(3): 1002-1007. |
| [80] | Masias A, Felten N, Garcia-Mendez R, et al. Elastic, plastic, and creep mechanical properties of lithium metal[J]. Journal of Materials Science, 2019, 54(3): 2585-2600. |
| [81] | Yu S, Siegel D J. Grain boundary softening: a potential mechanism for lithium metal penetration through stiff solid electrolytes[J]. ACS Applied Materials & Interfaces, 2018, 10(44): 38151-38158. |
| [82] | Doux J M, Nguyen H, Tan D H S, et al. Stack pressure considerations for room-temperature all-solid-state lithium metal batteries[J]. Advanced Energy Materials, 2020, 10(1): 1903253. |
| [83] | Wenzel S, Randau S, Leichtweiß T, et al. Direct observation of the interfacial instability of the fast ionic conductor Li10GeP2S12 at the lithium metal anode[J]. Chemistry of Materials, 2016, 28(7): 2400-2407. |
| [84] | Krauskopf T, Richter F H, Zeier W G, et al. Physicochemical concepts of the lithium metal anode in solid-state batteries[J]. Chemical Reviews, 2020, 120(15): 7745-7794. |
| [85] | Fan X L, Ji X, Han F D, et al. Fluorinated solid electrolyte interphase enables highly reversible solid-state Li metal battery[J]. Science Advances, 2018, 4(12): eaau9245. |
| [1] | Yufeng WANG, Xiaoxue LUO, Hongliang FAN, Baijing WU, Cunpu LI, Zidong WEI. Green organic electrosynthesis coupled with water electrolysis to produce hydrogen—overview of electrode interface regulation strategies [J]. CIESC Journal, 2025, 76(8): 3753-3771. |
| [2] | Guoqing SUN, Haibo LI, Zhiyang DING, Wenhui GUO, Hao XU, Yanxia ZHAO. Research progress of silicon based anode materials [J]. CIESC Journal, 2025, 76(7): 3197-3211. |
| [3] | Fengfeng GAO, Huifeng CHENG, Bo YANG, Xiaogang HAO. Electrically driven NiFeMn LDH/CNTs/PVDF film electrode for selective extraction of tungstate ions [J]. CIESC Journal, 2025, 76(7): 3350-3360. |
| [4] | Yinxiang TANG, Feng ZHU, Yingying FAN, Yuxin LONG, Yong DAI, Chunling DENG, Xiaofeng HUANG. Effect of preparation conditions on low-temperature co-removal of COS and CS2 from modified calcium carbide slag [J]. CIESC Journal, 2025, 76(7): 3639-3650. |
| [5] | Ziheng WANG, Wenhuai LI, Wei ZHOU. Application of patterned electrodes in solid oxide fuel cell [J]. CIESC Journal, 2025, 76(7): 3153-3171. |
| [6] | Junyi WANG, Zhangxun XIA, Fenning JING, Suli WANG. Study on the relaxation time distribution of electrochemical impedance spectroscopy in high temperature polymer electrolyte membrane fuel cells based on reformed hydrogen fuels [J]. CIESC Journal, 2025, 76(7): 3509-3520. |
| [7] | Quankang SHENG, Ao CHEN, Long CHEN, Yu ZHANG, Shaoyun CHEN, Chenglong HU. In situ growth of oriented polyaniline nanorod array on pencil core and its electrochemical energy storage [J]. CIESC Journal, 2025, 76(4): 1875-1884. |
| [8] | Ruixue CUI, Yuhan ZHANG, Fang SUN. Synthesis and photoinitiation property of a cinnamyl formamide disulfide compound [J]. CIESC Journal, 2025, 76(3): 1305-1311. |
| [9] | Guipei XU, Qian SUN, Jiewen LAI, Yifeng LU, Huifang DI, Hui HUANG, Zhenbing WANG. Research progress on failure mechanism of electrochemical double layer capacitors [J]. CIESC Journal, 2025, 76(3): 951-962. |
| [10] | Zhongqing LI, Zhiyuan WANG, Xiaojian LUAN, Sikai LIANG, Kai WANG. Preparation of MnO coating based on electroplating-low oxygen partial pressure treatment and coking inhibition properties during thermal cracking of naphtha [J]. CIESC Journal, 2025, 76(3): 1050-1063. |
| [11] | Xiaohang ZHONG, Wei XU, Wen ZHANG, Li XU, Yuxin WANG. A critical review on the effects of Fe impurity on H2 production via alkaline water electrolysis [J]. CIESC Journal, 2025, 76(2): 519-531. |
| [12] | Menghan WANG, Miao YU, Tong WU. Research progress of electrolyte for lithium-sulfur batteries: molecular design and application [J]. CIESC Journal, 2025, 76(12): 6196-6217. |
| [13] | Shuyan SHUANG, Wei ZHANG, Jiale WANG, Junfeng WANG. Experimental study on hydrogen production from methanol decomposition by liquid-phase discharge using porous nickel electrodes [J]. CIESC Journal, 2025, 76(11): 5753-5763. |
| [14] | Haixia ZHAO, Yang LIU, Lei WANG, Fengfeng GAO, Zhiyuan GUO, Panpan ZHANG, Jing WANG, Zhiyong JI. Electrochemical method for synchronous extraction of lithium and bromine from oil and gas field produced water to lithium bromide [J]. CIESC Journal, 2025, 76(10): 5213-5224. |
| [15] | Yabin ZHANG, Yang SU, Huirong ZHANG, Yipeng SONG, Jian LI, Yanxia GUO. Mechanism of enhanced arsenic sulfide stabilization/solidification by using steel slag and carbide slag [J]. CIESC Journal, 2024, 75(7): 2656-2669. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
