CIESC Journal ›› 2025, Vol. 76 ›› Issue (9): 4872-4881.DOI: 10.11949/0438-1157.20250139
• Separation engineering • Previous Articles Next Articles
Zhihong JIANG1,2( ), Qian LEI1,2, Yinjun ZHU1,2, Zhigang LEI3, Honglin CHEN1,2(
), Qian LEI1,2, Yinjun ZHU1,2, Zhigang LEI3, Honglin CHEN1,2( )
)
Received:2025-02-17
Revised:2025-05-30
Online:2025-10-23
Published:2025-09-25
Contact:
Honglin CHEN
蒋智洪1,2( ), 雷骞1,2, 朱引军1,2, 雷志刚3, 陈洪林1,2(
), 雷骞1,2, 朱引军1,2, 雷志刚3, 陈洪林1,2( )
)
通讯作者:
陈洪林
作者简介:蒋智洪(2000—),男,硕士研究生,jiangzhihong22@mails.ucas.ac.cn
基金资助:CLC Number:
Zhihong JIANG, Qian LEI, Yinjun ZHU, Zhigang LEI, Honglin CHEN. Study on physical property model and enrichment process of trioxane system[J]. CIESC Journal, 2025, 76(9): 4872-4881.
蒋智洪, 雷骞, 朱引军, 雷志刚, 陈洪林. 三聚甲醛体系物性模型和提浓工艺研究[J]. 化工学报, 2025, 76(9): 4872-4881.
Add to citation manager EndNote|Ris|BibTeX
| 反应 | A | B | C | D |
|---|---|---|---|---|
| 式(2) | -30.946 | 4819.0 | 3.7410 | -0.004534 |
| -30.941 | 5653.0 | 3.7410 | -0.004534 | |
| -30.933 | 5361.0 | 3.7410 | -0.004534 | |
| 式(4) | 1129.7 | -25100 | -198.40 | 0.3160 |
| 1129.0 | -25510 | -198.40 | 0.3160 | |
| 1129.0 | -25630 | -198.40 | 0.3160 |
Table 1 Related parameters of chemical reaction equilibrium constant ki[19]
| 反应 | A | B | C | D |
|---|---|---|---|---|
| 式(2) | -30.946 | 4819.0 | 3.7410 | -0.004534 |
| -30.941 | 5653.0 | 3.7410 | -0.004534 | |
| -30.933 | 5361.0 | 3.7410 | -0.004534 | |
| 式(4) | 1129.7 | -25100 | -198.40 | 0.3160 |
| 1129.0 | -25510 | -198.40 | 0.3160 | |
| 1129.0 | -25630 | -198.40 | 0.3160 |
| 物质 | 拆分基团 |
|---|---|
| CH2O | 1·CH2O |
| H2O | 1·H2O |
| HOCH2OH | 1·HOCH2OH |
| HO(CH2O) n H,n>1 | 1·H2O n·CH2O |
| CH3OH | 1·CH3OH |
| HOCH2OCH3 | 1·CH3O 1·CH2OH |
| HO(CH2O) n CH3,n>1 | 1·CH3O 1·CH2OH n-1·CH2O |
| (CH2O)3 | 1·(CH2O)3 |
Table 2 UNIFAC groups of components in trioxane solutions[13]
| 物质 | 拆分基团 |
|---|---|
| CH2O | 1·CH2O |
| H2O | 1·H2O |
| HOCH2OH | 1·HOCH2OH |
| HO(CH2O) n H,n>1 | 1·H2O n·CH2O |
| CH3OH | 1·CH3OH |
| HOCH2OCH3 | 1·CH3O 1·CH2OH |
| HO(CH2O) n CH3,n>1 | 1·CH3O 1·CH2OH n-1·CH2O |
| (CH2O)3 | 1·(CH2O)3 |
| 基团 | 编号 | R | Q | 文献 |
|---|---|---|---|---|
| —OH | 1 | 1 | 1.2 | [ |
| —CH2O— | 2 | 0.9183 | 0.78 | [ |
| —CH2— | 3 | 0.6744 | 0.54 | [ |
| H2O | 4 | 0.92 | 1.4 | [ |
| HOCH2OH | 5 | 2.6744 | 2.94 | [ |
| (CH2O)3 | 6 | 2.754 | 3.3 | [ |
| CH3OH | 7 | 1.4311 | 1.432 | [ |
| —CH3O | 8 | 1.1459 | 1.088 | [ |
| —CH2OH | 9 | 1.2044 | 1.124 | [ |
Table 3 Size and surface parameters of UNIFAC group
| 基团 | 编号 | R | Q | 文献 |
|---|---|---|---|---|
| —OH | 1 | 1 | 1.2 | [ |
| —CH2O— | 2 | 0.9183 | 0.78 | [ |
| —CH2— | 3 | 0.6744 | 0.54 | [ |
| H2O | 4 | 0.92 | 1.4 | [ |
| HOCH2OH | 5 | 2.6744 | 2.94 | [ |
| (CH2O)3 | 6 | 2.754 | 3.3 | [ |
| CH3OH | 7 | 1.4311 | 1.432 | [ |
| —CH3O | 8 | 1.1459 | 1.088 | [ |
| —CH2OH | 9 | 1.2044 | 1.124 | [ |
| i | j | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1 | — | 28.06 | 156.4 | 353.5 | 353.5 | 28.06 | -137.1 | 112.8 | -137.1 |
| 2 | 237.7 | — | 83.36 | 867.8 | 189.2 | a26 | 238.4 | 0 | 238.4 |
| 3 | 986.5 | 251.5 | — | 1318 | 1318 | 251.5 | 697.2 | 447.8 | 697.2 |
| 4 | -229.1 | -254.5 | 300 | — | 189.5 | 80.63 | 289.6 | -219.3 | a49 |
| 5 | -229.1 | 59.2 | 300 | -191.8 | — | 80.63 | 289.6 | -142.4 | 289.6 |
| 6 | 237.7 | a62 | 83.36 | 379.4 | 379.4 | — | 239.6 | 0 | 392.20 |
| 7 | 249.1 | -128.6 | 16.5 | -181.0 | -181 | -16.67 | — | -128.6 | 0 |
| 8 | 1164.8 | 0 | 273 | 423.8 | 774.8 | 0 | 238.4 | — | 238.4 |
| 9 | 249.1 | -128.6 | 16.5 | a94 | -181 | -187.7 | 0 | -128.6 | — |
Table 4 UNIFAC group interaction parameters aij[19]
| i | j | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1 | — | 28.06 | 156.4 | 353.5 | 353.5 | 28.06 | -137.1 | 112.8 | -137.1 |
| 2 | 237.7 | — | 83.36 | 867.8 | 189.2 | a26 | 238.4 | 0 | 238.4 |
| 3 | 986.5 | 251.5 | — | 1318 | 1318 | 251.5 | 697.2 | 447.8 | 697.2 |
| 4 | -229.1 | -254.5 | 300 | — | 189.5 | 80.63 | 289.6 | -219.3 | a49 |
| 5 | -229.1 | 59.2 | 300 | -191.8 | — | 80.63 | 289.6 | -142.4 | 289.6 |
| 6 | 237.7 | a62 | 83.36 | 379.4 | 379.4 | — | 239.6 | 0 | 392.20 |
| 7 | 249.1 | -128.6 | 16.5 | -181.0 | -181 | -16.67 | — | -128.6 | 0 |
| 8 | 1164.8 | 0 | 273 | 423.8 | 774.8 | 0 | 238.4 | — | 238.4 |
| 9 | 249.1 | -128.6 | 16.5 | a94 | -181 | -187.7 | 0 | -128.6 | — |
| 物质 | Ai | Bi | Ci | 文献 |
|---|---|---|---|---|
| CH2O | 14.4625 | -2204.13 | -30.15 | [ |
| H2O | 16.2886 | -3816.44 | -46.13 | [ |
| CH3OH | 16.5725 | -3626.55 | -34.29 | [ |
| HOCH2OH | 17.4364 | -4762.07 | -51.2 | [ |
| HOCH2OCH3 | 19.5736 | -5646.71 | 0 | [ |
| (CH2O)3 | 14.3796 | -3099.47 | -68.92 | [ |
Table 5 Parameters of pure component vapor pressure
| 物质 | Ai | Bi | Ci | 文献 |
|---|---|---|---|---|
| CH2O | 14.4625 | -2204.13 | -30.15 | [ |
| H2O | 16.2886 | -3816.44 | -46.13 | [ |
| CH3OH | 16.5725 | -3626.55 | -34.29 | [ |
| HOCH2OH | 17.4364 | -4762.07 | -51.2 | [ |
| HOCH2OCH3 | 19.5736 | -5646.71 | 0 | [ |
| (CH2O)3 | 14.3796 | -3099.47 | -68.92 | [ |
| 模型 | 体系 | Δ | Δ | Δ | Δp/% |
|---|---|---|---|---|---|
| 本模型 | 甲醛-水 | 4.64 | — | — | 0.81 |
| 甲醛-水-甲醇 | 9.15 | — | 5.26 | 2.35 | |
| 甲醛-水-三聚甲醛 | 7.31 | 7.93 | — | 0.97 | |
| Bongartz等[ | 甲醛-水 | 3.51 | — | — | 1.20 |
| 甲醛-水-甲醇 | 14.04 | — | 10.97 | 3.03 | |
| 甲醛-水-三聚甲醛 | 19.38 | 31.16 | — | 5.95 | |
| Schemme等[ | 甲醛-水 | 14.61 | — | — | 4.63 |
| 甲醛-水-甲醇 | 5.91 | — | 7.09 | 3.59 | |
| 甲醛-水-三聚甲醛 | 11.91 | 8.72 | — | 2.08 |
Table 6 Relative average deviation of different systems
| 模型 | 体系 | Δ | Δ | Δ | Δp/% |
|---|---|---|---|---|---|
| 本模型 | 甲醛-水 | 4.64 | — | — | 0.81 |
| 甲醛-水-甲醇 | 9.15 | — | 5.26 | 2.35 | |
| 甲醛-水-三聚甲醛 | 7.31 | 7.93 | — | 0.97 | |
| Bongartz等[ | 甲醛-水 | 3.51 | — | — | 1.20 |
| 甲醛-水-甲醇 | 14.04 | — | 10.97 | 3.03 | |
| 甲醛-水-三聚甲醛 | 19.38 | 31.16 | — | 5.95 | |
| Schemme等[ | 甲醛-水 | 14.61 | — | — | 4.63 |
| 甲醛-水-甲醇 | 5.91 | — | 7.09 | 3.59 | |
| 甲醛-水-三聚甲醛 | 11.91 | 8.72 | — | 2.08 |
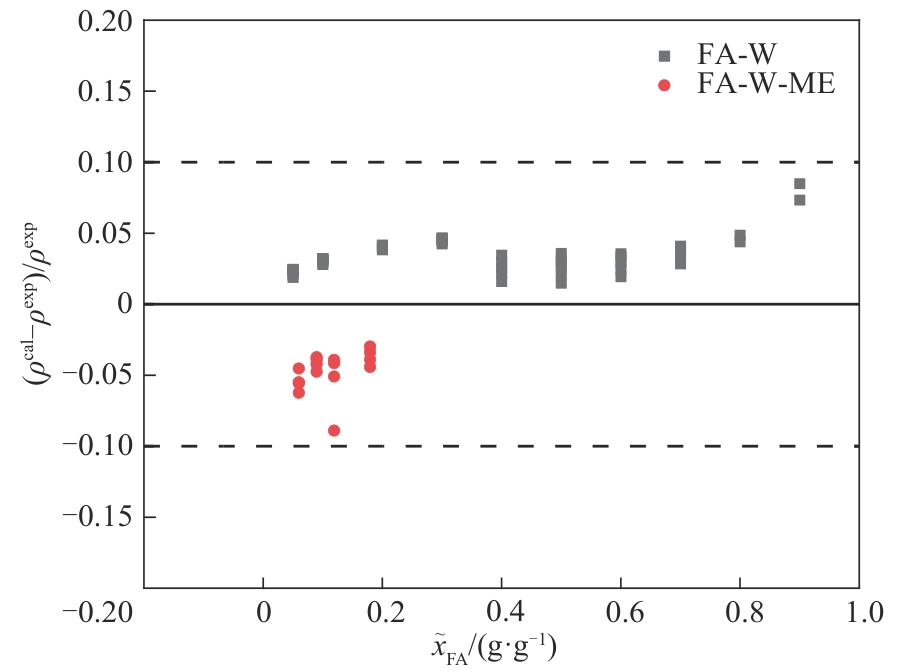
Fig.5 Relative deviation of calculated density and literature density[28] for the formaldehyde-water (293—383 K) and formaldehyde-water-methanol (283—333 K) systems
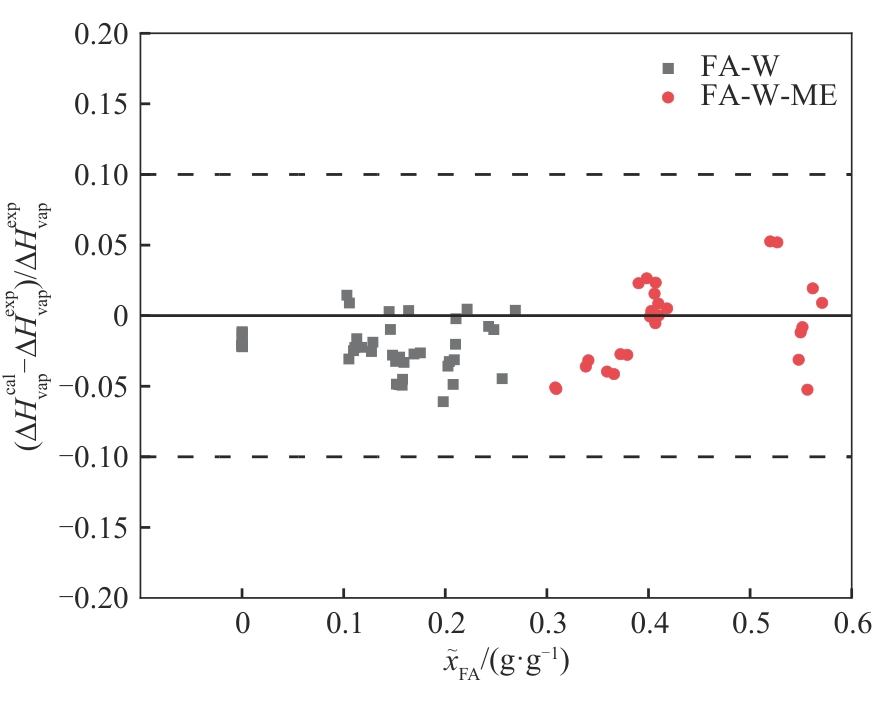
Fig.6 Relative deviation of calculated enthalpy of phase transition and literature values[29] for the formaldehyde-water (322—362 K) and formaldehyde-water-methanol (311—332 K) systems
| 进料 | 馏出物进料比/(g·g-1) | 回流比/(g·g-1) | 理论进料位置 | 理论塔板数 | 总能耗/kW |
|---|---|---|---|---|---|
| 不含甲醇 | 0.30 | 0.28 | 2 | 14 | 18.92 |
| 含甲醇 | 0.30 | 0.39 | 2 | 13 | 21.61 |
Table 7 Operating conditions of concentration column
| 进料 | 馏出物进料比/(g·g-1) | 回流比/(g·g-1) | 理论进料位置 | 理论塔板数 | 总能耗/kW |
|---|---|---|---|---|---|
| 不含甲醇 | 0.30 | 0.28 | 2 | 14 | 18.92 |
| 含甲醇 | 0.30 | 0.39 | 2 | 13 | 21.61 |
| 条件 | 流量/(kg·h-1) | 甲醛浓度/(g·g-1) | 水浓度/(g·g-1) | 甲醇浓度/(g·g-1) | 三聚甲醛浓度/(g·g-1) | |
|---|---|---|---|---|---|---|
| 含甲醇 | 进料 | 100 | 0.3933 | 0.4469 | 0.0107 | 0.1491 |
| 塔顶 | 29.52 | 0.1445 | 0.3355 | 0.0199 | 0.5001 | |
| 塔底 | 70.48 | 0.4975 | 0.4935 | 0.0069 | 0.0021 | |
| 不含甲醇 | 进料 | 100 | 0.3936 | 0.4548 | — | 0.1516 |
| 塔顶 | 30.02 | 0.1512 | 0.3487 | — | 0.5001 | |
| 塔底 | 69.98 | 0.4976 | 0.5003 | — | 0.0021 |
Table 8 Inlet and outlet flow rates and compositions of the concentration column
| 条件 | 流量/(kg·h-1) | 甲醛浓度/(g·g-1) | 水浓度/(g·g-1) | 甲醇浓度/(g·g-1) | 三聚甲醛浓度/(g·g-1) | |
|---|---|---|---|---|---|---|
| 含甲醇 | 进料 | 100 | 0.3933 | 0.4469 | 0.0107 | 0.1491 |
| 塔顶 | 29.52 | 0.1445 | 0.3355 | 0.0199 | 0.5001 | |
| 塔底 | 70.48 | 0.4975 | 0.4935 | 0.0069 | 0.0021 | |
| 不含甲醇 | 进料 | 100 | 0.3936 | 0.4548 | — | 0.1516 |
| 塔顶 | 30.02 | 0.1512 | 0.3487 | — | 0.5001 | |
| 塔底 | 69.98 | 0.4976 | 0.5003 | — | 0.0021 |
| 塔板 | 填料高度/m | 液泛率/% | 压降/kPa | 持液量/L |
|---|---|---|---|---|
| 2 | 0.5 | 77.45 | 0.14 | 9.90 |
| 3 | 1.0 | 75.78 | 0.13 | 9.80 |
| 4 | 1.5 | 73.91 | 0.12 | 9.70 |
| 5 | 2.0 | 72.02 | 0.11 | 9.60 |
| 6 | 2.5 | 70.23 | 0.10 | 9.51 |
| 7 | 3.0 | 68.69 | 0.09 | 9.44 |
| 8 | 3.5 | 67.43 | 0.09 | 9.37 |
| 9 | 4.0 | 66.45 | 0.08 | 9.33 |
| 10 | 4.5 | 65.72 | 0.08 | 9.29 |
| 11 | 5.0 | 65.15 | 0.08 | 9.27 |
| 12 | 5.5 | 64.65 | 0.08 | 9.27 |
Table 9 Hydraulic design of concentration column
| 塔板 | 填料高度/m | 液泛率/% | 压降/kPa | 持液量/L |
|---|---|---|---|---|
| 2 | 0.5 | 77.45 | 0.14 | 9.90 |
| 3 | 1.0 | 75.78 | 0.13 | 9.80 |
| 4 | 1.5 | 73.91 | 0.12 | 9.70 |
| 5 | 2.0 | 72.02 | 0.11 | 9.60 |
| 6 | 2.5 | 70.23 | 0.10 | 9.51 |
| 7 | 3.0 | 68.69 | 0.09 | 9.44 |
| 8 | 3.5 | 67.43 | 0.09 | 9.37 |
| 9 | 4.0 | 66.45 | 0.08 | 9.33 |
| 10 | 4.5 | 65.72 | 0.08 | 9.29 |
| 11 | 5.0 | 65.15 | 0.08 | 9.27 |
| 12 | 5.5 | 64.65 | 0.08 | 9.27 |
| [1] | Masamoto J, Hamanaka K, Yoshida K, et al. Synthesis of trioxane using heteropolyacids as catalyst[J]. Angewandte Chemie International Edition, 2000, 39(12): 2102-2104. |
| [2] | Curioni A, Sprik M, Andreoni W, et al. Density functional theory-based molecular dynamics simulation of acid-catalyzed chemical reactions in liquid trioxane[J]. Journal of the American Chemical Society, 1997, 119(31): 7218-7229. |
| [3] | Curioni A, Andreoni W, Hutter J, et al. Density-functional-theory-based molecular dynamics study of 1,3,5-trioxane and 1,3-dioxolane protolysis[J]. Journal of the American Chemical Society, 1994, 116(25): 11251-11255. |
| [4] | Ma W T, Hu Y F, Qi J G, et al. Acid-catalyzed synthesis of trioxane in aprotic media[J]. Industrial & Engineering Chemistry Research, 2017, 56(24): 6910-6915. |
| [5] | Pei X P, Li H, Zhang Z S, et al. Process intensification for energy efficient reactive distillation of trioxane production from aqueous formaldehyde[J]. Chemical Engineering and Processing-Process Intensification, 2022, 175: 108914. |
| [6] | 陈杰, 王洪波, 程锐. 离子液法与硫酸法生产三聚甲醛(TOX)工艺技术对比[J]. 化工技术与开发, 2013, 42(6): 60-62. |
| Chen J, Wang H B, Cheng R. Comparison between ionic liquid method and sulfuric acid method of trioxane preparation[J]. Technology & Development of Chemical Industry, 2013, 42(6): 60-62. | |
| [7] | 关键, 林陵, 曾崇余. PW12/AC催化剂在合成三聚甲醛中的催化性能研究[J]. 天然气化工, 2005, 30(4): 19-22. |
| Guan J, Lin L, Zeng C Y. Trioxane synthesis from formaldehyde over the supperted PW12/AC catalyst[J]. Natural Gas Chemical Industry, 2005, 30(4): 19-22. | |
| [8] | Qi J G, Hu Y F, Ma W T, et al. The reactions that determine the yield and selectivity of 1,3,5-trioxane[J]. Chemical Engineering Journal, 2018, 331: 311-316. |
| [9] | Augé J, Gil R. A convenient solvent-free preparation of 1,3,5-trioxanes[J]. Tetrahedron Letters, 2002, 43(44): 7919-7920. |
| [10] | 范娟娟, 王士明, 刘剡. 三聚甲醛生产装置工艺优化研究[J]. 当代化工研究, 2019(3): 142-143. |
| Fan J J, Wang S M, Liu Y. Research on process optimization of trioxane production unit[J]. Modern Chemical Research, 2019(3): 142-143. | |
| [11] | Maurer G. Vapor-liquid equilibrium of formaldehyde-and water-containing multicomponent mixtures[J]. AIChE Journal, 1986, 32(6): 932-948. |
| [12] | Grützner T, Hasse H, Lang N, et al. Development of a new industrial process for trioxane production[J]. Chemical Engineering Science, 2007, 62(18/19/20): 5613-5620. |
| [13] | Hasse H, Hahnenstein I, Maurer G. Revised vapor-liquid equilibrium model for multicomponent formaldehyde mixtures[J]. AIChE Journal, 1990, 36(12): 1807-1814. |
| [14] | Schemme S, Meschede S, Köller M, et al. Property data estimation for hemiformals, methylene glycols and polyoxymethylene dimethyl ethers and process optimization in formaldehyde synthesis[J]. Energies, 2020, 13(13): 3401. |
| [15] | Schmitz N, Friebel A, von Harbou E V, et al. Liquid-liquid equilibrium in binary and ternary mixtures containing formaldehyde, water, methanol, methylal, and poly(oxymethylene) dimethyl ethers[J]. Fluid Phase Equilibria, 2016, 425: 127-135. |
| [16] | Fredenslund A, Jones R L, Prausnitz J M. Group-contribution estimation of activity coefficients in nonideal liquid mixtures[J]. AIChE Journal, 1975, 21(6): 1086-1099. |
| [17] | Albert M. Thermodynamische eigenschaften formaldehydhaltiger mischungen[D]. Kaiserslautern: Technische Universität Kaiserslautern, 1999. |
| [18] | Kuhnert C. Dampf-flüssigkeits-gleichgewichte in mehrkomponentigen formaldhydhaltigen [formaldehydhaltigen] systemen[D]. Kaiserslautern: Technische Universität Kaiserslautern, 2004. |
| [19] | Schmitz N, Breitkreuz C F, Ströfer E, et al. Vapor-liquid equilibrium and distillation of mixtures containing formaldehdye and poly(oxymethylene) dimethyl ethers[J]. Chemical Engineering and Processing -Process Intensification, 2018, 131: 116-124. |
| [20] | Bai Z M, Liu H H, Liu Y S, et al. Prediction of the vapor-liquid equilibrium of chemical reactive systems containing formaldehyde using the COSMO-RS method[J]. Fluid Phase Equilibria, 2016, 415: 125-133. |
| [21] | Breitkreuz C F, Dyga M, Forte E, et al. Conceptual design of a crystallization-based trioxane production process[J]. Chemical Engineering and Processing-Process Intensification, 2022, 171: 108710. |
| [22] | Albert M, Hahnenstein I, Hasse H, et al. Vapor-liquid equilibrium of formaldehyde mixtures: new data and model revision[J]. AIChE Journal, 1996, 42(6): 1741-1752. |
| [23] | Albert M, García B C, Kuhnert C, et al. Vapor-liquid equilibrium of aqueous solutions of formaldehyde and methanol[J]. AIChE Journal, 2000, 46(8): 1676-1687. |
| [24] | Albert M, García B C, Kreiter C, et al. et al. Vapor-liquid and chemical equilibria of formaldehyde-water mixtures[J]. AIChE Journal, 1999, 45(9): 2024-2033. |
| [25] | Albert M, Hasse H, Kuhnert C, et al. New experimental results for the vapor-liquid equilibrium of the binary system (trioxane+ water) and the ternary system (formaldehyde+ trioxane+ water)[J]. Journal of Chemical & Engineering Data, 2005, 50(4): 1218-1223. |
| [26] | Kuhnert C, Albert M, Breyer S, et al. Phase equilibrium in formaldehyde containing multicomponent mixtures: experimental results for fluid phase equilibria of (formaldehyde+(water or methanol)+ methylal)) and (formaldehyde+ water+ methanol+ methylal) and comparison with predictions[J]. Industrial & Engineering Chemistry Research, 2006, 45(14): 5155-5164. |
| [27] | Bongartz D, Burre J, Mitsos A. Production of oxymethylene dimethyl ethers from hydrogen and carbon dioxide(part Ⅰ): Modeling and analysis for OME1 [J]. Industrial & Engineering Chemistry Research, 2019, 58(12): 4881-4889. |
| [28] | Dyga M, Keller A, Hasse H. Density of solutions of formaldehyde in water and alcohols[J]. AIChE Journal, 2022, 68(4): e17573. |
| [29] | Liu Y Q, Hasse H, Maurer G. Enthalpy change on vaporization of aqueous and methanolic formaldehyde solutions[J]. AIChE Journal, 1992, 38(11): 1693-1702. |
| [30] | Soboleva O, Blazhin Y M, Ogorodnikov S. Formaldehyde-water system(communication Ⅰ): Physicochemical properties of individual hydroxymethylene hydrates[J]. Zhurnal Pikladnoi Khimii, 1979, 52(7): 1519-1523. |
| [31] | Winkelman J G M, Beenackers A A C M. Correlations for the density and viscosity of aqueous formaldehyde solutions[J]. Industrial & Engineering Chemistry Research, 2000, 39(2): 557-562. |
| [32] | 中国科学院吉林应用化学研究所. 聚甲醛[M]. 北京: 燃料化学工业出版社, 1973: 38. |
| Jilin Institute of Applied Chemistry, Chinese Academy of Sciences. Polyformaldehyde[M]. Beijing: Fuel Chemical Industry Press, 1973: 38. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||