化工学报 ›› 2023, Vol. 74 ›› Issue (8): 3171-3192.DOI: 10.11949/0438-1157.20230338
郑玉圆1,2( ), 葛志伟1,2,3(
), 葛志伟1,2,3( ), 韩翔宇1,2, 王亮1,2,3, 陈海生1,2(
), 韩翔宇1,2, 王亮1,2,3, 陈海生1,2( )
)
收稿日期:2023-04-06
修回日期:2023-08-15
出版日期:2023-08-25
发布日期:2023-10-18
通讯作者:
葛志伟,陈海生
作者简介:郑玉圆(1999—),女,硕士研究生,zhengyuyuan@iet.cn
基金资助:
Yuyuan ZHENG1,2( ), Zhiwei GE1,2,3(
), Zhiwei GE1,2,3( ), Xiangyu HAN1,2, Liang WANG1,2,3, Haisheng CHEN1,2(
), Xiangyu HAN1,2, Liang WANG1,2,3, Haisheng CHEN1,2( )
)
Received:2023-04-06
Revised:2023-08-15
Online:2023-08-25
Published:2023-10-18
Contact:
Zhiwei GE, Haisheng CHEN
摘要:
热化学储热由于能量密度高,材料适宜于长时储存和远距离运输,成为高效储热新兴的研究热点。钙基材料热化学储热成本低且无毒无污染,具有广阔应用前景。总结了目前热化学储热的主要体系及分类,针对中高温钙基热化学储热技术从材料改性、反应器设计及系统集成应用三个层面的研究进展进行综述。探讨钙基热化学储热技术研究中面临的挑战与机遇,并对今后的研究与发展方向提出了建议。
中图分类号:
郑玉圆, 葛志伟, 韩翔宇, 王亮, 陈海生. 中高温钙基材料热化学储热的研究进展与展望[J]. 化工学报, 2023, 74(8): 3171-3192.
Yuyuan ZHENG, Zhiwei GE, Xiangyu HAN, Liang WANG, Haisheng CHEN. Progress and prospect of medium and high temperature thermochemical energy storage of calcium-based materials[J]. CIESC Journal, 2023, 74(8): 3171-3192.
| 反应类型 | 反应式 | ΔH/(kJ/mol) | T/℃ |
|---|---|---|---|
| 金属氢化物反应[ | 75 | 450 | |
| 88 | 947 | ||
| 氨类反应[ | 66 | 200 | |
| 335 | 467 | ||
| 氢氧化物反应[ | 84 | 330 | |
| 104 | 515 | ||
| 碳酸盐反应[ | 178 | 900 | |
| 125 | 400 | ||
| 氧化还原反应[ | 205 | 914 | |
| 910 | 948 |
表1 热化学储热的反应类型及反应条件
Table 1 The classification and reaction conditions for thermochemical energy storage
| 反应类型 | 反应式 | ΔH/(kJ/mol) | T/℃ |
|---|---|---|---|
| 金属氢化物反应[ | 75 | 450 | |
| 88 | 947 | ||
| 氨类反应[ | 66 | 200 | |
| 335 | 467 | ||
| 氢氧化物反应[ | 84 | 330 | |
| 104 | 515 | ||
| 碳酸盐反应[ | 178 | 900 | |
| 125 | 400 | ||
| 氧化还原反应[ | 205 | 914 | |
| 910 | 948 |
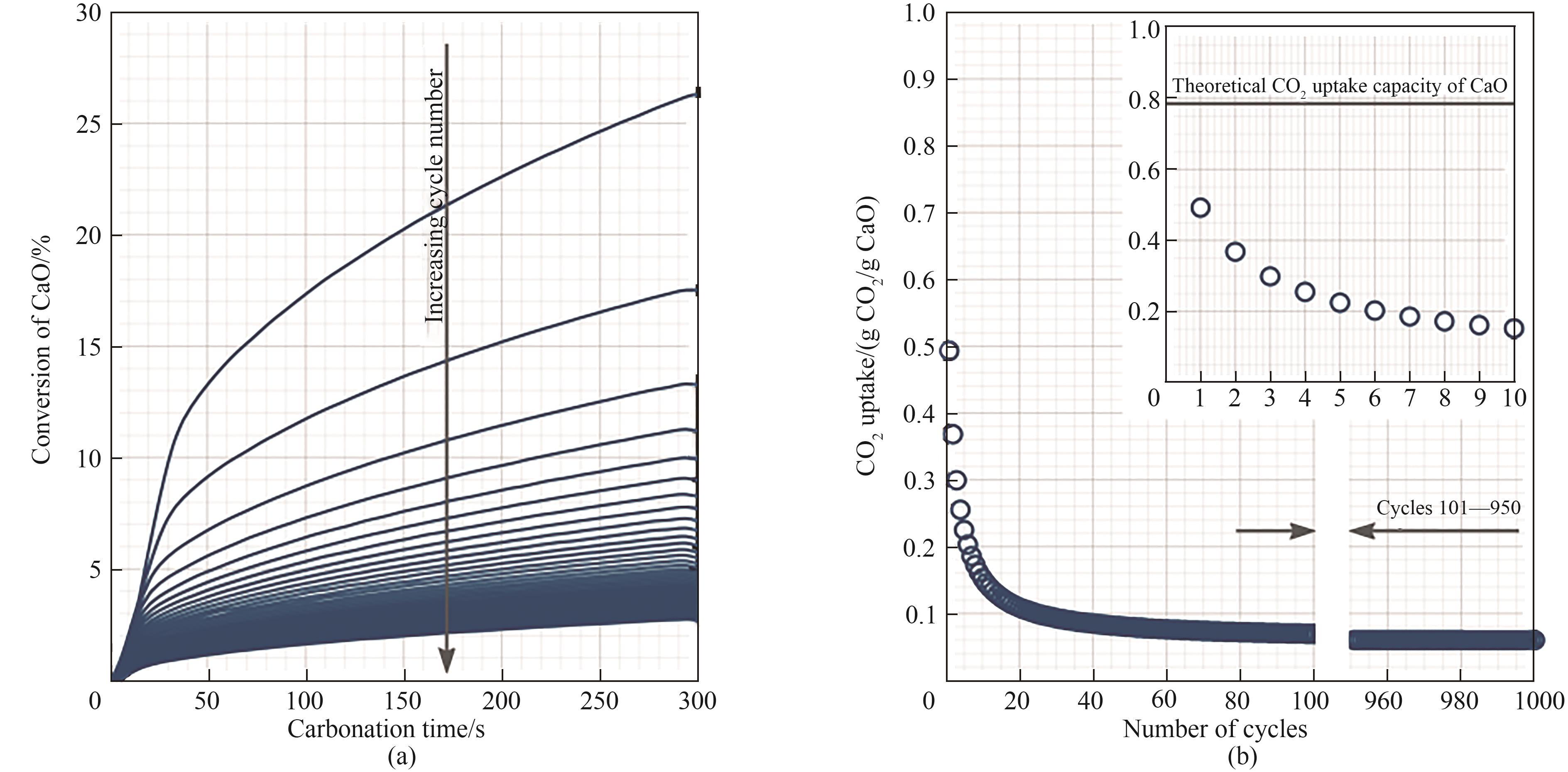
图2 (a)天然钙基材料在950℃下100个循环内的CaO转化率;(b)1000个循环内的CO2吸附量[37]
Fig.2 (a) CaO conversion of natural calcium-based materials within 100 cycles at 950℃; (b) CO2 adsorption within 1000 cycles[37]
| 分类 | 掺杂材料 | 特点 | 文献 |
|---|---|---|---|
| 活性掺杂 | Al2O3, ZrO2 | 在50次煅烧/碳酸化循环中保持80%以上吸附性;允许离子在整个晶体结构中迁移,对碳酸化反应起到催化作用;有效增强循环稳定性和反应活性 | [ |
| CeO2, Mn3O4 | 分散CaO颗粒,缓解烧结;Ce和Mn之间的电子转移促进了CO2扩散和O2-迁移;在40次循环中保持0.61 g/g的CO2吸附量 | [ | |
| Fe2O3, Mn3O4 | 薄片状多孔结构;促进氧空位产生并降低反应活化能;在20次循环中保持95%的转化率 | [ | |
| Al2O3, CeO2 | 掺杂量为5%时表现出最高的储热能力;30次循环有效转化率和能量密度仅下降7%;具有更大的比表面积和孔隙率,促进CO2吸附 | [ | |
| 惰性掺杂 | Al2O3 | 提高反应速率,缩短反应时间(减少42%);提高循环稳定性 | [ |
| SiO2 | 提高材料机械强度,在100次循环内保持28 N以上 | [ | |
| SiO2 | 提高材料导热性能,比热容提高20%;掺杂量为5%时材料反应动力学表现最佳;循环稳定性增强28% | [ | |
| TiO2 | 掺杂量为2.5% 储热密度达1256.68 kJ/kg,30次循环后仍为纯CaCO3的2.26倍;反应活化能从1117.39 kJ/mol降低到997.6 kJ/mol,脱碳温度从903.56℃降低到876.13℃;总转化率降低 | [ |
表2 氧化物掺杂改性的分类及特点
Table 2 Classification and characteristics of oxide doping modification
| 分类 | 掺杂材料 | 特点 | 文献 |
|---|---|---|---|
| 活性掺杂 | Al2O3, ZrO2 | 在50次煅烧/碳酸化循环中保持80%以上吸附性;允许离子在整个晶体结构中迁移,对碳酸化反应起到催化作用;有效增强循环稳定性和反应活性 | [ |
| CeO2, Mn3O4 | 分散CaO颗粒,缓解烧结;Ce和Mn之间的电子转移促进了CO2扩散和O2-迁移;在40次循环中保持0.61 g/g的CO2吸附量 | [ | |
| Fe2O3, Mn3O4 | 薄片状多孔结构;促进氧空位产生并降低反应活化能;在20次循环中保持95%的转化率 | [ | |
| Al2O3, CeO2 | 掺杂量为5%时表现出最高的储热能力;30次循环有效转化率和能量密度仅下降7%;具有更大的比表面积和孔隙率,促进CO2吸附 | [ | |
| 惰性掺杂 | Al2O3 | 提高反应速率,缩短反应时间(减少42%);提高循环稳定性 | [ |
| SiO2 | 提高材料机械强度,在100次循环内保持28 N以上 | [ | |
| SiO2 | 提高材料导热性能,比热容提高20%;掺杂量为5%时材料反应动力学表现最佳;循环稳定性增强28% | [ | |
| TiO2 | 掺杂量为2.5% 储热密度达1256.68 kJ/kg,30次循环后仍为纯CaCO3的2.26倍;反应活化能从1117.39 kJ/mol降低到997.6 kJ/mol,脱碳温度从903.56℃降低到876.13℃;总转化率降低 | [ |
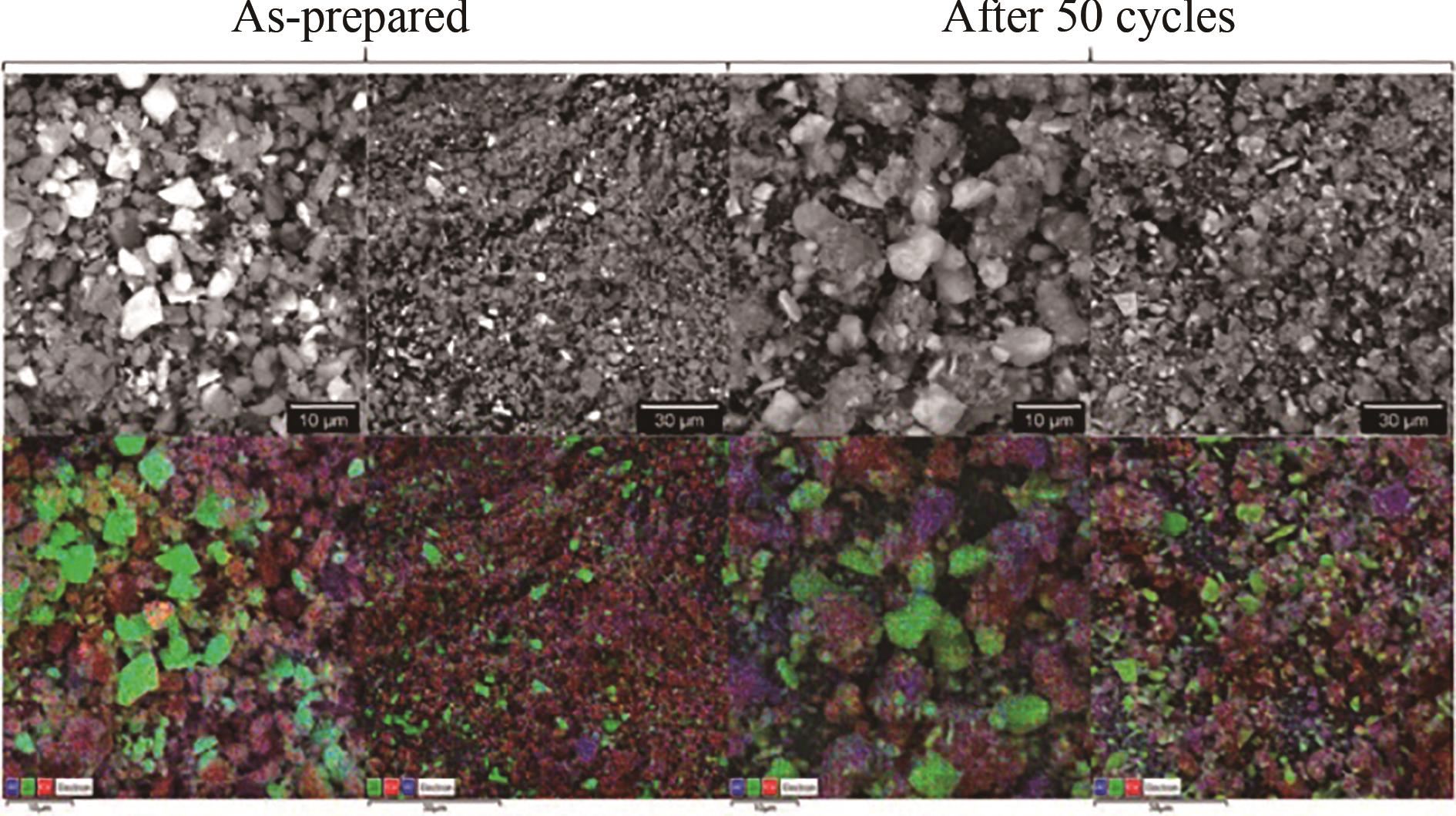
图3 50次循环前后CaCO3-Al2O3-ZrO2 (质量分数13.3%)样品的SEM图和EDS图(Al:蓝色;Zr:绿色;Ca:红色)[42]
Fig.3 SEM and EDS images of CaCO3-Al2O3-ZrO2 (mass fraction 13.3%) samples before and after 50 cycles (Al: blue; Zr: green; Ca: red) [42]

图7 封装在半透性陶瓷材料(外壳:棕色部分)中的CaO/Ca(OH)2(核芯:白色和灰色部分)体系的水合/脱水反应原理[58]
Fig.7 Principle of hydration/ dehydration reaction of CaO/Ca(OH)2 system encapsulated in semi-permeable ceramic material (shell: brown part; core: white and gray parts)[58]
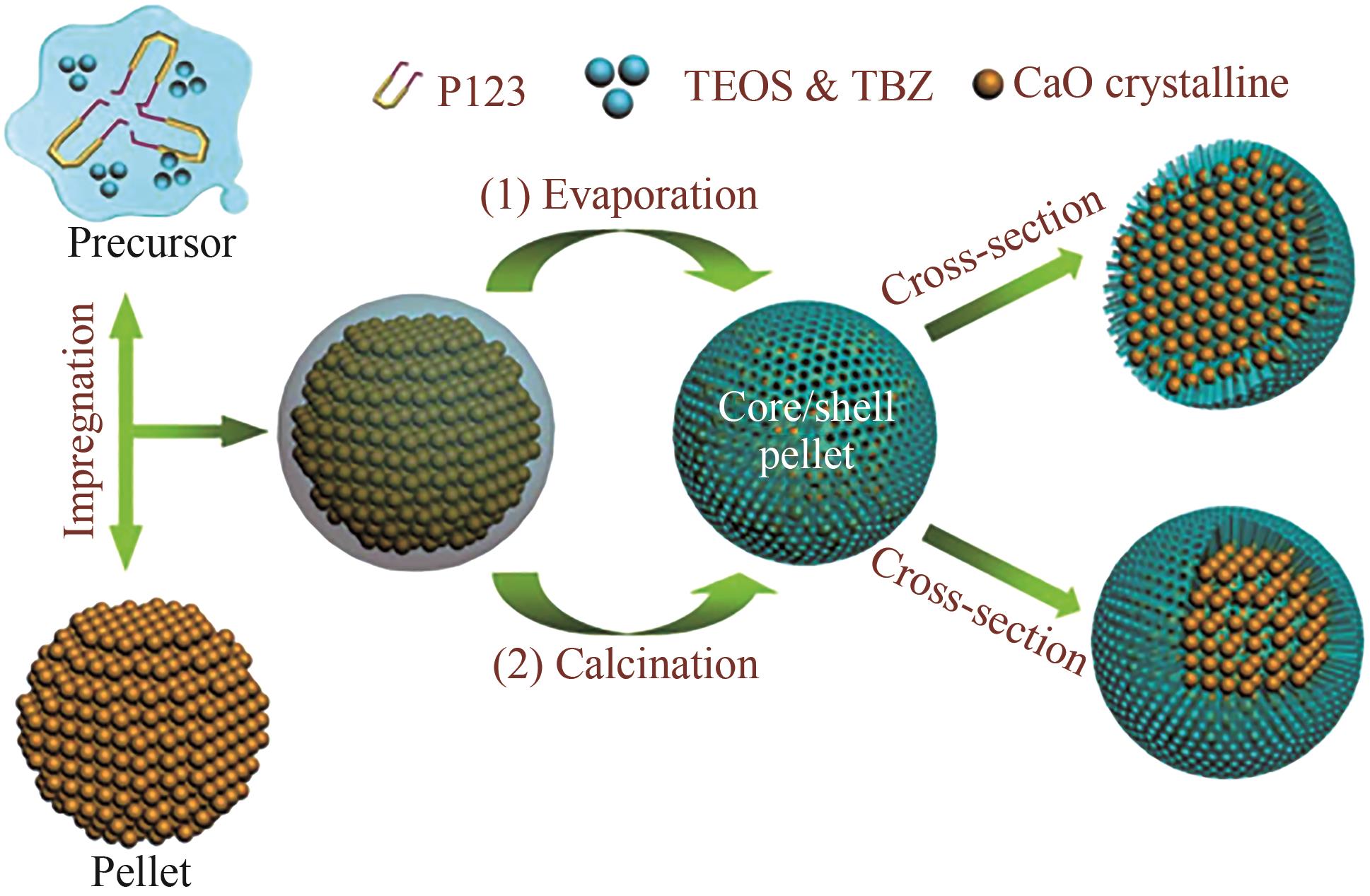
图8 采用重复浸渍-蒸发涂层工艺的核壳结构CaO基球团的形成过程[65]
Fig.8 Formation process of the core-shell-structured CaO-based pellets using the general repeated impregnation-evaporation coating process[65]
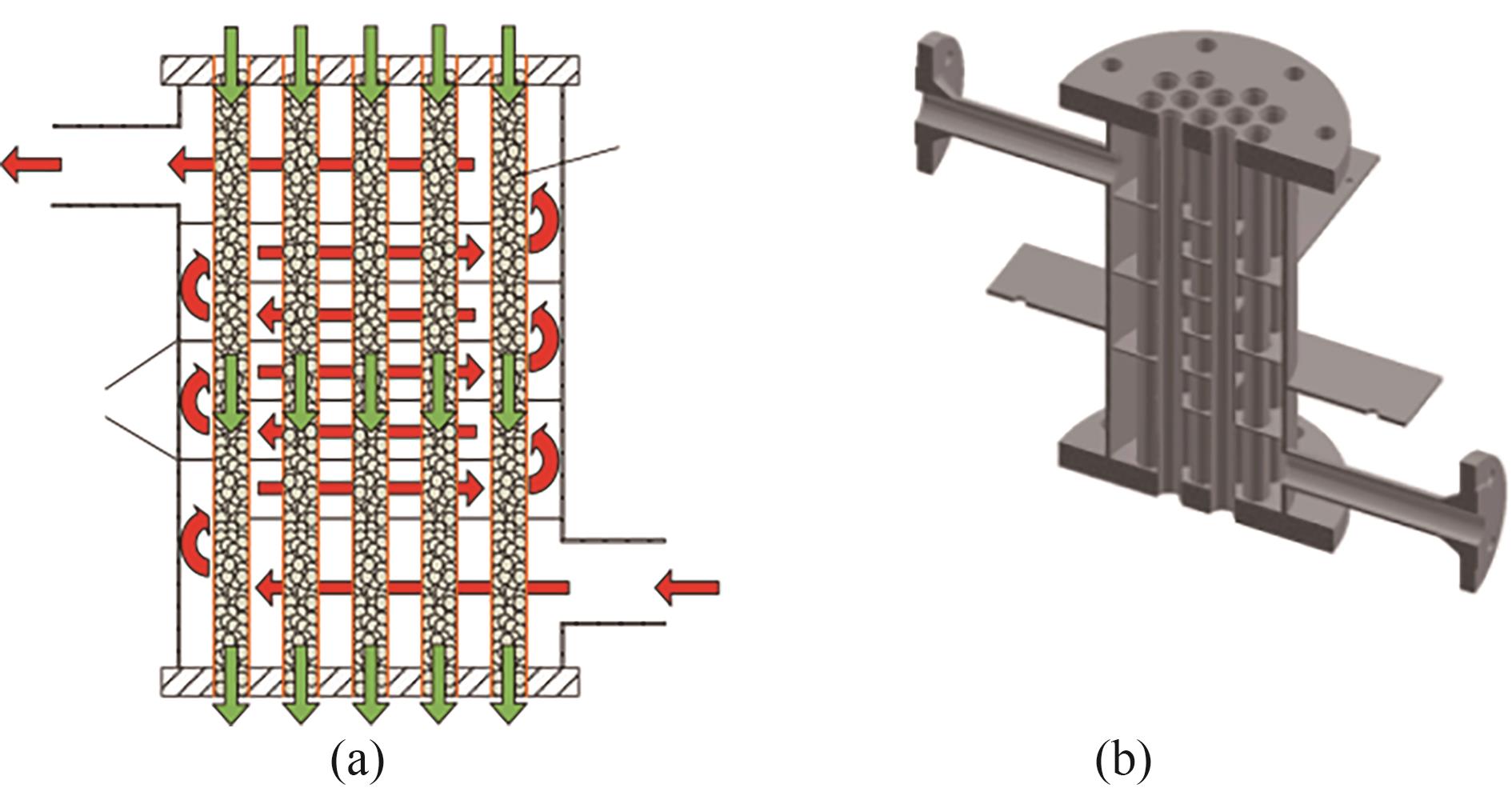
图20 传热流体在壳内(红色箭头)和储热材料在反应器管中(绿色箭头)的流动示意图(a)及反应器的3D图像(b)[60]
Fig.20 The flow routes of the heat transfer fluid in the shell (red arrows) and the heat storage material in the reactor tube (green arrows) (a) and 3D image of the reactor (b)[60]
| 1 | 陈海生, 刘畅, 徐玉杰, 等. 储能在碳达峰碳中和目标下的战略地位和作用[J]. 储能科学与技术, 2021, 10(5): 1477-1485. |
| Chen H S, Liu C, Xu Y J, et al. The strategic position and role of energy storage under the goal of carbon peak and carbon neutrality[J]. Energy Storage Science and Technology, 2021, 10(5): 1477-1485. | |
| 2 | Alvarez Rivero M, Rodrigues D, Pinheiro C I C, et al. Solid-gas reactors driven by concentrated solar energy with potential application to calcium looping: a comparative review[J]. Renewable and Sustainable Energy Reviews, 2022, 158: 112048 |
| 3 | 王泽众, 黄平瑞, 魏高升, 等. 太阳能热发电固-气两相化学储热技术研究进展[J]. 发电技术, 2021, 42(2): 238-246. |
| Wang Z Z, Huang P R, Wei G S, et al. Research progress of solid-gas two-phase chemical heat storage technology for solar thermal power generation[J]. Power Generation Technology, 2021, 42(2): 238-246. | |
| 4 | Vecchi A, Sciacovelli A. Long-duration thermo-mechanical energy storage—present and future techno-economic competitiveness[J]. Applied Energy, 2023, 334: 120628. |
| 5 | Borri E, Zsembinszki G, Cabeza L F. Recent developments of thermal energy storage applications in the built environment: a bibliometric analysis and systematic review[J]. Applied Thermal Engineering, 2021, 189: 116666. |
| 6 | Aydin D, Casey S P, Riffat S. The latest advancements on thermochemical heat storage systems[J]. Renewable and Sustainable Energy Reviews, 2015, 41: 356-367. |
| 7 | Salgado-Pizarro R, Calderón A, Svobodova-Sedlackova A, et al. The relevance of thermochemical energy storage in the last two decades: the analysis of research evolution[J]. Journal of Energy Storage, 2022, 51: 104377. |
| 8 | Desai F, Sunku Prasad J, Muthukumar P, et al. Thermochemical energy storage system for cooling and process heating applications: a review[J]. Energy Conversion and Management, 2021, 229: 113617. |
| 9 | Han X Y, Wang L, Ling H S, et al. Critical review of thermochemical energy storage systems based on cobalt, manganese, and copper oxides[J]. Renewable and Sustainable Energy Reviews, 2022, 158: 112076. |
| 10 | Pardo P, Deydier A, Anxionnaz-Minvielle Z, et al. A review on high temperature thermochemical heat energy storage[J]. Renewable and Sustainable Energy Reviews, 2014, 32: 591-610. |
| 11 | Carrillo A J, González-Aguilar J, Romero M, et al. Solar energy on demand: a review on high temperature thermochemical heat storage systems and materials[J]. Chemical Reviews, 2019, 119(7): 4777-4816. |
| 12 | Bhatnagar A, Shaz M A, Srivastava O N. Synthesis of MgH2 using autocatalytic effect of MgH2 [J]. International Journal of Hydrogen Energy, 2019, 44(13): 6738-6747. |
| 13 | Leng H Y, Pan Y B, Li Q, et al. Effect of LiH on hydrogen storage property of MgH2 [J]. International Journal of Hydrogen Energy, 2014, 39(25): 13622-13627. |
| 14 | Yan J, Pan Z H, Zhao C Y. Experimental study of MgO/Mg(OH)2 thermochemical heat storage with direct heat transfer mode[J]. Applied Energy, 2020, 275: 115356. |
| 15 | Bian Z G, Li Y J, Ren Y, et al. Thermochemical heat storage performance of CaO particles under fluidization in coupled CaO/Ca(OH)2 cycles and CaO/CaCO3 cycles[J]. Journal of Energy Storage, 2022, 56: 106045. |
| 16 | Shkatulov A I, Kim S T, Miura H, et al. Adapting the MgO-CO2 working pair for thermochemical energy storage by doping with salts[J]. Energy Conversion and Management, 2019, 185: 473-481. |
| 17 | Chen X, Dong Z, Zhu L, et al. Mass transfer performance inside Ca-based thermochemical energy storage materials under different operating conditions[J]. Renewable Energy, 2023, 205: 340-348. |
| 18 | Carrillo A J, Pizarro P, Coronado J M. Assessing Cr incorporation in Mn2O3/Mn3O4 redox materials for thermochemical heat storage applications[J]. Journal of Energy Storage, 2021, 33: 102028. |
| 19 | Fellet M, Buckley C E, Paskevicius M, et al. Research on metal hydrides revived for next-generation solutions to renewable energy storage[J]. MRS Bulletin, 2013, 38(12): 1012-1013. |
| 20 | Lovegrove K, Luzzi A, Soldiani I, et al. Developing ammonia based thermochemical energy storage for dish power plants[J]. Solar Energy, 2004, 76(1/2/3): 331-337. |
| 21 | Shkatulov A, Ryu J, Kato Y, et al. Composite material "Mg(OH)2/vermiculite": a promising new candidate for storage of middle temperature heat[J]. Energy, 2012, 44(1): 1028-1034. |
| 22 | Bian Z G, Li Y J, Zhang C X, et al. CaO/Ca(OH)2 heat storage performance of hollow nanostructured CaO-based material from Ca-looping cycles for CO2 capture[J]. Fuel Processing Technology, 2021, 217: 106834. |
| 23 | Criado Y A, Alonso M, Abanades J C. Enhancement of a CaO/Ca(OH)2 based material for thermochemical energy storage[J]. Solar Energy, 2016, 135: 800-809. |
| 24 | Wu S K, Zhou C, Doroodchi E, et al. A review on high-temperature thermochemical energy storage based on metal oxides redox cycle[J]. Energy Conversion and Management, 2018, 168: 421-453. |
| 25 | Chen X Y, Zhang Z, Qi C G, et al. State of the art on the high-temperature thermochemical energy storage systems[J]. Energy Conversion and Management, 2018, 177: 792-815. |
| 26 | Ortiz C, Valverde J M, Chacartegui R, et al. The calcium-looping (CaCO3/CaO) process for thermochemical energy storage in concentrating solar power plants[J]. Renewable and Sustainable Energy Reviews, 2019, 113: 109252. |
| 27 | Wu S K, Zhou C, Tremain P, et al. A phase change calcium looping thermochemical energy storage system based on CaCO3/CaO-CaCl2 [J]. Energy Conversion and Management, 2021, 227: 113503. |
| 28 | Wang K, Yan T, Li R K, et al. A review for Ca(OH)2/CaO thermochemical energy storage systems[J]. Journal of Energy Storage, 2022, 50: 104612. |
| 29 | Sunku Prasad J, Muthukumar P, Desai F, et al. A critical review of high-temperature reversible thermochemical energy storage systems[J]. Applied Energy, 2019, 254: 113733. |
| 30 | Hu M, Hu H Y, Ye Z H, et al. A review on turning sewage sludge to value-added energy and materials via thermochemical conversion towards carbon neutrality[J]. Journal of Cleaner Production, 2022, 379: 134657. |
| 31 | Li B, Magoua Mbeugang C F, Huang Y, et al. A review of CaO based catalysts for tar removal during biomass gasification[J]. Energy, 2022, 244: 123172. |
| 32 | Zhang X Y, Liu W Q, Zhou S M, et al. A review on granulation of CaO-based sorbent for carbon dioxide capture[J]. Chemical Engineering Journal, 2022, 446: 136880. |
| 33 | Yuan Y, Li Y J, Zhao J L. Development on thermochemical energy storage based on CaO-based materials: a review[J]. Sustainability, 2018, 10(8): 2660. |
| 34 | Yuan Y, Li Y J, Duan L B, et al. CaO/Ca(OH)2 thermochemical heat storage of carbide slag from calcium looping cycles for CO2 capture[J]. Energy Conversion and Management, 2018, 174: 8-19. |
| 35 | Hu Y C, Liu W Q, Chen H Q, et al. Screening of inert solid supports for CaO-based sorbents for high temperature CO2 capture[J]. Fuel, 2016, 181: 199-206. |
| 36 | Schaube F, Koch L, Wörner A, et al. A thermodynamic and kinetic study of the de- and rehydration of Ca(OH)2 at high H2O partial pressures for thermo-chemical heat storage[J]. Thermochimica Acta, 2012, 538: 9-20. |
| 37 | Dunstan M T, Donat F, Bork A H, et al. CO2 capture at medium to high temperature using solid oxide-based sorbents: fundamental aspects, mechanistic insights, and recent advances[J]. Chemical Reviews, 2021, 121(20): 12681-12745. |
| 38 | Fujii I, Ishino M, Akiyama S, et al. Behavior of Ca(OH)2/CaO pellet under dehydration and hydration[J]. Solar Energy, 1994, 53(4): 329-341. |
| 39 | Geng Y Q, Guo Y X, Fan B, et al. Research progress of calcium-based adsorbents for CO2 capture and anti-sintering modification[J]. Journal of Fuel Chemistry and Technology, 2021, 49(7): 998-1013. |
| 40 | Ridha F N, Manovic V, Wu Y H, et al. Post-combustion CO2 capture by formic acid-modified CaO-based sorbents[J]. International Journal of Greenhouse Gas Control, 2013, 16: 21-28. |
| 41 | Guo H X, Kou X C, Zhao Y J, et al. Effect of synergistic interaction between Ce and Mn on the CO2 capture of calcium-based sorbent: textural properties, electron donation, and oxygen vacancy[J]. Chemical Engineering Journal, 2018, 334: 237-246. |
| 42 | Møller K T, Berger A, Paskevicius M, et al. Synergetic effect of multicomponent additives on limestone when assessed as a thermochemical energy storage material[J]. Journal of Alloys and Compounds, 2022, 891: 161954. |
| 43 | Guo H, Kou X, Zhao Y, et al. Role of microstructure, electron transfer, and coordination state in the CO2 capture of calcium-based sorbent by doping (Zr-Mn) [J]. Chemical Engineering Journal, 2018, 336: 376-385. |
| 44 | Guo H X, Wang X, Wang H, et al. Double-exchange-induced effective increased CO2 capture of CaO by doping bimetallic oxides with variable valence state[J]. Chemical Engineering Journal, 2022, 433: 134490. |
| 45 | Sun H, Li Y J, Yan X Y, et al. Thermochemical energy storage performance of Al2O3/CeO2 co-doped CaO-based material under high carbonation pressure[J]. Applied Energy, 2020, 263: 114650. |
| 46 | Mathew A, Nadim N, Chandratilleke T T, et al. Kinetic investigation and numerical modelling of CaCO3/Al2O3 reactor for high-temperature thermal energy storage application[J]. Solar Energy, 2022, 241: 262-274. |
| 47 | Chen X Y, Jin X G, Liu Z M, et al. Experimental investigation on the CaO/CaCO3 thermochemical energy storage with SiO2 doping[J]. Energy, 2018, 155: 128-138. |
| 48 | Xu T X, Tian X K, Khosa A A, et al. Reaction performance of CaCO3/CaO thermochemical energy storage with TiO2 dopant and experimental study in a fixed-bed reactor[J]. Energy, 2021, 236: 121451. |
| 49 | Guo H X, Feng J Q, Zhao Y J, et al. Effect of micro-structure and oxygen vacancy on the stability of (Zr-Ce)-additive CaO-based sorbent in CO2 adsorption[J]. Journal of CO2 Utilization, 2017, 19: 165-176. |
| 50 | Sakellariou K G, Karagiannakis G, Criado Y A, et al. Calcium oxide based materials for thermochemical heat storage in concentrated solar power plants[J]. Solar Energy, 2015, 122: 215-230. |
| 51 | Salvador C, Lu D, Anthony E J, et al. Enhancement of CaO for CO2 capture in an FBC environment[J]. Chemical Engineering Journal, 2003, 96(1/2/3): 187-195. |
| 52 | Shkatulov A, Aristov Y. Modification of magnesium and calcium hydroxides with salts: an efficient way to advanced materials for storage of middle-temperature heat[J]. Energy, 2015, 85: 667-676. |
| 53 | Shkatulov A, Aristov Y. Calcium hydroxide doped by KNO3 as a promising candidate for thermochemical storage of solar heat[J]. RSC Advances, 2017, 7(68): 42929-42939. |
| 54 | Lee C H, Choi S W, Yoon H J, et al. Na2CO3-doped CaO-based high-temperature CO2 sorbent and its sorption kinetics[J]. Chemical Engineering Journal, 2018, 352: 103-109. |
| 55 | Xu Y Q, Lu B W, Luo C, et al. Na2CO3 promoted CaO-based heat carrier for thermochemical energy storage in concentrated solar power plants[J]. Chemical Engineering Journal, 2022, 435: 134852. |
| 56 | Wang T, Zhao C Y, Yan J. Investigation on the Ca(OH)2/CaO thermochemical energy storage system with potassium nitrate addition[J]. Solar Energy Materials and Solar Cells, 2020, 215: 110646. |
| 57 | Xu Y Q, Ding H R, Luo C, et al. Potential synergy of chlorine and potassium and sodium elements in carbonation enhancement of CaO-based sorbents[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 11677-11684. |
| 58 | Afflerbach S, Afflerbach K, Trettin R, et al. Improvement of a semipermeable shell for encapsulation of calcium hydroxide for thermochemical heat storage solutions[J]. Solar Energy, 2021, 217: 208-222. |
| 59 | Afflerbach S, Kappes M, Gipperich A, et al. Semipermeable encapsulation of calcium hydroxide for thermochemical heat storage solutions[J]. Solar Energy, 2017, 148: 1-11. |
| 60 | Mejia A C, Afflerbach S, Linder M, et al. Experimental analysis of encapsulated CaO/Ca(OH)2 granules as thermochemical storage in a novel moving bed reactor[J]. Applied Thermal Engineering, 2020, 169: 114961. |
| 61 | Chen Y N, Long Y, Sun J A, et al. Core-in-shell, cellulose-templated CaO-based sorbent pellets for CO2 capture at elevated temperatures[J]. Energy & Fuels, 2021, 35(16): 13215-13223. |
| 62 | Liu F J, Chou K S, Huang Y K. A novel method to make regenerable core-shell calcium-based sorbents[J]. Journal of Environmental Management, 2006, 79(1): 51-56. |
| 63 | Sun J A, Liu W Q, Hu Y C, et al. Structurally improved, core-in-shell, CaO-based sorbent pellets for CO2 capture[J]. Energy & Fuels, 2015, 29(10): 6636-6644. |
| 64 | Jin D L, Yu X J, Yue L H, et al. Decomposition kinetics study of AlOOH coated calcium carbonate[J]. Materials Chemistry and Physics, 2009, 115(1): 418-422. |
| 65 | Sun Z K, Sedghkerdar M H, Saayman J, et al. A facile fabrication of mesoporous core-shell CaO-based pellets with enhanced reactive stability and resistance to attrition in cyclic CO2 capture[J]. Journal of Materials Chemistry A, 2014, 2(39): 16577-16588. |
| 66 | Ogura H, Miyazaki M, Matsuda H, et al. Experimental study on heat transfer enhancement of the solid reactant particle bed in a chemical heat pump using Ca(OH)2/CaO reaction[J]. Kagaku Kogaku Ronbunshu, 1991, 17(5): 916-923. |
| 67 | Wokon M, Block T, Nicolai S, et al. Thermodynamic and kinetic investigation of a technical grade manganese-iron binary oxide for thermochemical energy storage[J]. Solar Energy, 2017, 153: 471-485. |
| 68 | Schaube F, Utz I, Wörner A, et al. De- and rehydration of Ca(OH)2 in a reactor with direct heat transfer for thermo-chemical heat storage(Part B): Validation of model[J]. Chemical Engineering Research and Design, 2013, 91(5): 865-873. |
| 69 | Yan J, Zhao C Y. Experimental study of CaO/Ca(OH)2 in a fixed-bed reactor for thermochemical heat storage[J]. Applied Energy, 2016, 175: 277-284. |
| 70 | Schmidt M, Gutierrez A, Linder M. Thermochemical energy storage with CaO/Ca(OH)2—experimental investigation of the thermal capability at low vapor pressures in a lab scale reactor[J]. Applied Energy, 2017, 188: 672-681. |
| 71 | Wang B Y, Wang Z Y, Ma Y, et al. Heat transfer enhancement of indirect heat transfer reactors for Ca(OH)2/CaO thermochemical energy storage system[J]. Processes, 2021, 9(7): 1136. |
| 72 | Wang M Y, Chen L, He P, et al. Numerical study and enhancement of Ca(OH)2/CaO dehydration process with porous channels embedded in reactors[J]. Energy, 2019, 181: 417-428. |
| 73 | Han X C, Xu H J, Zhao C Y. Design and performance evaluation of multi-layered reactor for calcium-based thermochemical heat storage with multi-physics coupling[J]. Renewable Energy, 2022, 195: 1324-1340. |
| 74 | Funayama S, Takasu H, Kim S T, et al. Thermochemical storage performance of a packed bed of calcium hydroxide composite with a silicon-based ceramic honeycomb support[J]. Energy, 2020, 201: 117673. |
| 75 | Kato Y, Yamada M, Kanie T, et al. Calcium oxide/carbon dioxide reactivity in a packed bed reactor of a chemical heat pump for high-temperature gas reactors[J]. Nuclear Engineering and Design, 2001, 210(1/2/3): 1-8. |
| 76 | Scaltsoyiannes A, Lemonidou A. CaCO3 decomposition for calcium-looping applications: kinetic modeling in a fixed-bed reactor[J]. Chemical Engineering Science: X, 2020, 8: 100071. |
| 77 | Sun H, Li Y J, Bian Z G, et al. Thermochemical energy storage performances of Ca-based natural and waste materials under high pressure during CaO/CaCO3 cycles[J]. Energy Conversion and Management, 2019, 197: 111885. |
| 78 | Criado Y A, Alonso M, Abanades J C, et al. Conceptual process design of a CaO/Ca(OH)2 thermochemical energy storage system using fluidized bed reactors[J]. Applied Thermal Engineering, 2014, 73(1): 1087-1094. |
| 79 | Criado Y A, Huille A, Rougé S, et al. Experimental investigation and model validation of a CaO/Ca(OH)2 fluidized bed reactor for thermochemical energy storage applications[J]. Chemical Engineering Journal, 2017, 313: 1194-1205. |
| 80 | Pardo P, Anxionnaz-Minvielle Z, Rougé S, et al. Ca(OH)2/CaO reversible reaction in a fluidized bed reactor for thermochemical heat storage[J]. Solar Energy, 2014, 107: 605-616. |
| 81 | Kai R, Marc L, Matthias S. Experimental investigation of a novel mechanically fluidized bed reactor for thermochemical energy storage with calcium hydroxide/calcium oxide[J]. Applied Energy, 2022, 315:118976. |
| 82 | Uchino T, Fushimi C. Fluidized bed reactor for thermochemical heat storage using Ca(OH)2/CaO to absorb the fluctuations of electric power supplied by variable renewable energy sources: a dynamic model[J]. Chemical Engineering Journal, 2021, 419: 129571. |
| 83 | Shimizu T, Hirama T, Hosoda H, et al. A twin fluid-bed reactor for removal of CO2 from combustion processes[J]. Chemical Engineering Research and Design, 1999, 77(1): 62-68. |
| 84 | Grasa G S, Abanades J C. CO2 capture capacity of CaO in long series of carbonation/calcination cycles[J]. Industrial & Engineering Chemistry Research, 2006, 45(26): 8846-8851. |
| 85 | Arias B, Diego M E, Abanades J C, et al. Demonstration of steady state CO2 capture in a 1.7 MWth calcium looping pilot[J]. International Journal of Greenhouse Gas Control, 2013, 18: 237-245. |
| 86 | Blamey J, Al-Jeboori M J, Manovic V, et al. CO2 capture by calcium aluminate pellets in a small fluidized bed[J]. Fuel Processing Technology, 2016, 142: 100-106. |
| 87 | Blamey J, Manovic V, Anthony E J, et al. On steam hydration of CaO-based sorbent cycled for CO2 capture[J]. Fuel, 2015, 150: 269-277. |
| 88 | Zheng H B, Liu X L, Xuan Y M, et al. Thermochemical heat storage performances of fluidized black CaCO3 pellets under direct concentrated solar irradiation[J]. Renewable Energy, 2021, 178: 1353-1369. |
| 89 | Nikulshina V, Gebald C, Steinfeld A. CO2 capture from atmospheric air via consecutive CaO-carbonation and CaCO3-calcination cycles in a fluidized-bed solar reactor[J]. Chemical Engineering Journal, 2009, 146(2): 244-248. |
| 90 | Tregambi C, Padula S, Galbusieri M, et al. Directly irradiated fluidized bed reactor for thermochemical energy storage and solar fuels production[J]. Powder Technology, 2020, 366: 460-469. |
| 91 | Farcot L, Le Pierrès N, Michel B, et al. Numerical investigations of a continuous thermochemical heat storage reactor[J]. Journal of Energy Storage, 2018, 20: 109-119. |
| 92 | Roßkopf C, Afflerbach S, Schmidt M, et al. Investigations of nano coated calcium hydroxide cycled in a thermochemical heat storage[J]. Energy Conversion and Management, 2015, 97: 94-102. |
| 93 | Schmidt M, Szczukowski C, Roßkopf C, et al. Experimental results of a 10 kW high temperature thermochemical storage reactor based on calcium hydroxide[J]. Applied Thermal Engineering, 2014, 62(2): 553-559. |
| 94 | Chen X Y, Jin X G, Zhang Z H, et al. Experimental investigation of CaCO3/CaO in a spiral coil reactor for thermochemical energy storage[J]. Chemical Engineering Journal, 2022, 428: 131971. |
| 95 | Barker R. The reactivity of calcium oxide towards carbon dioxide and its use for energy storage[J]. Journal of Applied Chemistry and Biotechnology, 1974, 24(4/5): 221-227. |
| 96 | Pascual S, Lisbona P, Bailera M, et al. Design and operational performance maps of calcium looping thermochemical energy storage for concentrating solar power plants[J]. Energy, 2021, 220: 119715. |
| 97 | Hughes R W, Lu D Y, Anthony E J, et al. Design, process simulation and construction of an atmospheric dual fluidized bed combustion system for in situ CO2 capture using high-temperature sorbents[J]. Fuel Processing Technology, 2005, 86(14/15): 1523-1531. |
| 98 | Lu D Y, Hughes R W, Anthony E J. Ca-based sorbent looping combustion for CO2 capture in pilot-scale dual fluidized beds[J]. Fuel Processing Technology, 2008, 89(12): 1386-1395. |
| 99 | Ströhle J, Hilz J, Epple B. Performance of the carbonator and calciner during long-term carbonate looping tests in a 1 MWth pilot plant[J]. Journal of Environmental Chemical Engineering, 2020, 8(1): 103578. |
| 100 | Ströhle J, Junk M, Kremer J, et al. Carbonate looping experiments in a 1 MWth pilot plant and model validation[J]. Fuel, 2014, 127: 13-22. |
| 101 | Reitz M, Junk M, Ströhle J, et al. Design and operation of a 300 kWth indirectly heated carbonate looping pilot plant[J]. International Journal of Greenhouse Gas Control, 2016, 54: 272-281. |
| 102 | Ogura H, Yamamoto T, Kage H, et al. Effects of heat exchange condition on hot air production by a chemical heat pump dryer using CaO/H2O/Ca(OH)2 reaction[J]. Chemical Engineering Journal, 2002, 86(1/2): 3-10. |
| 103 | Ogura H, Yamamoto T, Kage H. Efficiencies of CaO/H2O/Ca(OH)2 chemical heat pump for heat storing and heating/cooling[J]. Energy, 2003, 28(14): 1479-1493. |
| 104 | Ogura H, Yasuda S, Otsubo Y, et al. Continuous operation of a chemical heat pump[J]. Asia-Pacific Journal of Chemical Engineering, 2007, 2(2): 118-123. |
| 105 | Ogura H, Shimojyo R, Kage H, et al. Simulation of hydration/dehydration of CaO/Ca(OH)2 chemical heat pump reactor for cold/hot heat generation[J]. Drying Technology, 1999, 17(7/8): 1579-1592. |
| 106 | Kato Y, Saku D, Harada N, et al. Utilization of high temperature heat from nuclear reactor using inorganic chemical heat pump[J]. Progress in Nuclear Energy, 1998, 32(3/4): 563-570. |
| 107 | Fujimoto S, Bilgen E, Ogura H. Dynamic simulation of CaO/Ca(OH)2 chemical heat pump systems[J]. Exergy, An International Journal, 2002, 2(1): 6-14. |
| 108 | Fujimoto S, Bilgen E, Ogura H. CaO/Ca(OH)2 chemical heat pump system[J]. Energy Conversion and Management, 2002, 43(7): 947-960. |
| 109 | Ogura H, Kubota M, Suzuki H, et al. Fundamental experimental study on chemical heat pump for storing low-temperature waste heat and releasing cold-heat[J]. Kagaku Kogaku Ronbunshu, 2009, 35(5): 506-510. |
| 110 | Zhang H, Ogura H, Umezu M, et al. Hydration reaction characteristics of CaO from various local limestone samples as chemical heat pump/storage materials[J]. Journal of Materials Science, 2017, 52(19): 11360-11369. |
| 111 | Arjmand M, Liu L C, Neretnieks I. Exergetic efficiency of high-temperature-lift chemical heat pump (CHP) based on CaO/CO2 and CaO/H2O working pairs[J]. International Journal of Energy Research, 2013, 37(9): 1122-1131. |
| 112 | Cerkvenik B, Kato Y, Storkenmaier F. Applicability of calcium oxide in a cascading sorption cooling system[J]. Journal of Chemical Engineering of Japan, 2002, 35(10): 969-976. |
| 113 | Alovisio A, Chacartegui R, Ortiz C, et al. Optimizing the CSP-calcium looping integration for thermochemical energy storage[J]. Energy Conversion and Management, 2017, 136: 85-98. |
| 114 | Tregambi C, Montagnaro F, Salatino P, et al. A model of integrated calcium looping for CO2 capture and concentrated solar power[J]. Solar Energy, 2015, 120: 208-220. |
| 115 | Edwards S E B, Materić V. Calcium looping in solar power generation plants[J]. Solar Energy, 2012, 86(9): 2494-2503. |
| 116 | Chacartegui R, Alovisio A, Ortiz C, et al. Thermochemical energy storage of concentrated solar power by integration of the calcium looping process and a CO2 power cycle[J]. Applied Energy, 2016, 173: 589-605. |
| 117 | Tregambi C, Bareschino P, Mancusi E, et al. Modelling of a concentrated solar power-photovoltaics hybrid plant for carbon dioxide capture and utilization via calcium looping and methanation[J]. Energy Conversion and Management, 2021, 230: 113792. |
| 118 | Binotti M, Astolfi M, Campanari S, et al. Preliminary assessment of sCO2 cycles for power generation in CSP solar tower plants[J]. Applied Energy, 2017, 204: 1007-1017. |
| 119 | Hanak D P, Manovic V. Calcium looping with supercritical CO2 cycle for decarbonisation of coal-fired power plant[J]. Energy, 2016, 102: 343-353. |
| 120 | Ortiz C, Romano M C, Valverde J M, et al. Process integration of calcium-looping thermochemical energy storage system in concentrating solar power plants[J]. Energy, 2018, 155: 535-551. |
| 121 | Fernández R, Ortiz C, Chacartegui R, et al. Dispatchability of solar photovoltaics from thermochemical energy storage[J]. Energy Conversion and Management, 2019, 191: 237-246. |
| 122 | Colelli G, Chacartegui R, Ortiz C, et al. Life cycle and environmental assessment of calcium looping (CaL) in solar thermochemical energy storage[J]. Energy Conversion and Management, 2022, 257: 115428. |
| 123 | Karasavvas E, Panopoulos K D, Papadopoulou S, et al. Energy and exergy analysis of the integration of concentrated solar power with calcium looping for power production and thermochemical energy storage[J]. Renewable Energy, 2020, 154: 743-753. |
| 124 | Tesio U, Guelpa E, Verda V. Integration of thermochemical energy storage in concentrated solar power (Part 1): Energy and economic analysis/optimization[J]. Energy Conversion and Management: X, 2020, 6: 100039. |
| 125 | Ortiz C, Tejada C, Chacartegui R, et al. Solar combined cycle with high-temperature thermochemical energy storage[J]. Energy Conversion and Management, 2021, 241: 114274. |
| 126 | Bravo R, Ortiz C, Chacartegui R, et al. Hybrid solar power plant with thermochemical energy storage: a multi-objective operational optimisation[J]. Energy Conversion and Management, 2020, 205: 112421. |
| 127 | Khosa A A, Xu T X, Xia B Q, et al. Technological challenges and industrial applications of CaCO3/CaO based thermal energy storage system—a review[J]. Solar Energy, 2019, 193: 618-636. |
| [1] | 江河, 袁俊飞, 王林, 邢谷雨. 均流腔结构对微细通道内相变流动特性影响的实验研究[J]. 化工学报, 2023, 74(S1): 235-244. |
| [2] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [3] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [4] | 李贵贤, 曹阿波, 孟文亮, 王东亮, 杨勇, 周怀荣. 耦合固体氧化物电解槽的CO2制甲醇过程设计与评价研究[J]. 化工学报, 2023, 74(7): 2999-3009. |
| [5] | 蔡斌, 张效林, 罗倩, 党江涛, 左栗源, 刘欣梅. 导电薄膜材料的研究进展[J]. 化工学报, 2023, 74(6): 2308-2321. |
| [6] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [7] | 邵远哲, 赵忠盖, 刘飞. 基于共同趋势模型的非平稳过程质量相关故障检测方法[J]. 化工学报, 2023, 74(6): 2522-2537. |
| [8] | 徐文超, 孙志高, 李翠敏, 李娟, 黄海峰. 静态条件下表面活性剂E-1310对HCFC-141b水合物生成的影响[J]. 化工学报, 2023, 74(5): 2179-2185. |
| [9] | 王子健, 柯明, 李佳涵, 李舒婷, 孙巾茹, 童燕兵, 赵治平, 刘加英, 任璐. 短b轴ZSM-5分子筛制备方法及应用研究进展[J]. 化工学报, 2023, 74(4): 1457-1473. |
| [10] | 贠程, 王倩琳, 陈锋, 张鑫, 窦站, 颜廷俊. 基于社团结构的化工过程风险演化路径深度挖掘[J]. 化工学报, 2023, 74(4): 1639-1650. |
| [11] | 陈向上, 马振杰, 任希华, 贾悦, 吕晓龙, 陈华艳. 三维网络萃取膜的制备及传质效率研究[J]. 化工学报, 2023, 74(3): 1126-1133. |
| [12] | 王子宗, 索寒生, 赵学良. 数字孪生智能乙烯工厂研究与构建[J]. 化工学报, 2023, 74(3): 1175-1186. |
| [13] | 刘润竹, 储甜甜, 张孝阿, 王成忠, 张军营. α,ω-端羟基亚苯基氟硅聚合物的合成及性能[J]. 化工学报, 2023, 74(3): 1360-1369. |
| [14] | 王雅琳, 潘雨晴, 刘晨亮. 基于GSA-LSTM动态结构特征提取的间歇过程监测方法[J]. 化工学报, 2022, 73(9): 3994-4002. |
| [15] | 杨宏欣, 李兴亚, 葛亮, 徐铜文. 含哌啶阳离子侧长链型一/二价阴离子选择性分离膜的制备[J]. 化工学报, 2022, 73(8): 3739-3748. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号