化工学报 ›› 2023, Vol. 74 ›› Issue (3): 1360-1369.DOI: 10.11949/0438-1157.20221455
收稿日期:2022-11-08
修回日期:2023-02-06
出版日期:2023-03-05
发布日期:2023-04-19
通讯作者:
张孝阿,张军营
作者简介:刘润竹(1997—),女,硕士研究生,1062655630@qq.com
基金资助:
Runzhu LIU( ), Tiantian CHU, Xiaoa ZHANG(
), Tiantian CHU, Xiaoa ZHANG( ), Chengzhong WANG, Junying ZHANG(
), Chengzhong WANG, Junying ZHANG( )
)
Received:2022-11-08
Revised:2023-02-06
Online:2023-03-05
Published:2023-04-19
Contact:
Xiaoa ZHANG, Junying ZHANG
摘要:
在非平衡催化剂作用下,通过α,ω-端羟基聚甲基三氟丙基硅氧烷和亚苯基二硅醇单体的脱水缩聚反应,制得一系列α,ω-端羟基亚苯基氟硅聚合物,并研究了反应时间、催化剂用量、反应物浓度、单体比例等因素对所得聚合物的状态、特性黏度、分子量及分布、共聚组成的影响。采用红外、核磁、流变仪、热分析等手段对聚合物的结构、室温交联反应性、力学性能、耐高温性能和玻璃化转变进行了研究。结果表明,向主链中引入亚苯基在显著提高聚合物耐高温性能的同时,还有助于交联反应速率和力学性能的改善,并且当亚苯基含量低于30%(mol)时不会对聚合物的玻璃化温度(Tg)产生不利影响。分析原因,亚苯基的插入抑制了有机硅链的成环降解且提高了链的刚性(有利于耐高温性),客观上隔开并降低了大体积三氟丙基侧基对端羟基的屏蔽效应(有利于交联反应性),因而将聚合物制备成氟硅密封胶,具有较好的交联性能、力学性能和耐高低温性能。
中图分类号:
刘润竹, 储甜甜, 张孝阿, 王成忠, 张军营. α,ω-端羟基亚苯基氟硅聚合物的合成及性能[J]. 化工学报, 2023, 74(3): 1360-1369.
Runzhu LIU, Tiantian CHU, Xiaoa ZHANG, Chengzhong WANG, Junying ZHANG. Synthesis and properties of phenylene-containing α,ω-hydroxy-terminated fluorosilicone polymers[J]. CIESC Journal, 2023, 74(3): 1360-1369.

图1 α,ω-端羟基亚苯基氟硅聚合物(O1、O2、O3和O4)的合成路线
Fig.1 Synthetic route to α,ω-hydroxy-terminated phenylene-containing fluorosilicone polymers (O1, O2, O3, and O4)
| 序号 | 聚合物 | 氟硅原料 | 亚苯基单体 | 投料比① | 产率/% | 聚合物形态 | [η]/(ml/g) | Mn | PDI | 含量/%(mol)② | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 氟硅 | 亚苯基 | ||||||||||
| 1 | O1 | FMS-9921 | BHSPE | 2:1 | 94 | 黏稠液体 | 32 | 22700 | 1.84 | 89.30 | 21.40 |
| 2 | O1 | FMS-9921 | BHSPE | 1:1 | 92 | 半固体 | 87 | 36800 | 1.61 | 82.90 | 34.21 |
| 3 | O1 | FMS-9921 | BHSPE | 1:2 | 93 | 固体 | 132 | 33500 | 1.86 | 74.49 | 51.03 |
| 4 | O1 | FMS-9921 | BHSPE | 1:4 | 94 | 固体 | 158 | 46700 | 2.01 | 66.42 | 67.17 |
| 5 | O1 | FMS-9921 | BHSPE | 1:6 | 94 | 固体 | 184 | 60400 | 2.17 | 62.36 | 75.29 |
| 6 | O2 | FMS-9922 | BHSPE | 1:1 | 93 | 半固体 | 56 | 33000 | 1.71 | 83.48 | 33.05 |
| 7 | O3 | FMS-9921 | BHSB | 2:1 | 93 | 黏稠液体 | 30 | 15300 | 1.32 | 89.08 | 10.92 |
| 8 | O3 | FMS-9921 | BHSB | 1:1 | 94 | 黏稠液体 | 89 | 39100 | 1.56 | 72.89 | 27.11 |
| 9 | O3 | FMS-9921 | BHSB | 1:2 | 92 | 半固体 | 100 | 45300 | 1.68 | 61.69 | 38.31 |
| 10 | O3 | FMS-9921 | BHSB | 1:4 | 96 | 固体 | 158 | 50900 | 1.83 | 47.96 | 52.04 |
| 11 | O3 | FMS-9921 | BHSB | 1:6 | 95 | 固体 | 179 | 97800 | 2.22 | 37.60 | 62.40 |
| 12 | O4 | FMS-9922 | BHSB | 1:1 | 92 | 黏稠液体 | 69 | 24100 | 1.60 | 76.96 | 23.04 |
表1 单体投料比对聚合物合成的影响
Table 1 Effect of monomer ratio on the synthesis of α,ω-hydroxy-terminated phenylene fluorosilicone polymers
| 序号 | 聚合物 | 氟硅原料 | 亚苯基单体 | 投料比① | 产率/% | 聚合物形态 | [η]/(ml/g) | Mn | PDI | 含量/%(mol)② | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 氟硅 | 亚苯基 | ||||||||||
| 1 | O1 | FMS-9921 | BHSPE | 2:1 | 94 | 黏稠液体 | 32 | 22700 | 1.84 | 89.30 | 21.40 |
| 2 | O1 | FMS-9921 | BHSPE | 1:1 | 92 | 半固体 | 87 | 36800 | 1.61 | 82.90 | 34.21 |
| 3 | O1 | FMS-9921 | BHSPE | 1:2 | 93 | 固体 | 132 | 33500 | 1.86 | 74.49 | 51.03 |
| 4 | O1 | FMS-9921 | BHSPE | 1:4 | 94 | 固体 | 158 | 46700 | 2.01 | 66.42 | 67.17 |
| 5 | O1 | FMS-9921 | BHSPE | 1:6 | 94 | 固体 | 184 | 60400 | 2.17 | 62.36 | 75.29 |
| 6 | O2 | FMS-9922 | BHSPE | 1:1 | 93 | 半固体 | 56 | 33000 | 1.71 | 83.48 | 33.05 |
| 7 | O3 | FMS-9921 | BHSB | 2:1 | 93 | 黏稠液体 | 30 | 15300 | 1.32 | 89.08 | 10.92 |
| 8 | O3 | FMS-9921 | BHSB | 1:1 | 94 | 黏稠液体 | 89 | 39100 | 1.56 | 72.89 | 27.11 |
| 9 | O3 | FMS-9921 | BHSB | 1:2 | 92 | 半固体 | 100 | 45300 | 1.68 | 61.69 | 38.31 |
| 10 | O3 | FMS-9921 | BHSB | 1:4 | 96 | 固体 | 158 | 50900 | 1.83 | 47.96 | 52.04 |
| 11 | O3 | FMS-9921 | BHSB | 1:6 | 95 | 固体 | 179 | 97800 | 2.22 | 37.60 | 62.40 |
| 12 | O4 | FMS-9922 | BHSB | 1:1 | 92 | 黏稠液体 | 69 | 24100 | 1.60 | 76.96 | 23.04 |
| 序号 | 反应时间/h | 产率/% | Mn① | PDI① | [η]/(ml/g)② |
|---|---|---|---|---|---|
| 1 | 4.5 | 88 | 9500 | 2.16 | 48 |
| 2 | 6.5 | 94 | 39100 | 1.56 | 89 |
| 3 | 8.5 | 95 | 77200 | 1.55 | 117 |
| 4 | 10.5 | 95 | 77500 | 1.68 | 121 |
表2 反应时间对聚合物合成的影响
Table 2 Effect of reaction time on the synthesis of α,ω-hydroxy-terminated phenylene fluorosilicone polymers
| 序号 | 反应时间/h | 产率/% | Mn① | PDI① | [η]/(ml/g)② |
|---|---|---|---|---|---|
| 1 | 4.5 | 88 | 9500 | 2.16 | 48 |
| 2 | 6.5 | 94 | 39100 | 1.56 | 89 |
| 3 | 8.5 | 95 | 77200 | 1.55 | 117 |
| 4 | 10.5 | 95 | 77500 | 1.68 | 121 |
| 序号 | TMG∶Si—OH/ %(mol) | 产率/% | Mn | PDI | [η] /(ml/g) |
|---|---|---|---|---|---|
| 1 | 1 | 94 | 39100 | 1.56 | 89 |
| 2 | 2 | 95 | 76700 | 1.67 | 110 |
| 3 | 4 | 96 | 94700 | 1.66 | 132 |
| 4 | 6 | 96 | 102100 | 1.80 | 141 |
表3 TMG用量对聚合物合成的影响
Table 3 Effect of TMG doasage on the synthesis of α,ω-hydroxy-terminated phenylene fluorosilicone polymers
| 序号 | TMG∶Si—OH/ %(mol) | 产率/% | Mn | PDI | [η] /(ml/g) |
|---|---|---|---|---|---|
| 1 | 1 | 94 | 39100 | 1.56 | 89 |
| 2 | 2 | 95 | 76700 | 1.67 | 110 |
| 3 | 4 | 96 | 94700 | 1.66 | 132 |
| 4 | 6 | 96 | 102100 | 1.80 | 141 |
| 序号 | 反应物浓度/ %(质量) | 产率/% | Mn | PDI | [η] /(ml/g) |
|---|---|---|---|---|---|
| 1 | 45 | 65 | 27300 | 1.46 | 62 |
| 2 | 55 | 76 | 32800 | 1.49 | 78 |
| 3 | 65 | 94 | 39100 | 1.56 | 89 |
| 4 | 75 | 85 | 3100 | 1.47 | 38 |
| 5① | 100 | 0 | 800 | 1.10 | 6 |
表4 反应物浓度对聚合物合成的影响
Table 4 Effect of reactant concentration on the synthesis of α,ω-hydroxy-terminated phenylene fluorosilicone polymers
| 序号 | 反应物浓度/ %(质量) | 产率/% | Mn | PDI | [η] /(ml/g) |
|---|---|---|---|---|---|
| 1 | 45 | 65 | 27300 | 1.46 | 62 |
| 2 | 55 | 76 | 32800 | 1.49 | 78 |
| 3 | 65 | 94 | 39100 | 1.56 | 89 |
| 4 | 75 | 85 | 3100 | 1.47 | 38 |
| 5① | 100 | 0 | 800 | 1.10 | 6 |
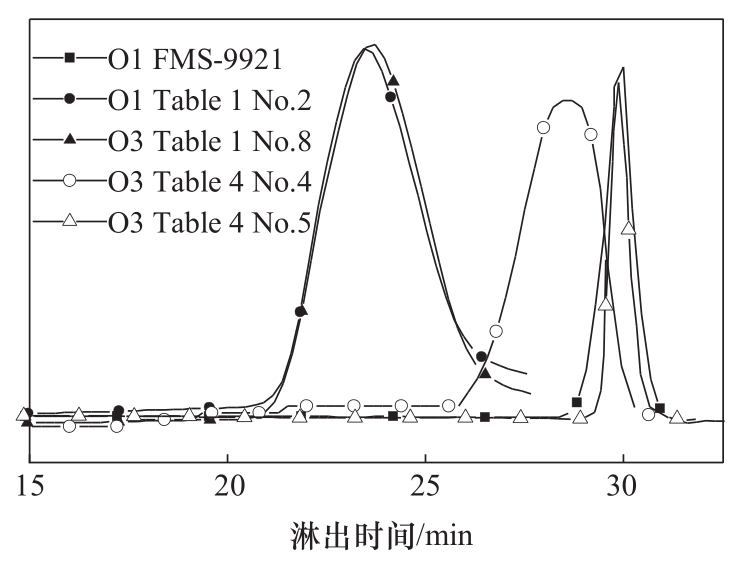
图2 聚合物O1(表1序号2)、O3(表1序号8、表4序号4和5)和FMS-9921的GPC曲线
Fig.2 GPC traces of polymer O1 (Table 1 No. 2), O3 (Table 1 No. 8, Table 4 No. 4 and 5), and FMS-9921
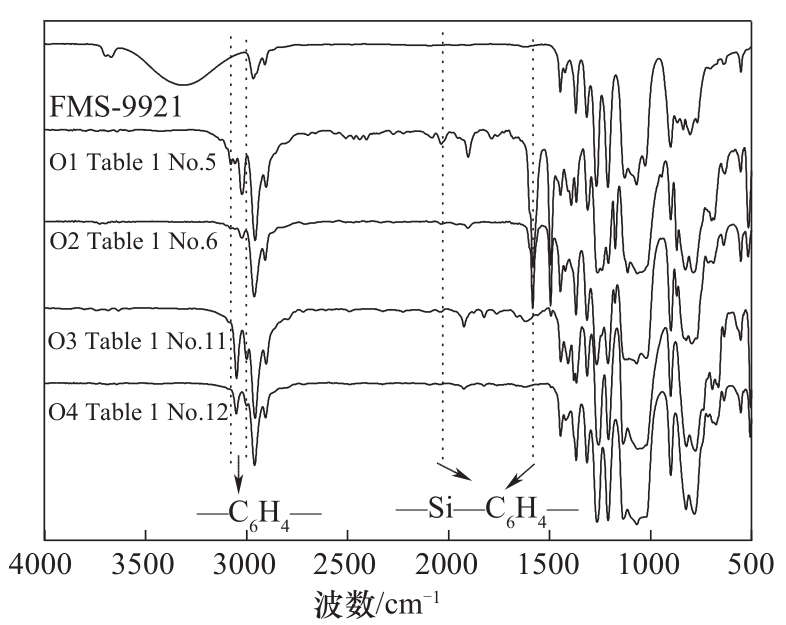
图3 聚合物O1(表1序号5)、O2(表1序号6)、O3(表1序号11)、O4(表1序号12)和FMS-9921的FTIR光谱
Fig.3 FTIR spectra of polymer O1 (Table 1 No. 5), O2 (Table 1 No. 6), O3 (Table 1 No. 11), O4 (Table 1 No. 12) and FMS-9921
| 基础聚合物 | 交联密度/ (10-5 mol/ml) | 邵A硬度 | 拉伸强度/ MPa | 拉断 伸长率/% |
|---|---|---|---|---|
| AFS-R-H1101 | 11.50 | 52±3 | 2.95±0.20 | 165±5 |
| O4 | 7.29 | 59±3 | 3.34±0.20 | 199±2 |
表5 AFS-R-H1101和O4硫化胶的硬度和拉伸性能
Table 5 Hardness and tensile properties of AFS-R-H1101 and O4 vulcanizates
| 基础聚合物 | 交联密度/ (10-5 mol/ml) | 邵A硬度 | 拉伸强度/ MPa | 拉断 伸长率/% |
|---|---|---|---|---|
| AFS-R-H1101 | 11.50 | 52±3 | 2.95±0.20 | 165±5 |
| O4 | 7.29 | 59±3 | 3.34±0.20 | 199±2 |
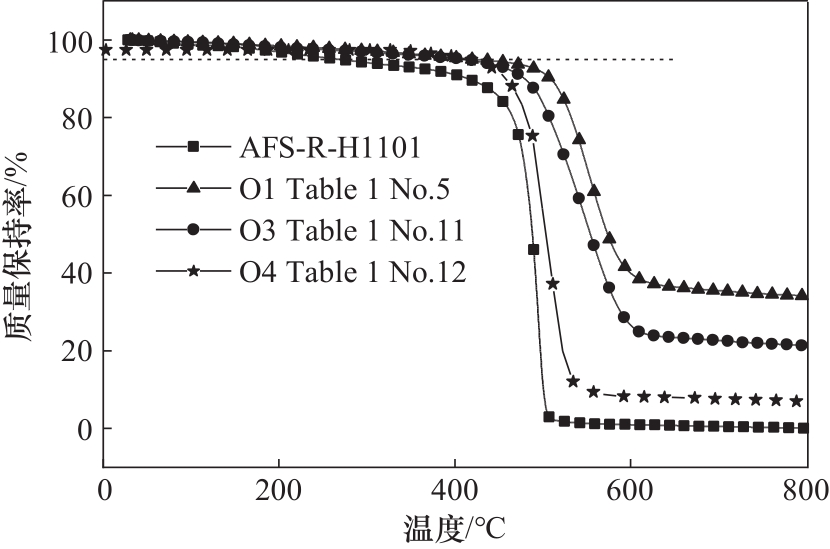
图8 聚合物O1(表1序号5)、O3(表1序号11)、O4(表1序号12)及AFS-R-H1101的热失重曲线
Fig.8 TGA curves of polymer O1 (Table 1 No. 5), O3 (Table 1 No. 11), O4 (Table 1 No. 12) and AFS-R-H1101
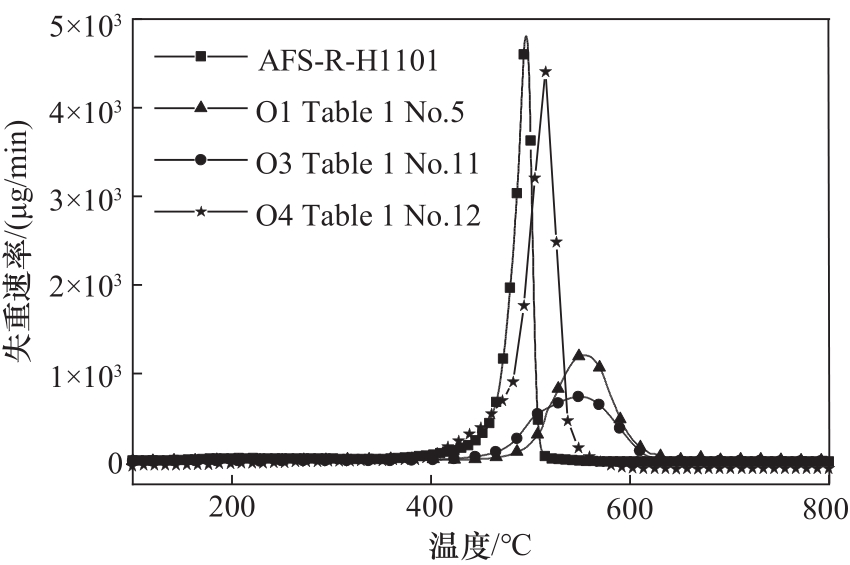
图9 聚合物O1(表1序号5)、O3(表1序号11)、O4(表1序号12)及AFS-R-H1101的微分失重曲线
Fig.9 DTG curves of polymer O1 (Table 1 No. 5), O3 (Table 1 No. 11), O4 (Table 1 No. 12) and AFS-R-H1101
| 16 | Andrianov K A. Rearrangements and polymerization of cyclic organosilicon compounds[J]. Polymer Science U.S.S.R., 1971, 13(2): 284-298. |
| 17 | 刘润竹, 张猛, 刘振超, 等. 亚芳基改性聚硅氧烷的制备及其热稳定性的研究进展[J]. 当代化工研究, 2021(23): 1-3. |
| Liu R Z, Zhang M, Liu Z C, et al. Research progress on the preparation and thermal stability of arylene-modified polysiloxane[J]. Modern Chemical Research, 2021(23): 1-3. | |
| 18 | Omietanski G M. Random siloxane copolymers containing phenylene and phenyl ether chain linkages: US3287310[P]. 1966-11-22. |
| 19 | Singh N, Leman J T, Whitney J M, et al. Copolymer sealant compositions and method for making: US20030130466[P]. 2003-07-10. |
| 20 | Zhao D, Shan S X, Zhang M, et al. Preparation of titanium-silphenylene-siloxane hybrid polymers with high refractive index, transmittance, and thermal stability[J]. Chinese Journal of Polymer Science, 2020, 38(9): 973-982. |
| 21 | 张猛, 王伟, 赵栋, 等. 高折射率钛-二苯醚-硅氧烷聚合物的合成表征和性能[J]. 高分子材料科学与工程, 2021, 37(8): 1-9, 18. |
| Zhang M, Wang W, Zhao D, et al. Synthesis, characterization and properties of titanium-diphenylether-siloxane polymers with high refractive index[J]. Polymer Materials Science & Engineering, 2021, 37(8): 1-9, 18. | |
| 22 | Dvornic P R. Degradative side reactions in the syntheses of exactly alternating silarylene-siloxane polymers[J]. Polymer Bulletin, 1992, 28(3): 339-344. |
| 23 | Liang S, Wong M Y, Schneider A, et al. Transparent silphenylene elastomers from highly branched monomers[J]. Polymer Chemistry, 2021, 12(2): 209-215. |
| 24 | Chen X J, Cui Y D, Yin G Q, et al. Thermooxidative degradation behavior of poly(silphenylene-siloxane)s[J]. Journal of Applied Polymer Science, 2010, 117(2): 926-933. |
| 25 | Chen X J, Cui Y D, Yin G Q, et al. Synthesis of vinyl substitute poly(silphenylene-siloxane) via silyl hydride-dialkoxysilane process[J]. Journal of Applied Polymer Science, 2007, 106(2): 1007-1013. |
| 26 | Li Y N, Kawakami Y. Catalytic cross-dehydrocoupling polymerization of 1, 4-bis(dimethylsilyl)benzene with water. A new approach to poly[(oxydimethylsilylene)(1,4-phenylene)(dimethylsilylene)][J]. Macromolecules, 1999, 32(10): 3540-3542. |
| 27 | Li Y N, Kawakami Y. Synthesis and properties of polymers containing silphenylene moiety via catalytic cross-dehydrocoupling polymerization of 1, 4-bis(dimethylsilyl)benzene[J]. Macromolecules, 1999, 32(26): 8768-8773. |
| 28 | Zhang R Z, Mark J E, Pinhas A R. Dehydrocoupling polymerization of bis-silanes and disilanols to poly(silphenylenesiloxane) as catalyzed by rhodium complexes[J]. Macromolecules, 2000, 33(10): 3508-3510. |
| 29 | Kawakita T, Oh H S, Moon J Y, et al. Synthesis, characterization and thermal properties of phenylene-disiloxane polymers obtained from catalytic cross-dehydrocoupling polymerization of bis(dimethylsilyl)benzene isomers and water[J]. Polymer International, 2001, 50(12): 1346-1351. |
| 30 | Noll W. Chemistry and Technology of Silicones[M]. New York: Academic Press, 1968. |
| 31 | Merker R L, Scott M J, Haberland G G. Random and block copolymers of poly(tetramethyl-p-silphenylene-siloxane) and polydimethylsiloxane[J]. Journal of Polymer Science Part A: General Papers, 1964, 2(1): 31-44. |
| 32 | 来国桥, 幸松民. 有机硅产品合成工艺及应用[M]. 2版. 北京: 化学工业出版社, 2010: 607. |
| Lai G Q, Xing S M. Synthesis Technology and Application of Silicone Products[M]. 2nd ed. Beijing: Chemical Industry Press, 2010: 607. | |
| 33 | 张欢欢, 许东华, 管东波, 等. 双组分加成型硅橡胶交联固化过程的流变学研究[J]. 高等学校化学学报, 2015, 36(4): 788-793. |
| Zhang H H, Xu D H, Guan D B, et al. Rheological properties of two-component silicon rubber during cross-linking by addition reaction[J]. Chemical Journal of Chinese Universities, 2015, 36(4): 788-793. | |
| 34 | 周传健, 尤加健, 张晨, 等. 甲基苯基硅橡胶的制备及应用研究[J]. 有机硅材料, 2017, 31(S1): 17-22. |
| Zhou C J, You J J, Zhang C, et al. Study on preparation and application of methylphenyl silicone rubber[J]. Silicone Material, 2017, 31(S1): 17-22. | |
| 35 | 黄艳华, 薛磊, 苏正涛, 等. 航空用氟硅橡胶和氟醚橡胶的性能对比研究[J]. 有机硅材料, 2021, 35(3): 1-4, 16. |
| Huang Y H, Xue L, Su Z T, et al. Study on performance comparison of fluorosilicone rubber and fluoroether rubber for aviation[J]. Silicone Material, 2021, 35(3): 1-4, 16. | |
| 1 | 刘嘉, 苏正涛, 栗付平. 航空橡胶与密封材料[M]. 北京: 国防工业出版社, 2011: 400. |
| Liu J, Su Z T, Li F P. Aeronautical Rubber and Sealing Materials[M]. Beijing: National Defense Industry Press, 2011: 400. | |
| 2 | 陈天运, 赵文斌, 吴松华. 脱酸型氟硅密封剂耐温耐油性能的研究[J]. 粘接, 2019, 40(6): 5-8. |
| Chen T Y, Zhao W B, Wu S H. Study on the heat and oil resistance of deacidified fluorosilicone sealant[J]. Adhesion, 2019, 40(6): 5-8. | |
| 3 | 吴松华, 高元峰, 秦蓬波, 等. 脱氢型氟硅密封剂耐温耐油性能的研究[J]. 中国胶粘剂, 2019, 28(12): 20-23. |
| Wu S H, Gao Y F, Qin P B, et al. Study on temperature and oil resistance of dehydrogenated fluorosilicone sealant[J]. China Adhesives, 2019, 28(12): 20-23. | |
| 4 | 唐斌, 吴松华, 刘刚. 氟硅生胶中甲基三氟丙基含量对密封剂性能的影响[J]. 粘接, 2013, 34(6): 34-37. |
| Tang B, Wu S H, Liu G. Effect of (γ-trifluropropyl methyl) siloxy-unit content of fluorosilicone rubber on performance of RTV foam sealant[J]. Adhesion, 2013, 34(6): 34-37. | |
| 5 | 刘刚, 索军营, 吴松华, 等. 室温硫化氟硅密封剂耐喷气燃料性能的研究[J]. 有机硅材料, 2011, 25(2): 76-79. |
| Liu G, Suo J Y, Wu S H, et al. Study on jet fuels resistance of RTV flurosilicone sealants[J]. Silicone Material, 2011, 25(2): 76-79. | |
| 6 | Fei H F, Xie W C, Wang Q, et al. Controlled synthesis and characterization of poly[methyl(3, 3, 3-trifluoropropyl)siloxane]with selective end groups[J]. RSC Advances, 2014, 4(99): 56279-56287. |
| 7 | Zhang G D, Hu Y Q, Wu J R, et al. Improved synthesis and properties of hydroxyl-terminated liquid fluorosilicone[J]. Journal of Applied Polymer Science, 2016, 133(12): 43220. |
| 8 | Evans E R. Curable fluorosilicone rubber composition: US4742101[P]. 1988-05-03. |
| 9 | Singh N, Leman J T, Whitney J M. Polyfunctional fluorosilicone composition, method for making, and use: US6479610[P]. 2002-11-12. |
| 10 | 刘润竹, 张猛, 张天福, 等. 端烷氧基聚甲基三氟丙基硅氧烷的合成表征和室温交联特性[J]. 高分子材料科学与工程, 2022, 38(4): 1-9, 19. |
| Liu R Z, Zhang M, Zhang T F, et al. Synthesis, characterization and room temperature crosslinking characteristics of alkoxy terminated poly(methyltrifluoropropyl)siloxanes[J]. Polymer Materials Science & Engineering, 2022, 38(4): 1-9, 19. | |
| 11 | 朱良波, 孙挚, 唐小斗. 室温硫化甲基苯基硅橡胶的研制[J]. 有机硅材料, 2017, 31(S1): 69-71. |
| Zhu L B, Sun Z, Tang X D. Preparation of methyl phenyl RTV silicone rubber[J]. Silicone Material, 2017, 31(S1): 69-71. | |
| 12 | 沙艳松, 张长生, 罗世凯. 硅橡胶耐热性能的研究进展[J]. 有机硅材料, 2012, 26(2): 122-126. |
| Sha Y S, Zhang C S, Luo S K. Research progress of thermal resistant silicone rubber[J]. Silicone Material, 2012, 26(2): 122-126. | |
| 13 | 孙全吉, 黄艳华, 吴娜, 等. 苯基硅橡胶性能的研究进展[J]. 有机硅材料, 2018, 32(1): 71-76. |
| Sun Q J, Huang Y H, Wu N, et al. Research progress on performance of phenyl silicone rubber[J]. Silicone Material, 2018, 32(1): 71-76. | |
| 14 | Zlatanic A, Radojcic D, Wan X M, et al. Monitoring of the course of the silanolate-initiated polymerization of cyclic siloxanes. A mechanism for the copolymerization of dimethyl and diphenyl monomers[J]. Macromolecules, 2018, 51(3): 895-905. |
| 15 | Zlatanic A, Radojcic D, Wan X M, et al. Suppression of crystallization in polydimethylsiloxanes and chain branching in their phenyl-containing copolymers[J]. Macromolecules, 2017, 50(9): 3532-3543. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [3] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [4] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [5] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [6] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [7] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [8] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [9] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [10] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [11] | 陈雅鑫, 袁航, 刘冠章, 毛磊, 杨纯, 张瑞芳, 张光亚. 蛋白质纳米笼介导的酶自固定化研究进展[J]. 化工学报, 2023, 74(7): 2773-2782. |
| [12] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [13] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [14] | 李彬, 徐正虎, 姜爽, 张天永. 双氧水催化氧化法清洁高效合成促进剂CBS[J]. 化工学报, 2023, 74(7): 2919-2925. |
| [15] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号