化工学报 ›› 2024, Vol. 75 ›› Issue (4): 1222-1240.DOI: 10.11949/0438-1157.20231268
李云璇1( ), 刘新悦1(
), 刘新悦1( ), 陈熙2, 刘文2, 周明月1(
), 陈熙2, 刘文2, 周明月1( ), 蓝兴英1
), 蓝兴英1
收稿日期:2023-12-04
修回日期:2024-03-04
出版日期:2024-04-25
发布日期:2024-06-06
通讯作者:
周明月
作者简介:李云璇(2001—),女,硕士研究生,2654336380@qq.com基金资助:
Yunxuan LI1( ), Xinyue LIU1(
), Xinyue LIU1( ), Xi CHEN2, Wen LIU2, Mingyue ZHOU1(
), Xi CHEN2, Wen LIU2, Mingyue ZHOU1( ), Xingying LAN1
), Xingying LAN1
Received:2023-12-04
Revised:2024-03-04
Online:2024-04-25
Published:2024-06-06
Contact:
Mingyue ZHOU
摘要:
随着双碳政策的推进和可再生能源的快速发展,能源存储技术成为解决能源转型和可持续发展的关键支撑。氧化还原液流电池(RFB)是一种用于大规模储能的电化学器件,具有设计灵活、存储容量大、循环寿命长、安全性高等特点。然而受氧化还原介质分子溶解度的限制,其能量密度相对较低,同时可实用化的体系较少。为解决这一问题,基于固液氧化还原靶向反应的能量存储技术应运而生。氧化还原靶向液流电池(RTFB)在提高能量密度的同时保持良好流动性,克服了传统RFB的限制。综述了近年来该技术在材料、器件及动力学方面的研究进展,包括各种材料的特点、器件的设计及性能以及动力学过程的表征与建模。最后总结当前研究的不足之处并展望未来发展趋势。
中图分类号:
李云璇, 刘新悦, 陈熙, 刘文, 周明月, 蓝兴英. 基于固液氧化还原靶向反应的能量存储技术:材料、器件及动力学[J]. 化工学报, 2024, 75(4): 1222-1240.
Yunxuan LI, Xinyue LIU, Xi CHEN, Wen LIU, Mingyue ZHOU, Xingying LAN. Energy storage technologies based on solid-liquid redox-targeting reactions: materials, devices, and kinetics[J]. CIESC Journal, 2024, 75(4): 1222-1240.
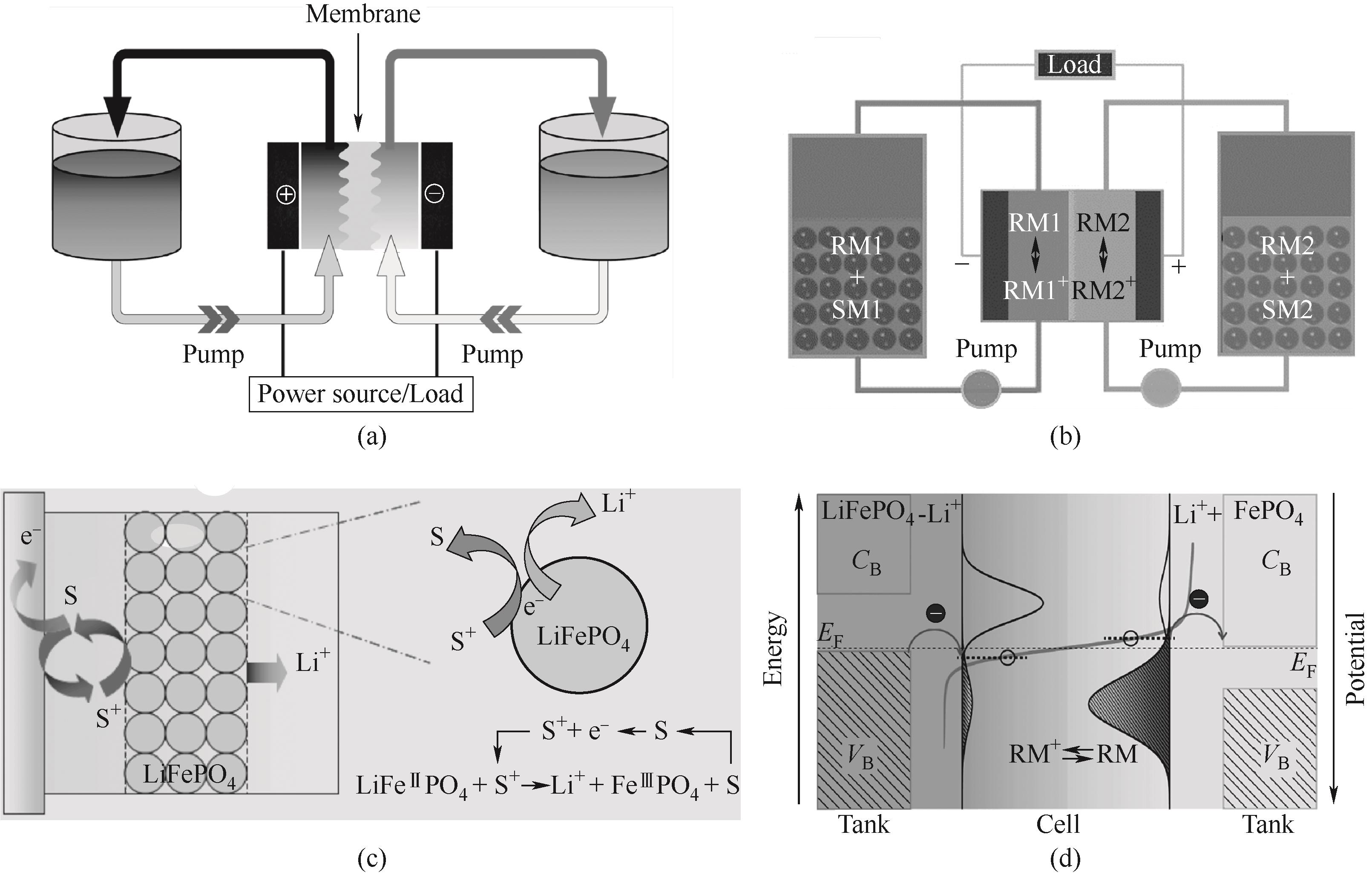
图1 (a)RFB示意图[7];(b)RTFB示意图;(c)氧化还原介质分子S与LiFePO4等绝缘电极材料发生氧化还原靶向反应的示意图[8];(d)氧化还原介质RM+与LiFePO4的SMRT反应的能量和电荷转移过程示意图[9]
Fig.1 (a) Schematic diagram of RFB[7];(b) Schematic diagram of RTFB; (c) The redox-targeting reaction principle of freely diffusing shuttle molecules S and insulating electrode materials such as LiFePO4[8]; (d) Energy diagram and charge transfer of SMRT reaction between RM+ and LiFePO4[9]
| 场景 | 电对的作用 | 电对的溶解度要求 | 电对与电池性能关联 |
|---|---|---|---|
| 常规RFB | 储能介质 | 大于1 mol/L | 决定能量密度、功率性能 |
| 氧化还原靶向RFB | 传递能量+储能 | 大于0.05 mol/L | 决定功率性能 |
表1 氧化还原电对在常规和氧化还原靶向RFB中的特点
Table 1 Characteristics of redox pairs in conventional RFB and RTFB
| 场景 | 电对的作用 | 电对的溶解度要求 | 电对与电池性能关联 |
|---|---|---|---|
| 常规RFB | 储能介质 | 大于1 mol/L | 决定能量密度、功率性能 |
| 氧化还原靶向RFB | 传递能量+储能 | 大于0.05 mol/L | 决定功率性能 |
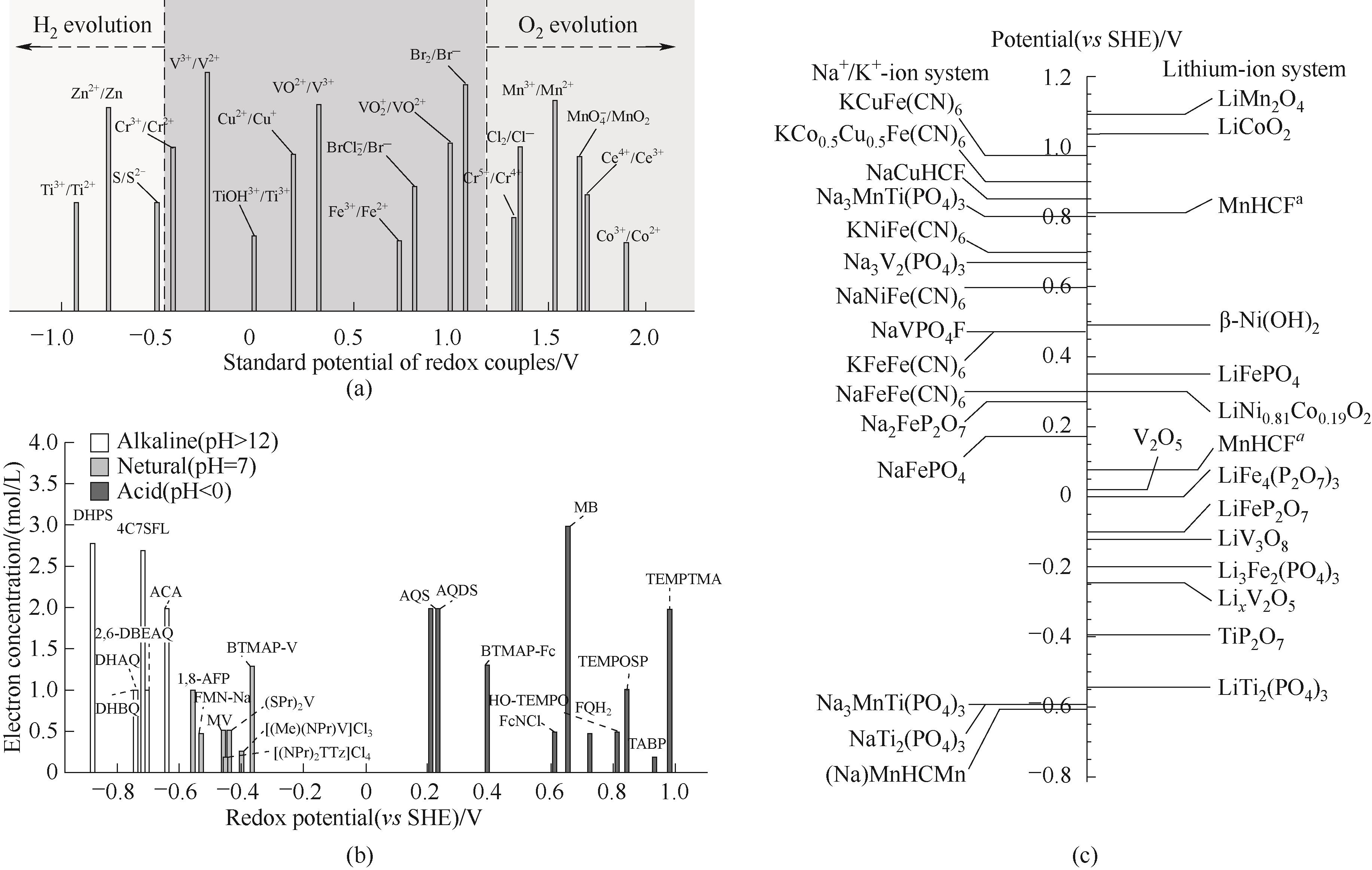
图2 (a)部分金属离子的标准电极电势[16];(b)部分有机小分子的电位及等效电子浓度对比[33];(c)部分插层材料的标准电极电势
Fig.2 (a) Redox potential of metal ions[16]; (b) Comparison of redox potential and equivalent electron concentration of organic small molecules[33]; (c) Redox potential of intercalation materials
| 指标 | 全钒 电池[ | 铁铬 电池[ | 锌铁 电池[ | 锌溴 电池[ |
|---|---|---|---|---|
| 能量密度①/(W·h/L) | 约39.5 | 约31.2 | 约25.4 | 65 |
| 能量效率/% | 83 | 70~75 | 83 | 80 |
表2 传统液流电池电化学性能
Table 2 Electrochemical performance of traditional flow batteries
| 指标 | 全钒 电池[ | 铁铬 电池[ | 锌铁 电池[ | 锌溴 电池[ |
|---|---|---|---|---|
| 能量密度①/(W·h/L) | 约39.5 | 约31.2 | 约25.4 | 65 |
| 能量效率/% | 83 | 70~75 | 83 | 80 |
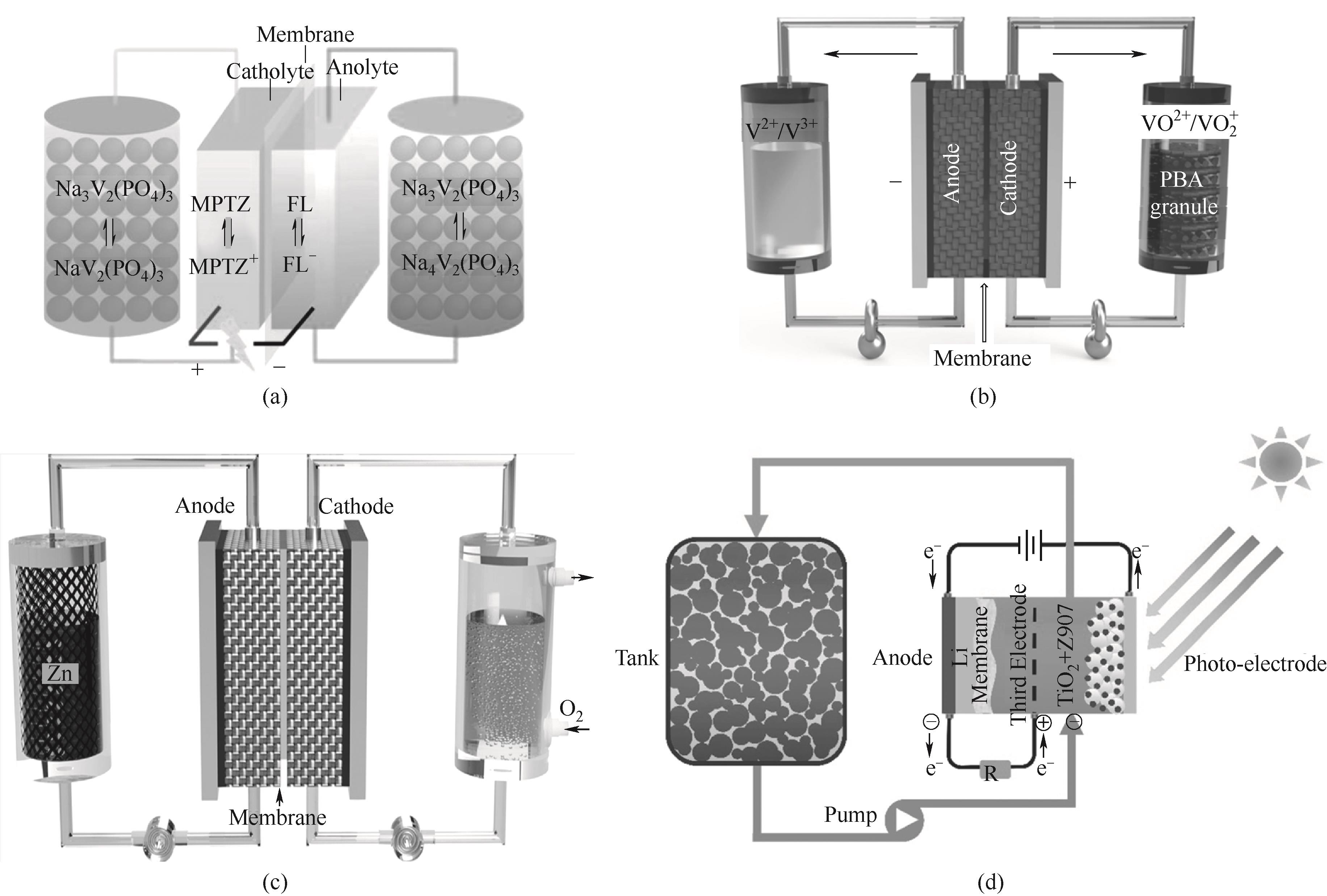
图3 (a)基于氧化还原靶向反应的氧化还原钠离子液流电池示意图[45];(b)氧化还原靶向钒液流电池(RT-VRB)示意图[58];(c)氧化还原介导的锌-空气燃料电池(RM-ZAFC)的结构和操作示意图[69];(d)基于氧化还原靶向反应的太阳能可充电电池示意图[70]
Fig.3 (a) Schematic diagram of a redox flow sodium-ion battery based on redox-targeting reaction[45]; (b) Schematic diagram of redox-targeting vanadium flow battery (RT-VRB)[58]; (c) Schematic diagram of the structure and operation of a redox mediated zinc air fuel cell (RM-ZAFC)[69]; (d) Schematic diagram of solar rechargeable batteries based on redox-targeting reaction[70]
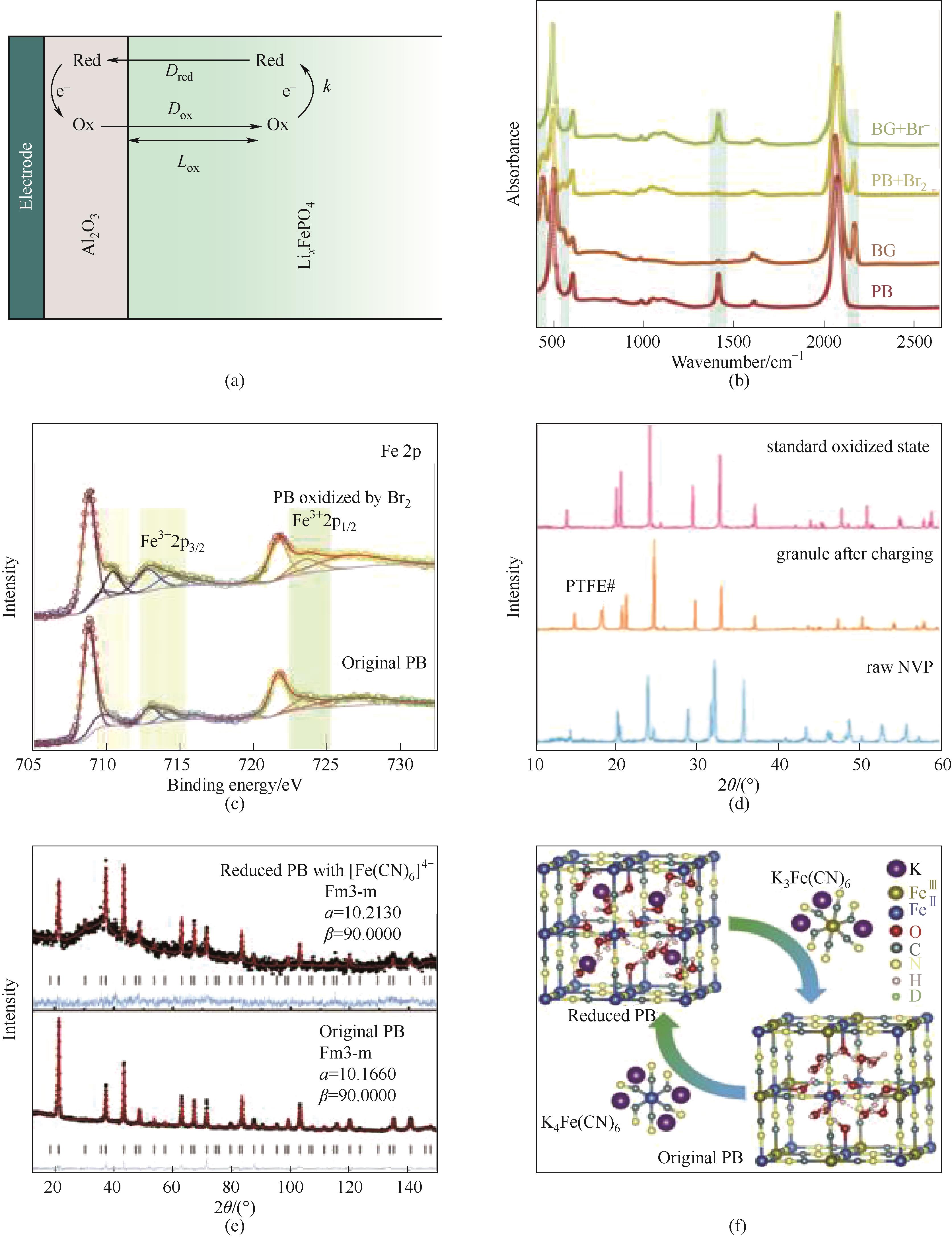
图4 (a)基于双层电极电化学方法的反应动力学测定方法[82];(b)标准PB和BG、Br2氧化PB和Br-还原BG的FTIR光谱[66];(c)PB和Br2氧化PB的Fe 2p XPS光谱[66];(d)充电前后的正极罐中NVP的XRD谱图[45];(e)[Fe(CN)6]4-原始和部分还原PB的ND模式[66];(f)从精修ND结果中获得的原始(底部)和还原的PB(或PW,顶部)的最可能结构[66]
Fig.4 (a) An electrochemical approach based on a double-layer electrode to determine the reaction kinetics[82]; (b) FTIR spectra of standard PB and BG, Br2-oxidized PB, and Br--reduced BG[66]; (c) Fe 2p XPS spectra of PB and Br2-oxidized PB[66]; (d) XRD patterns of NVP collected from the catholyte tank before and after charging[45]; (e) ND patterns of pristine and partially reduced PB by [Fe(CN)6]4-[66]; (f) The most probable structures of pristine (bottom) and reduced PB (or PW, top) unit cells obtained from the refinement of ND patterns[66]
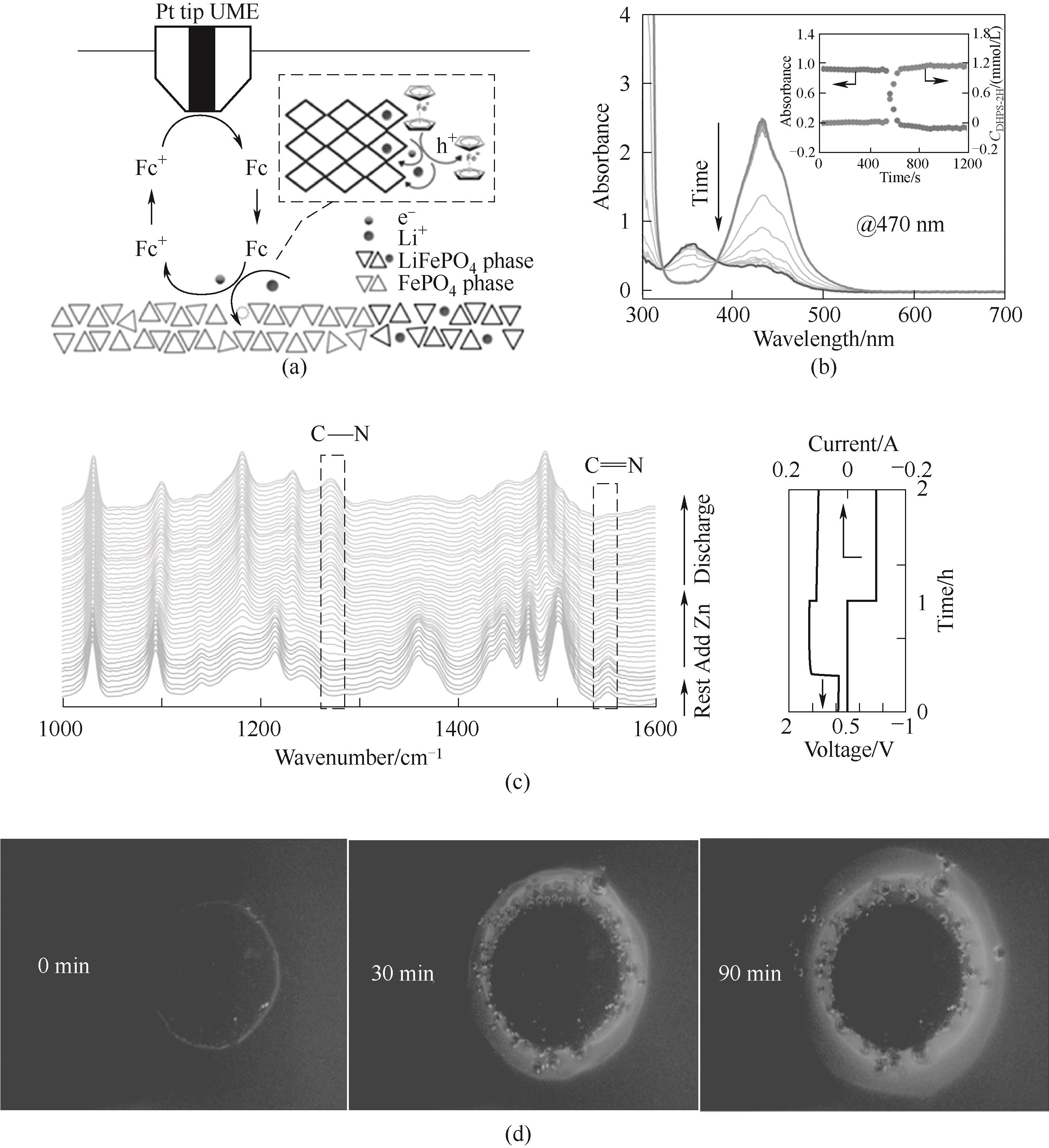
图5 (a)SECM法测定氧化还原靶向反应的界面电荷转移动力学[83];(b)添加过量锌前后DHPS电解液的原位紫外-可见光谱[84];(c)DHPS电解液在静置、添加过量锌和放电过程中的ATR-FTIR光谱[84];(d)平面石英反应器中还原态FL与NVP颗粒反应后的荧光显微镜图像[45]
Fig.5 (a) Interfacial charge-transfer kinetics of redox-targeting reactions measured by SECM[83]; (b) In situ UV-Vis spectra of DHPS electrolyte before and after addition of excess zinc[84]; (c) Operando ATR-FTIR spectra of DHPS electrolyte recorded during resting, after adding excess zinc and during discharge process[84]; (d) Fluorescence microscopic images of a planar quartz reactor filled with 20 mmol/L fully reduced FL upon reacting with a compact NVP granule assembly[45]
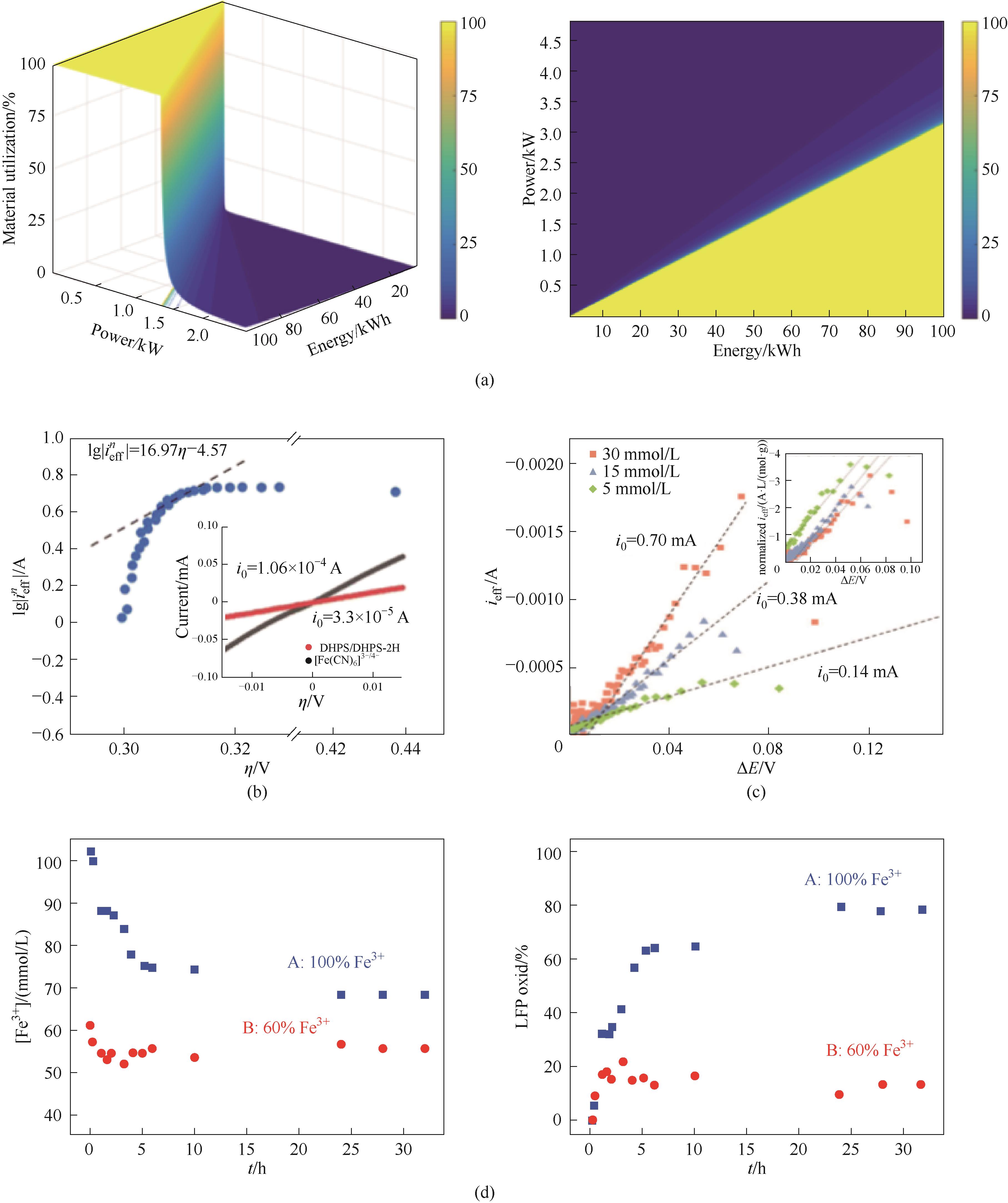
图6 (a)在SMRT系统中LiFePO4固体材料利用率与标称功率和能量的相关性[9];(b)lgieffn-η的关系图(含极化曲线)[84];(c)NVP/MPTZ体系中SMRT反应的动力学数据[45];(d)LFP30被[Fe(CN)6]3-氧化的动力学[85]
Fig.6 (a) Utilization of LiFePO4 as a function of power in an SMRT system and correlation of power and energy at different utilizations of solid material[9]; (b) lgieffn- η relationship (including polarization curve)[84]; (c) Kinetic data of SMRT reaction in NVP/MPTZ system[45];(d) Oxidation kinetics of LFP30 oxidized by [Fe(CN)6]3- [85]
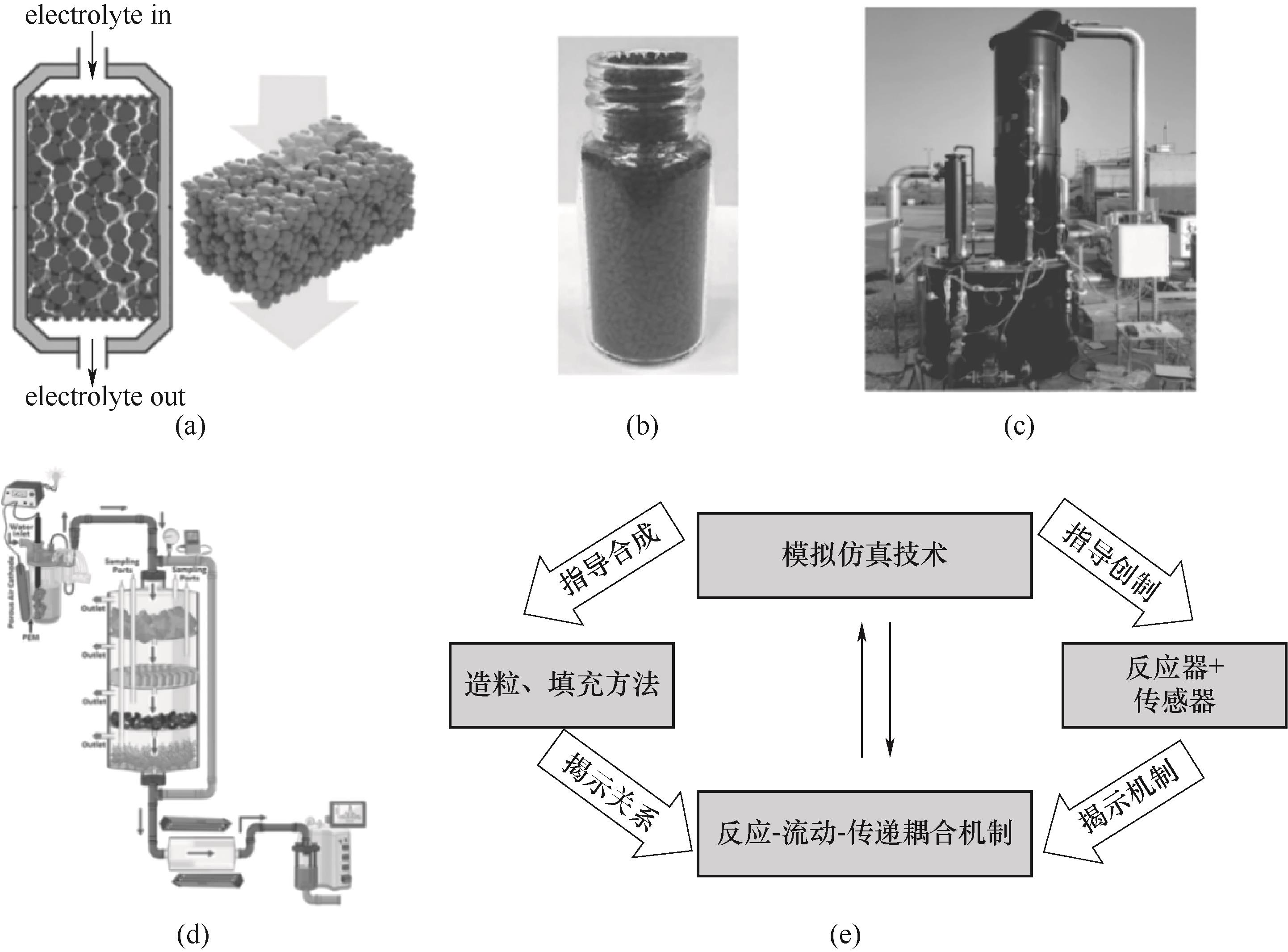
图7 (a)活性材料储能罐[10];(b)一个装满LiFePO4颗粒瓶子的照片[9];(c)去除某种物质的吸附柱[88];(d)一种污水处理装置[89];(e)氧化还原靶向液流电池储能技术研究的展望
Fig.7 (a) An energy-storage tank filled with active materials[10]; (b) Photograph of a bottle filled with LiFePO4 processed into granules[9]; (c) Adsorption column to remove a substance[88]; (d) The utility model relates to a sewage treatment device[89]; (e) Outlook for future study of redox-targeting flow battery energy storage technology
| 62 | Li G C, Yang L Q, Jiang X, et al. A multi-electron redox mediator for redox-targeting lithium-sulfur flow batteries[J]. Journal of Power Sources, 2018, 378: 418-422. |
| 63 | Self E C, Delnick F M, Ruther R E, et al. High-capacity organic radical mediated phosphorus anode for sodium-based redox flow batteries[J]. ACS Energy Letters, 2019, 4(11): 2593-2600. |
| 64 | Yu J Z, Fan L, Yan R T, et al. Redox targeting-based aqueous redox flow lithium battery[J]. ACS Energy Letters, 2018, 3(10): 2314-2320. |
| 65 | 吴晓宏, 贾鑫, 秦伟, 等. 一种基于氧化还原靶向反应的中性水系液流锂电池: 113258114B[P]. 2022-04-08. |
| Wu X H, Jia X, Qin W, et al. Stable and high-capacity neutral aqueous liquid flow lithium battery based on redox targeting reaction: 113258114B[P]. 2022-04-08. | |
| 66 | Chen Y, Zhou M Y, Xia Y H, et al. A stable and high-capacity redox targeting-based electrolyte for aqueous flow batteries[J]. Joule, 2019, 3(9): 2255-2267. |
| 67 | Zhang J L, Li L Y, Nie Z M, et al. Effects of additives on the stability of electrolytes for all-vanadium redox flow batteries[J]. Journal of Applied Electrochemistry, 2011, 41(10): 1215-1221. |
| 68 | Páez T, Zhang F F, Muñoz M Á, et al. The redox-mediated nickel-metal hydride flow battery[J]. Advanced Energy Materials, 2022, 12(1): 2102866. |
| 69 | Zhang H, Huang S Q, Salla M, et al. A redox-mediated zinc-air fuel cell[J]. ACS Energy Letters, 2022, 7(8): 2565-2575. |
| 70 | Fan L, Jia C K, Zhu Y G, et al. Redox targeting of Prussian blue: toward low-cost and high energy density redox flow battery and solar rechargeable battery[J]. ACS Energy Letters, 2017, 2(3): 615-621. |
| 71 | Rahman M A, Wang X J, Wen C E. High energy density metal-air batteries: a review[J]. Journal of the Electrochemical Society, 2013, 160(10): A1759-A1771. |
| 72 | Zhu Y G, Jia C K, Yang J, et al. Dual redox catalysts for oxygen reduction and evolution reactions: towards a redox flow Li-O2 battery[J]. Chemical Communications, 2015, 51(46): 9451-9454. |
| 73 | Zhang F F, Gao M Q, Huang S Q, et al. Redox targeting of energy materials for energy storage and conversion[J]. Advanced Materials, 2022, 34(25): 2104562. |
| 74 | Amunátegui B, Ibáñez A, Sierra M, et al. Electrochemical energy storage for renewable energy integration: zinc-air flow batteries[J]. Journal of Applied Electrochemistry, 2018, 48(6): 627-637. |
| 75 | Gao M Q, Song Y X, Zou X, et al. A redox-mediated iron-air fuel cell for sustainable and scalable power generation[J]. Advanced Energy Materials, 2023, 13(38): 2301868. |
| 76 | Akbarzadeh A, Wadowski T. Heat pipe-based cooling systems for photovoltaic cells under concentrated solar radiation[J]. Applied Thermal Engineering, 1996, 16(1): 81-87. |
| 77 | Zhang H, Zhang F F, Yu J Z, et al. Redox targeting-based thermally regenerative electrochemical cycle flow cell for enhanced low-grade heat harnessing[J]. Advanced Materials, 2021, 33(5): e2006234. |
| 78 | Zhang H, Lek D G, Huang S Q, et al. Efficient low-grade heat conversion and storage with an activity-regulated redox flow cell via a thermally regenerative electrochemical cycle[J]. Advanced Materials, 2022, 34(34): e2202266. |
| 79 | Yan S, Huang S P, Xu H, et al. Redox targeting-based neutral aqueous flow battery with high energy density and low cost[J]. ChemSusChem, 2023, 16(19): e202300710. |
| 80 | Vorotyntsev M A, Zader P A. Simulation of mediator-catalysis process inside redox flow battery[J]. Russian Journal of Electrochemistry, 2022, 58(11): 1041-1056. |
| 81 | Lee G, Wong C M, Sevov C S. Single vs dual shuttle cycling of polyferrocenyl cathodes for redox targeting flow batteries[J]. ACS Energy Letters, 2022, 7(10): 3337-3344. |
| 82 | Jennings J R, Huang Q Z, Wang Q. Kinetics of Li x FePO4 lithiation/delithiation by ferrocene-based redox mediators: an electrochemical approach[J]. The Journal of Physical Chemistry C, 2015, 119(31): 17522-17528. |
| 83 | Yan R T, Ghilane J, Phuah K C, et al. Determining Li+-coupled redox targeting reaction kinetics of battery materials with scanning electrochemical microscopy[J]. The Journal of Physical Chemistry Letters, 2018, 9(3): 491-496. |
| 84 | Huang S Q, Yuan Z Z, Salla M, et al. A redox-mediated zinc electrode for ultra-robust deep-cycle redox flow batteries[J]. Energy & Environmental Science, 2023, 16(2): 438-445. |
| 1 | Yang Z G, Zhang J L, Kintner-Meyer M C, et al. Electrochemical energy storage for green grid[J]. Chemical Reviews, 2011, 111(5): 3577-3613. |
| 2 | Krishna R, Dhass A D, Arya A, et al. An assessment of the strategies for the energy-critical elements necessary for the development of sustainable energy sources[J]. Environmental Science and Pollution Research, 2023, 30(39): 90276-90297. |
| 3 | Chen H, Zhang X Y, Zhang S R, et al. A comparative study of iron-vanadium and all-vanadium flow battery for large scale energy storage[J]. Chemical Engineering Journal, 2022, 429: 132403. |
| 4 | Soloveichik G L. Flow batteries: current status and trends[J]. Chemical Reviews, 2015, 115(20): 11533-11558. |
| 5 | Thaller L H. Electrically rechargeable REDOX flow cell: US3996064[P]. 1976-12-07. |
| 6 | Huang Q Z, Wang Q. Next-generation, high-energy-density redox flow batteries[J]. ChemPlusChem, 2015, 80(2): 312-322. |
| 7 | Pan F, Wang Q. Redox species of redox flow batteries: a review[J]. Molecules, 2015, 20(11): 20499-20517. |
| 8 | Wang Q, Zakeeruddin S M, Wang D Y, et al. Redox targeting of insulating electrode materials: a new approach to high-energy-density batteries[J]. Angewandte Chemie (International Ed. in English), 2006, 45(48): 8197-8200. |
| 9 | Zhou M Y, Huang Q Z, Pham T T N, et al. Nernstian-potential-driven redox-targeting reactions of battery materials[J]. Chem, 2017, 3(6): 1036-1049. |
| 10 | Yan R T, Wang Q. Redox-targeting-based flow batteries for large-scale energy storage[J]. Advanced Materials, 2018, 30(47): e1802406. |
| 11 | Huang Q Z, Li H, Grätzel M, et al. Reversible chemical delithiation/lithiation of LiFePO4: towards a redox flow lithium-ion battery[J]. Physical Chemistry Chemical Physics, 2013, 15(6): 1793-1797. |
| 12 | Wang Q, Evans N, Zakeeruddin S M, et al. Molecular wiring of insulators: charging and discharging electrode materials for high-energy lithium-ion batteries by molecular charge transport layers[J]. Journal of the American Chemical Society, 2007, 129(11): 3163-3167. |
| 13 | Li Z N, Jiang T L, Ali M, et al. Recent progress in organic species for redox flow batteries[J]. Energy Storage Materials, 2022, 50: 105-138. |
| 14 | Dunn B, Kamath H, Tarascon J M. Electrical energy storage for the grid: a battery of choices[J]. Science, 2011, 334(6058): 928-935. |
| 15 | Wei X L, Xu W, Huang J H, et al. Radical compatibility with nonaqueous electrolytes and its impact on an all-organic redox flow battery[J]. Angewandte Chemie (International Ed. in English), 2015, 54(30): 8684-8687. |
| 16 | Wang W, Luo Q T, Li B, et al. Recent progress in redox flow battery research and development[J]. Advanced Functional Materials, 2013, 23(8): 970-986. |
| 17 | Zhang H M, Lu W J, Li X F. Progress and perspectives of flow battery technologies[J]. Electrochemical Energy Reviews, 2019, 2(3): 492-506. |
| 18 | Gong K, Xu F, Grunewald J B, et al. All-soluble all-iron aqueous redox-flow battery[J]. ACS Energy Letters, 2016, 1(1): 89-93. |
| 19 | Bae C, Roberts E P L, Chakrabarti M H, et al. All-chromium redox flow battery for renewable energy storage[J]. International Journal of Green Energy, 2011, 8(2): 248-264. |
| 20 | Sanz L, Lloyd D, Magdalena E, et al. Description and performance of a novel aqueous all-copper redox flow battery[J]. Journal of Power Sources, 2014, 268: 121-128. |
| 21 | Rahbani N, de Silva P, Baudrin E. Density functional theory-based protocol to calculate the redox potentials of first-row transition metal complexes for aqueous redox targeting flow batteries[J]. ChemSusChem, 2023, 16(18): e202300482. |
| 22 | Modiba P, Matoetoe M, Crouch A M. Kinetics study of transition metal complexes (Ce-DTPA, Cr-DTPA and V-DTPA) for redox flow battery applications[J]. Electrochimica Acta, 2013, 94: 336-343. |
| 23 | Cappillino P J, Pratt H D III, Hudak N S, et al. Application of redox non-innocent ligands to non-aqueous flow battery electrolytes[J]. Advanced Energy Materials, 2014, 4(1): 1300566. |
| 24 | Holland-Cunz M V, Cording F, Friedl J, et al. Redox flow batteries— concepts and chemistries for cost-effective energy storage[J]. Frontiers in Energy, 2018, 12(2): 198-224. |
| 85 | Vivo-Vilches J F, Nadeina A, Rahbani N, et al. LiFePO4-ferri/ferrocyanide redox targeting aqueous posolyte: set-up, efficiency and kinetics[J]. Journal of Power Sources, 2021, 488: 229387. |
| 86 | Zhang L Y, Feng R Z, Wang W, et al. Emerging chemistries and molecular designs for flow batteries[J]. Nature Reviews Chemistry, 2022, 6: 524-543. |
| 87 | Xia L, Long T, Li W Y, et al. Highly stable vanadium redox-flow battery assisted by redox-mediated catalysis[J]. Small, 2020, 16(38): e2003321. |
| 88 | Pallavicini J, Fedeli M, Scolieri G D, et al. Digital twin-based optimization and demo-scale validation of absorption columns using sodium hydroxide/water mixtures for the purification of biogas streams subject to impurity fluctuations[J]. Renewable Energy, 2023, 219: 119466. |
| 89 | Naha A, Antony S, Nath S, et al. A hypothetical model of multi-layered cost-effective wastewater treatment plant integrating microbial fuel cell and nanofiltration technology: a comprehensive review on wastewater treatment and sustainable remediation[J]. Environmental Pollution, 2023, 323: 121274. |
| 25 | Giri S, Dash I. Ferrocene to functionalized ferrocene: a versatile redox-active electrolyte for high-performance aqueous and non-aqueous organic redox flow batteries[J]. Journal of Materials Chemistry A, 2023, 11(31): 16458-16493. |
| 26 | Ding Y, Zhang C K, Zhang L Y, et al. Molecular engineering of organic electroactive materials for redox flow batteries[J]. Chemical Society Reviews, 2018, 47(1): 69-103. |
| 27 | Wei X L, Cosimbescu L, Xu W, et al. Towards high-performance nonaqueous redox flow electrolyte via ionic modification of active species[J]. Advanced Energy Materials, 2015, 5(1): 1400678. |
| 28 | Cong G T, Zhou Y C, Li Z J, et al. A highly concentrated catholyte enabled by a low-melting-point ferrocene derivative[J]. ACS Energy Letters, 2017, 2(4): 869-875. |
| 29 | Pan F, Yang J, Huang Q, et al. Redox targeting of anatase TiO2 for redox flow lithium-ion batteries[J]. Advanced Energy Materials, 2014, 4(15): 1400567. |
| 30 | Skyllas-Kazacos M, Kazacos G, Poon G, et al. Recent advances with UNSW vanadium-based redox flow batteries[J]. International Journal of Energy Research, 2010, 34(2): 182-189. |
| 31 | Shin K, Lee J H, Heo J, et al. Current status and challenges for practical flowless Zn-Br batteries[J]. Current Opinion in Electrochemistry, 2022, 32: 100898. |
| 32 | Huskinson B, Marshak M P, Suh C, et al. A metal-free organic-inorganic aqueous flow battery[J]. Nature, 2014, 505: 195-198. |
| 33 | Zhang C K, Li X F. Perspective on organic flow batteries for large-scale energy storage[J]. Current Opinion in Electrochemistry, 2021, 30: 100836. |
| 34 | Wang W, Xu W, Cosimbescu L, et al. Anthraquinone with tailored structure for a nonaqueous metal-organic redox flow battery[J]. Chemical Communications, 2012, 48(53): 6669-6671. |
| 35 | DeBruler C, Hu B, Moss J, et al. A sulfonate-functionalized viologen enabling neutral cation exchange, aqueous organic redox flow batteries toward renewable energy storage[J]. ACS Energy Letters, 2018, 3(3): 663-668. |
| 36 | Zhao Z M, Liu X H, Zhang M Q, et al. Development of flow battery technologies using the principles of sustainable chemistry[J]. Chemical Society Reviews, 2023, 52(17): 6031-6074. |
| 37 | Zhang J, Lai L, Wang H, et al. Energy storage mechanisms of anode materials for potassium ion batteries[J]. Materials Today Energy, 2021, 21: 100747. |
| 38 | Zanzola E, Gentil S, Gschwend G, et al. Solid electrochemical energy storage for aqueous redox flow batteries: the case of copper hexacyanoferrate[J]. Electrochimica Acta, 2019, 321: 134704. |
| 39 | Meng Y T, Nie C H, Guo W J, et al. Inorganic cathode materials for potassium ion batteries[J]. Materials Today Energy, 2022, 25: 100982. |
| 40 | Wang X, Zhou M Y, Zhang F F, et al. Redox targeting of energy materials[J]. Current Opinion in Electrochemistry, 2021, 29: 100743. |
| 41 | Braconnier J J, Delmas C, Fouassier C, et al. Comportement electrochimique des phases NaxCoO2 [J]. Materials Research Bulletin, 1980, 15(12): 1797-1804. |
| 42 | Padhi A K, Nanjundaswamy K S, Goodenough J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries[J]. Journal of the Electrochemical Society, 1997, 144(4): 1188. |
| 43 | Huang Q Z, Yang J, Ng C B, et al. A redox flow lithium battery based on the redox targeting reactions between LiFePO4 and iodide[J]. Energy & Environmental Science, 2016, 9(3): 917-921. |
| 44 | Zhu Y G, Du Y H, Jia C K, et al. Unleashing the power and energy of LiFePO4-based redox flow lithium battery with a bifunctional redox mediator[J]. Journal of the American Chemical Society, 2017, 139(18): 6286-6289. |
| 45 | Zhou M Y, Chen Y, Zhang Q Q, et al. Na3V2(PO4)3 as the sole solid energy storage material for redox flow sodium-ion battery[J]. Advanced Energy Materials, 2019, 9(30): 1901188. |
| 46 | Shea J J, Luo C. Organic electrode materials for metal ion batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(5): 5361-5380. |
| 47 | Visco S J, Mailhe C C, de Jonghe L C, et al. A novel class of organosulfur electrodes for energy storage[J]. Journal of the Electrochemical Society, 1989, 136(3): 661. |
| 48 | Su Y Z, Dong W, Zhang J H, et al. Poly[bis(2-aminophenyloxy)disulfide]: a polyaniline derivative containing disulfide bonds as a cathode material for lithium battery[J]. Polymer, 2007, 48(1): 165-173. |
| 49 | Schröter E, Stolze C, Meyer J, et al. Organic redox targeting flow battery utilizing a hydrophilic polymer and its in-operando characterization via state-of-charge monitoring of the redox mediator[J]. ChemSusChem, 2023, 16(14): e202300296. |
| 50 | Schröter E, Stolze C, Saal A, et al. All-organic redox targeting with a single redox moiety: combining organic radical batteries and organic redox flow batteries[J]. ACS Applied Materials & Interfaces, 2022, 14(5): 6638-6648. |
| 51 | Lakraychi A E, Deunf E, Fahsi K, et al. An air-stable lithiated cathode material based on a 1,4-benzenedisulfonate backbone for organic Li-ion batteries[J]. Journal of Materials Chemistry A, 2018, 6(39): 19182-19189. |
| 52 | Zhang H C, Huang Q H, Xia X, et al. Aqueous organic redox-targeting flow battery based on Nernstian-potential-driven anodic redox-targeting reactions[J]. Journal of Materials Chemistry A, 2022, 10(12): 6740-6747. |
| 53 | Zanzola E, Dennison C R, Battistel A, et al. Redox solid energy boosters for flow batteries: polyaniline as a case study[J]. Electrochimica Acta, 2017, 235: 664-671. |
| 54 | Li L, Kim S, Wang W, et al. A stable vanadium redox‐flow battery with high energy density for large‐scale energy storage[J]. Advanced Energy Materials, 2011, 1(3): 394-400. |
| 55 | Waters S E, Robb B H, Marshak M P. Effect of chelation on iron-chromium redox flow batteries[J]. ACS Energy Letters, 2020, 5(6): 1758-1762. |
| 56 | Yuan Z Z, Duan Y Q, Liu T, et al. Toward a low-cost alkaline zinc-iron flow battery with a polybenzimidazole custom membrane for stationary energy storage[J]. iScience, 2018, 3: 40-49. |
| 57 | Mahmood A, Zheng Z, Chen Y. Zinc-bromine batteries: challenges, prospective solutions, and future[J]. Advanced Science, 2024, 11(3): e2305561. |
| 58 | Cheng Y H, Wang X, Huang S P, et al. Redox targeting-based vanadium redox-flow battery[J]. ACS Energy Letters, 2019, 4(12): 3028-3035. |
| 59 | Jia C K, Pan F, Zhu Y G, et al. High-energy density nonaqueous all redox flow lithium battery enabled with a polymeric membrane[J]. Science Advances, 2015, 1(10): e1500886. |
| 60 | Pan F, Huang Q Z, Huang H, et al. High-energy density redox flow lithium battery with unprecedented voltage efficiency[J]. Chemistry of Materials, 2016, 28(7): 2052-2057. |
| 61 | Li J F, Yang L Q, Yang S L, et al. The application of redox targeting principles to the design of rechargeable Li-S flow batteries[J]. Advanced Energy Materials, 2015, 5(24): 1501808. |
| [1] | 吴希, 孙博, 刘银东, 齐传磊, 陈凯毅, 王路海, 许崇, 李永峰. 钠离子电池沥青基碳负极材料制备技术研究进展[J]. 化工学报, 2024, 75(4): 1270-1283. |
| [2] | 贾旭东, 杨博龙, 程前, 李雪丽, 向中华. 分步负载金属法制备铁钴双金属位点高效氧还原电催化剂[J]. 化工学报, 2024, 75(4): 1578-1593. |
| [3] | 严孝清, 赵瑛, 张宇哲, 欧鸿辉, 黄起中, 胡华贵, 杨贵东. 五重孪晶铜纳米线@聚吡咯制备及其电催化硝酸盐还原制氨[J]. 化工学报, 2024, 75(4): 1519-1532. |
| [4] | 孙铭泽, 黄鹤来, 牛志强. 铂基氧还原催化剂:从单晶电极到拓展表面纳米材料[J]. 化工学报, 2024, 75(4): 1256-1269. |
| [5] | 李昂, 赵振宇, 李洪, 高鑫. 微波诱导高分散Pd/FeP催化剂构筑及其电催化性能研究[J]. 化工学报, 2024, 75(4): 1594-1606. |
| [6] | 潘娜, 田昌, 怀兰坤, 刘玉玉, 张芬芬, 高晓梅, 刘伟, 闫良国, 赵艳侠. 聚合铝钛基絮凝剂的合成与应用[J]. 化工学报, 2024, 75(3): 1009-1018. |
| [7] | 吴吉昊, 陈涛, 刘思宇, 刘梦柯, 杨卷. 双功能活化制备沥青基硬炭用于钠离子电池负极[J]. 化工学报, 2024, 75(3): 1019-1027. |
| [8] | 尹玉华, 方灿, 易清风, 李广. 不同碳导电剂对铁-空气电池性能的影响[J]. 化工学报, 2024, 75(2): 685-694. |
| [9] | 闻文, 王慧艳, 周静红, 曹约强, 周兴贵. 石墨负极颗粒对锂离子电池容量衰减及SEI膜生长影响的模拟研究[J]. 化工学报, 2024, 75(1): 366-376. |
| [10] | 齐元帅, 彭文朝, 李阳, 张凤宝, 范晓彬. 电化学脱盐机理及相关研究进展[J]. 化工学报, 2024, 75(1): 171-189. |
| [11] | 王婷, 王忠东, 项星宇, 何呈祥, 朱春英, 马友光, 付涛涛. 微反应器内环酯类锂电池添加剂合成研究进展[J]. 化工学报, 2024, 75(1): 95-109. |
| [12] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [13] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [14] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [15] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号