化工学报 ›› 2024, Vol. 75 ›› Issue (4): 1578-1593.DOI: 10.11949/0438-1157.20240073
收稿日期:2024-01-15
修回日期:2024-03-12
出版日期:2024-04-25
发布日期:2024-06-06
通讯作者:
李雪丽,向中华
作者简介:贾旭东(1998—),男,硕士研究生,307952430@qq.com
基金资助:
Xudong JIA( ), Bolong YANG, Qian CHENG, Xueli LI(
), Bolong YANG, Qian CHENG, Xueli LI( ), Zhonghua XIANG(
), Zhonghua XIANG( )
)
Received:2024-01-15
Revised:2024-03-12
Online:2024-04-25
Published:2024-06-06
Contact:
Xueli LI, Zhonghua XIANG
摘要:
过渡金属和氮掺杂的碳(M-N-C)由于优异的电催化活性以及较低的生产成本,成为铂基催化剂的替代。然而,目前的M-N-C催化剂通常涉及金属盐、含氮物质和碳载体的结合,在经过热处理和酸洗过程后得到的催化剂在活性位点密度和传质能力上性能不足。采用分步负载金属法制备了Fe、Co双金属掺杂的M-N-C催化剂。利用Zn2+和Co2+的竞争效应,成功合成了小尺寸且均匀的Zn-Co-ZIFs双金属沸石咪唑骨架。随后,在不形成金属团簇的前提下将最大量的Fe原子嵌入C-Zn-Co-ZIFs-H+前体结构中,使其在热解后能产生大量的FeCo—N x 活性位点。这种改进显著增加了FeCo-N-C-2催化剂中Fe活性位点的含量(1.9%,质量分数),并深度优化了其微孔和介孔结构(860 m2·g-1)。该催化剂在0.1 mol·L-1 HClO4和0.1 mol·L-1 KOH溶液中,氧还原反应(ORR)活性展现出了0.806 V和0.921 V的半波电位,并分别在50000、45000 s恒定电位测试后保持了91.21%和95.32%的活性。将其组装于质子交换膜燃料电池(PEMFCs)和碱性锌-空气液流电池(ZAFBs)中,峰值功率密度分别达到了746、164 mW·cm-2,显示出优越的性能。
中图分类号:
贾旭东, 杨博龙, 程前, 李雪丽, 向中华. 分步负载金属法制备铁钴双金属位点高效氧还原电催化剂[J]. 化工学报, 2024, 75(4): 1578-1593.
Xudong JIA, Bolong YANG, Qian CHENG, Xueli LI, Zhonghua XIANG. Preparation of high-efficiency iron-cobalt bimetallic site oxygen reduction electrocatalysts by step-by-step metal loading method[J]. CIESC Journal, 2024, 75(4): 1578-1593.

图A7 Fe-N-C(a)、Co-N-C(b)、FeCo-N-C-1(c)、FeCo-N-C-2(d)、20% Pt/C(e)催化剂在1.103~1.203 V范围内的酸性CV曲线和酸性双电层电容曲线(f)
Fig.A7 Acid CV curve of Fe-N-C (a), Co-N-C (b), FeCo-N-C-1 (c), FeCo-N-C-2 (d) and 20% Pt/C (e) catalysts at 1.103—1.203 V, and acid Cdl curve (f)
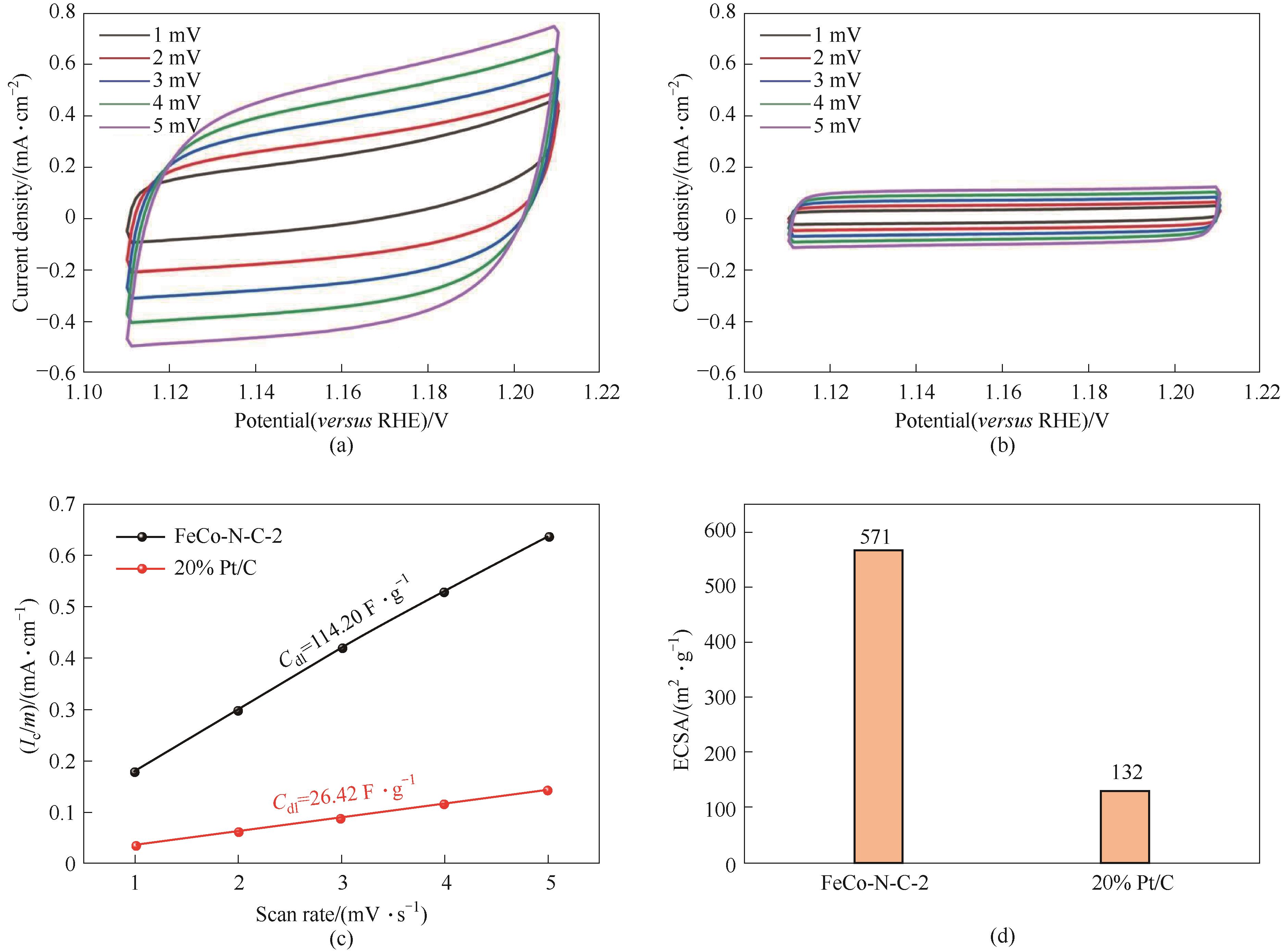
图A8 FeCo-N-C-2(a)、20% Pt/C(b)催化剂在1.111~1.211 V范围内的碱性CV曲线;碱性双电层电容曲线(c);ECSA(d)
Fig.A8 Alkaline CV curve of FeCo-N-C-2 (a), 20% Pt/C (b) catalysts at 1.111—1.211 V; alkaline Cdl curve (c); ECSA (d)
| 催化剂 | E1/2/V | 文献 |
|---|---|---|
| FeCo-N-C-2 | 0.921 | 本文 |
| f-FeCoNC900 | 0.890 | [ |
| FeCo2-NPC-900 | 0.870 | [ |
| Fe-Zn-SA/NC | 0.850 | [ |
| FeCo-ISAs/CN | 0.920 | [ |
表A2 FeCo-N-C-2催化剂与其他催化剂的碱性ORR性能比较
Table A2 Alkaline ORR properties of FeCo-N-C-2 catalyst compared with other catalysts
| 催化剂 | E1/2/V | 文献 |
|---|---|---|
| FeCo-N-C-2 | 0.921 | 本文 |
| f-FeCoNC900 | 0.890 | [ |
| FeCo2-NPC-900 | 0.870 | [ |
| Fe-Zn-SA/NC | 0.850 | [ |
| FeCo-ISAs/CN | 0.920 | [ |
| 催化剂 | 过电位/V | Tafel斜率/(mV·dec-1) | 文献 |
|---|---|---|---|
| FeCo-N-C-2 | 0.339 | 65.59 | 本文 |
| Co-Fe-N-C | 0.309 | 37.00 | [ |
| Fe2/Co1-GNCL | 0.350 | 70.00 | [ |
| CoDNi-N/C | 0.360 | 72.00 | [ |
| Fe-NiNC-50 | 0.340 | 54.00 | [ |
表A3 FeCo-N-C-2催化剂与其他催化剂的碱性OER性能比较
Table A3 Alkaline OER properties of FeCo-N-C-2 catalyst compared with other catalysts
| 催化剂 | 过电位/V | Tafel斜率/(mV·dec-1) | 文献 |
|---|---|---|---|
| FeCo-N-C-2 | 0.339 | 65.59 | 本文 |
| Co-Fe-N-C | 0.309 | 37.00 | [ |
| Fe2/Co1-GNCL | 0.350 | 70.00 | [ |
| CoDNi-N/C | 0.360 | 72.00 | [ |
| Fe-NiNC-50 | 0.340 | 54.00 | [ |
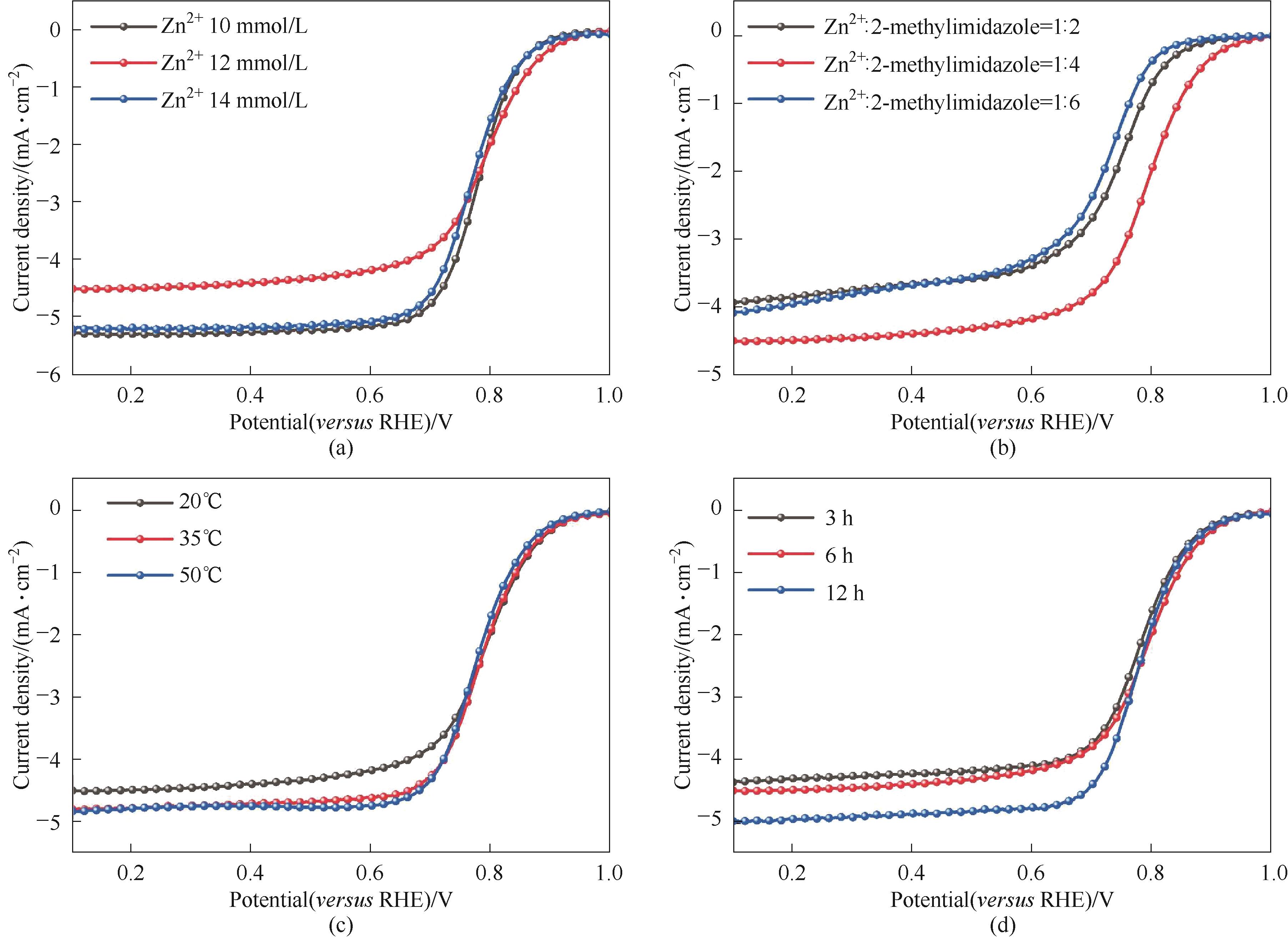
图A5 反应物浓度(a)、反应物比例(b)、反应温度(c)、反应时间(d)的参数优化
Fig.A5 Parameters optimization of reactant concentration (a), reactant ratio (b), reaction temperature (c) and reaction time (d)
| 催化剂 | Eonset/ V | E1/2/V | 文献 |
|---|---|---|---|
| FeCo-N-C-2 | 0.999 | 0.806 | 本文 |
| f-FeCoNC900 | 0.870 | 0.810 | [ |
| FeCo2-NPC-900 | 0.850 | 0.740 | [ |
| FeNi-N6 | 0.900 | 0.790 | [ |
| Fe-Zn-SA/NC | 0.870 | 0.780 | [ |
表A1 FeCo-N-C-2催化剂与其他催化剂的酸性ORR性能比较
Table A1 Acid ORR properties of FeCo-N-C-2 catalyst compared with other catalysts
| 催化剂 | Eonset/ V | E1/2/V | 文献 |
|---|---|---|---|
| FeCo-N-C-2 | 0.999 | 0.806 | 本文 |
| f-FeCoNC900 | 0.870 | 0.810 | [ |
| FeCo2-NPC-900 | 0.850 | 0.740 | [ |
| FeNi-N6 | 0.900 | 0.790 | [ |
| Fe-Zn-SA/NC | 0.870 | 0.780 | [ |
| 1 | Liu M Y, Xiao X D, Li Q, et al. Recent progress of electrocatalysts for oxygen reduction in fuel cells[J]. Journal of Colloid and Interface Science, 2022, 607(Pt 1): 791-815. |
| 2 | Wang X X, Cullen D A, Pan Y T, et al. Nitrogen-coordinated single cobalt atom catalysts for oxygen reduction in proton exchange membrane fuel cells[J]. Advanced Materials, 2018, 30(11)1706758 |
| 3 | Wang M M, Lin M T, Li J T, et al. Metal-organic framework derived carbon-confined Ni2P nanocrystals supported on graphene for an efficient oxygen evolution reaction[J]. Chemical Communications, 2017, 53(59): 8372-8375. |
| 4 | Wang Q, Meng Z, Li J T, et al. High retention volume covalent organic polymer for xenon capture: dynamic separation of Xe and Kr[J]. Green Energy and Environment, 2022, 7: 948-956. |
| 5 | Zhao Y, Zhang M, Wen X, et al. Microfluidic interface boosted synthesis of covalent organic polymer capsule[J]. Green Chemical Engineering, 2020, 1(1): 63-69. |
| 6 | Ding R, Zhang S Q, Chen Y W, et al. Application of machine learning in optimizing proton exchange membrane fuel cells: a review[J]. Energy and AI, 2022, 9: 100170. |
| 7 | Kodama K, Nagai T, Kuwaki A, et al. Challenges in applying highly active Pt-based nanostructured catalysts for oxygen reduction reactions to fuel cell vehicles[J]. Nature Nanotechnology, 2021, 16: 140-147. |
| 8 | Zhang J W, Yuan Y L, Gao L, et al. Stabilizing Pt-based electrocatalysts for oxygen reduction reaction: fundamental understanding and design strategies[J]. Advanced Materials, 2021, 33(20): e2006494. |
| 9 | Zhang B H, An G B, Chen J, et al. Surface state engineering of carbon dot/carbon nanotube heterojunctions for boosting oxygen reduction performance[J]. Journal of Colloid and Interface Science, 2023, 637: 173-181. |
| 10 | Hong Y S, Li L B, Huang B Y, et al. Molecular control of carbon-based oxygen reduction electrocatalysts through metal macrocyclic complexes functionalization[J]. Advanced Energy Materials, 2021, 11(33): 2100866. |
| 11 | Huang L, Zaman S, Tian X L, et al. Advanced platinum-based oxygen reduction electrocatalysts for fuel cells[J]. Accounts of Chemical Research, 2021, 54(2): 311-322. |
| 12 | Wang Y L, Shi R, Shang L, et al. Vertical graphene array for efficient electrocatalytic reduction of oxygen to hydrogen peroxide[J]. Nano Energy, 2022, 96: 107046. |
| 13 | Wang Y L, Waterhouse G I N, Shang L, et al. Electrocatalytic oxygen reduction to hydrogen peroxide: from homogeneous to heterogeneous electrocatalysis[J]. Advanced Energy Materials, 2021, 11(15): 2003323. |
| 14 | Li Y, Wang H H, Priest C, et al. Advanced electrocatalysis for energy and environmental sustainability via water and nitrogen reactions[J]. Advanced Materials, 2021, 33(6): e2000381. |
| 15 | Zhang B, Zheng Y J, Ma T, et al. Designing MOF nanoarchitectures for electrochemical water splitting[J]. Advanced Materials, 2021, 33(17): e2006042. |
| 16 | Xie X H, He C, Li B Y, et al. Performance enhancement and degradation mechanism identification of a single-atom Co-N-C catalyst for proton exchange membrane fuel cells[J]. Nature Catalysis, 2020, 3: 1044-1054. |
| 17 | Li J Z, Zhang H G, Samarakoon W, et al. Thermally driven structure and performance evolution of atomically dispersed FeN4 sites for oxygen reduction[J]. Angewandte Chemie International Edition, 2019, 58(52): 18971-18980. |
| 18 | Pavlenko V, Khosravi H S, Żółtowska S, et al. A comprehensive review of template-assisted porous carbons: modern preparation methods and advanced applications[J]. Materials Science and Engineering R: Reports, 2022, 149: 100682. |
| 19 | Sequeira C A C. Carbon anode in carbon history[J]. Molecules, 2020, 25(21): 4996. |
| 20 | Zhang H G, Osgood H, Xie X H, et al. Engineering nanostructures of PGM-free oxygen-reduction catalysts using metal-organic frameworks[J]. Nano Energy, 2017, 31: 331-350. |
| 21 | Ajmal S, Kumar A, Tabish M, et al. Modulating the microenvironment of single atom catalysts with tailored activity to benchmark the CO2 reduction[J]. Materials Today, 2023, 67: 203-228. |
| 22 | Dong F, Wu M J, Zhang G X, et al. Defect engineering of carbon-based electrocatalysts for rechargeable zinc-air batteries[J]. Chemistry, 2020, 15(22): 3737-3751. |
| 23 | Sui X L, Zhang L, Li J J, et al. Advanced support materials and interactions for atomically dispersed noble-metal catalysts: from support effects to design strategies[J]. Advanced Energy Materials, 2022, 12(1): 2102556. |
| 24 | Maiti K, Maiti S, Curnan M T, et al. Engineering single atom catalysts to tune properties for electrochemical reduction and evolution reactions[J]. Advanced Energy Materials, 2021, 11(38): 2101670. |
| 25 | Menga D, Low J L, Li Y S, et al. Resolving the dilemma of Fe-N-C catalysts by the selective synthesis of tetrapyrrolic active sites via an imprinting strategy[J]. Journal of the American Chemical Society, 2021, 143(43): 18010-18019. |
| 26 | Aslam S, Khanna M, Kuanr B K. Fabrication of CoFe2O4/reduced graphene oxide nanocomposite as a microwave absorber[J]. Advanced Science Letters, 2018, 24(2): 903-906. |
| 27 | Yuldashova I I, Tashmetov M Y. The influence of electron beams to structure parameters of multi walled carbon nanotube[J]. Physica B: Condensed Matter, 2019, 571: 280-284. |
| 28 | Qiu T, Yang J G, Bai X J. Insight into the change in carbon structure and thermodynamics during anthracite transformation into graphite[J]. International Journal of Minerals, Metallurgy and Materials, 2020, 27(2): 162-172. |
| 29 | Ghosh A, do Amaral Razzino C, Dasgupta A, et al. Structural and electrochemical properties of babassu coconut mesocarp-generated activated carbon and few-layer graphene[J]. Carbon, 2019, 145: 175-186. |
| 30 | Zhao J, Mao J Y, Li Y R, et al. Friction-induced nano-structural evolution of graphene as a lubrication additive[J]. Applied Surface Science, 2018, 434: 21-27. |
| 31 | Maiti K, Balamurugan J, Peera S G, et al. Highly active and durable core-shell fct-PdFe@Pd nanoparticles encapsulated NG as an efficient catalyst for oxygen reduction reaction[J]. ACS Applied Materials & Interfaces, 2018, 10(22): 18734-18745. |
| 32 | Dong X B, Zhao C Y, Guan Q X, et al. Metal-organic framework-derived cobalt and nitrogen Co-doped porous carbon with four-coordinated Co-N x for efficient acetylene hydrochlorination[J]. Applied Organometallic Chemistry, 2018, 32(12): e4570. |
| 33 | Singh S K, Takeyasu K, Nakamura J. Active sites and mechanism of oxygen reduction reaction electrocatalysis on nitrogen-doped carbon materials[J]. Advanced Materials, 2019, 31(13): e1804297. |
| 34 | Huang J W, Cheng Q Q, Huang Y C, et al. Highly efficient Fe— N—C electrocatalyst for oxygen reduction derived from core-shell-structured Fe(OH)3@zeolitic imidazolate framework[J]. ACS Applied Energy Materials, 2019, 2(5): 3194-3203. |
| 35 | Cui X Y, Yang S B, Yan X X, et al. Pyridinic-nitrogen-dominated graphene aerogels with Fe—N—C coordination for highly efficient oxygen reduction reaction[J]. Advanced Functional Materials, 2016, 26(31): 5708-5717. |
| 36 | Cui X, Gao L K, Lei S, et al. Simultaneously crafting single-atomic Fe sites and graphitic layer-wrapped Fe3C nanoparticles encapsulated within mesoporous carbon tubes for oxygen reduction[J]. Advanced Functional Materials, 2021, 31(10): 2009197. |
| 37 | Tang F, Lei H T, Wang S J, et al. A novel Fe—N—C catalyst for efficient oxygen reduction reaction based on polydopamine nanotubes[J]. Nanoscale, 2017, 9(44): 17364-17370. |
| 38 | Jiang H L, Yao Y F, Zhu Y H, et al. Iron carbide nanoparticles encapsulated in mesoporous Fe—N-doped graphene-like carbon hybrids as efficient bifunctional oxygen electrocatalysts[J]. ACS Applied Materials & Interfaces, 2015, 7(38): 21511-21520. |
| 39 | Li X L, Liu Q B, Yang B L, et al. An initial covalent organic polymer with closed-F edges directly for proton-exchange-membrane fuel cells[J]. Advanced Materials, 2022, 34(36): e2204570. |
| 40 | Jiang J, Zhang A L, Li L L, et al. Nickel-cobalt layered double hydroxide nanosheets as high-performance electrocatalyst for oxygen evolution reaction[J]. Journal of Power Sources, 2015, 278: 445-451. |
| 41 | Manivasakan P, Ramasamy P, Kim J. Use of urchin-like Ni x Co3- x O4 hierarchical nanostructures based on non-precious metals as bifunctional electrocatalysts for anion-exchange membrane alkaline alcohol fuel cells[J]. Nanoscale, 2014, 6(16): 9665-9672. |
| 42 | Wang J, Li L Q, Chen X, et al. A Co-N/C hollow-sphere electrocatalyst derived from a metanilic CoAl layered double hydroxide for the oxygen reduction reaction, and its active sites in various pH media[J]. Nano Research, 2017, 10(7): 2508-2518. |
| 43 | Zhang G X, Jia Y, Zhang C, et al. A general route via formamide condensation to prepare atomically dispersed metal-nitrogen-carbon electrocatalysts for energy technologies[J]. Energy & Environmental Science, 2019, 12(4): 1317-1325. |
| 44 | Fang X Z, Jiao L, Yu S H, et al. Metal-organic framework-derived FeCo-N-doped hollow porous carbon nanocubes for electrocatalysis in acidic and alkaline media[J]. ChemSusChem, 2017, 10(15): 3019-3024. |
| 45 | Zhou Y D, Yang W, Utetiwabo W, et al. Revealing of active sites and catalytic mechanism in N-coordinated Fe, Ni dual-doped carbon with superior acidic oxygen reduction than single-atom catalyst[J]. The Journal of Physical Chemistry Letters, 2020, 11(4): 1404-1410. |
| 46 | Xu J, Lai S H, Qi D F, et al. Atomic Fe-Zn dual-metal sites for high-efficiency pH-universal oxygen reduction catalysis[J]. Nano Research, 2021, 14(5): 1374-1381. |
| 47 | Zhang D Y, Chen W X, Li Z, et al. Isolated Fe and Co dual active sites on nitrogen-doped carbon for a highly efficient oxygen reduction reaction[J]. Chemical Communications, 2018, 54(34): 4274-4277. |
| 48 | Bai L C, Hsu C S, Alexander D T L, et al. A cobalt-iron double-atom catalyst for the oxygen evolution reaction[J]. Journal of the American Chemical Society, 2019, 141(36): 14190-14199. |
| 49 | Wei Y S, Sun L M, Wang M, et al. Fabricating dual-atom iron catalysts for efficient oxygen evolution reaction: a heteroatom modulator approach[J]. Angewandte Chemie International Edition, 2020, 59(37): 16013-16022. |
| 50 | Li Z H, He H Y, Cao H B, et al. Atomic Co/Ni dual sites and Co/Ni alloy nanoparticles in N-doped porous Janus-like carbon frameworks for bifunctional oxygen electrocatalysis[J]. Applied Catalysis B: Environmental, 2019, 240: 112-121. |
| 51 | Zhu X F, Zhang D T, Chen C J, et al. Harnessing the interplay of Fe-Ni atom pairs embedded in nitrogen-doped carbon for bifunctional oxygen electrocatalysis[J]. Nano Energy, 2020, 71: 104597. |
| [1] | 王天闻, 闫肃, 赵梦园, 杨天让, 刘建国. 固体氧化物电池空气电极铬中毒机理及抗铬性能研究进展[J]. 化工学报, 2024, 75(6): 2091-2108. |
| [2] | 江洋, 彭长宏, 陈伟, 周豪, 马忠彬, 李洪博, 邱在容, 张国鹏, 周康根. 废旧磷酸铁锂粉料综合回收中试研究[J]. 化工学报, 2024, 75(6): 2353-2361. |
| [3] | 丁禹, 杨昌泽, 李军, 孙会东, 商辉. 原子尺度钼系加氢脱硫催化剂的研究进展与展望[J]. 化工学报, 2024, 75(5): 1735-1749. |
| [4] | 裴欣哲, 孙朱行, 林钰翔, 张朝阳, 钱勇, 吕兴才. 电催化分解液氨阳极材料的研究[J]. 化工学报, 2024, 75(5): 1843-1854. |
| [5] | 赵亭亭, 鄢立祥, 唐福利, 肖敏之, 谭烨, 宋刘斌, 肖忠良, 李灵均. 光辅助锂-二氧化碳电池催化剂的设计策略与反应机理研究进展[J]. 化工学报, 2024, 75(5): 1750-1764. |
| [6] | 王金山, 王世学, 朱禹. 冷却表面温差对高温质子交换膜燃料电池性能的影响[J]. 化工学报, 2024, 75(5): 2026-2035. |
| [7] | 莫锦洪, 韩雪, 朱毅翔, 李菁, 王旭裕, 纪红兵. Pt-Ga/CeO2-ZrO2-Al2O3脱氢裂解双功能催化剂用于正丁烷催化制烯烃研究[J]. 化工学报, 2024, 75(5): 1855-1869. |
| [8] | 李云璇, 刘新悦, 陈熙, 刘文, 周明月, 蓝兴英. 基于固液氧化还原靶向反应的能量存储技术:材料、器件及动力学[J]. 化工学报, 2024, 75(4): 1222-1240. |
| [9] | 范以薇, 刘威, 李盈盈, 王培霞, 张吉松. 有机液体储氢中全氢化乙基咔唑催化脱氢研究进展[J]. 化工学报, 2024, 75(4): 1198-1208. |
| [10] | 冯彬彬, 卢明佳, 黄志宏, 常译文, 崔志明. 碳载体在质子交换膜燃料电池中的应用及优化[J]. 化工学报, 2024, 75(4): 1469-1484. |
| [11] | 严孝清, 赵瑛, 张宇哲, 欧鸿辉, 黄起中, 胡华贵, 杨贵东. 五重孪晶铜纳米线@聚吡咯制备及其电催化硝酸盐还原制氨[J]. 化工学报, 2024, 75(4): 1519-1532. |
| [12] | 程骁恺, 历伟, 王靖岱, 阳永荣. 镍催化可控/活性自由基聚合反应研究进展[J]. 化工学报, 2024, 75(4): 1105-1117. |
| [13] | 吴希, 孙博, 刘银东, 齐传磊, 陈凯毅, 王路海, 许崇, 李永峰. 钠离子电池沥青基碳负极材料制备技术研究进展[J]. 化工学报, 2024, 75(4): 1270-1283. |
| [14] | 韩宇, 周乐, 张鑫, 罗勇, 孙宝昌, 邹海魁, 陈建峰. 高黏附性Pd/SiO2/NF整体式催化剂的制备及加氢性能研究[J]. 化工学报, 2024, 75(4): 1533-1542. |
| [15] | 李昂, 赵振宇, 李洪, 高鑫. 微波诱导高分散Pd/FeP催化剂构筑及其电催化性能研究[J]. 化工学报, 2024, 75(4): 1594-1606. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号