化工学报 ›› 2024, Vol. 75 ›› Issue (11): 4286-4297.DOI: 10.11949/0438-1157.20240600
收稿日期:2024-06-03
修回日期:2024-08-14
出版日期:2024-11-25
发布日期:2024-12-26
通讯作者:
吕兴梅
作者简介:冯咪(1992—),女,博士研究生,副研究员,fengmi@ipe.ac.cn基金资助:
Mi FENG1,2,4( ), Jie ZHANG3(
), Jie ZHANG3( ), Xingmei LYU1(
), Xingmei LYU1( )
)
Received:2024-06-03
Revised:2024-08-14
Online:2024-11-25
Published:2024-12-26
Contact:
Xingmei LYU
摘要:
针对传统甲壳素制备工艺污染大、水耗高等缺点,提出了基于离子液体的高纯甲壳素制备绿色新方法。结果表明,所用离子液体中1-乙基-3-甲基咪唑醋酸盐([Emim]OAc)、甲烷磺酸胆碱([Ch]Ms)可分别通过溶解-再生两步法、溶解-分离一步法制备得甲壳素,纯度分别达到90.7%、97.4%,收率分别达到38.1%、63.9%。相比于[Emim]OAc提取体系,水含量对[Ch]Ms提取甲壳素影响较小,当水含量增大至50%时,甲壳素纯度仍达94.3%,收率维持在60%。此外,[Ch]Ms分离所得甲壳素结构未发生明显变化,仍保持为α构型,但[Emim]OAc分离所得甲壳素构型转变为半α构型。提出了[Ch]Ms阴、阳离子协同作用脱除蛋白质、碳酸钙,制备甲壳素机理。相比于[Emim]OAc体系,[Ch]Ms提取甲壳素具有低成本、易分离、流程短及产品纯度高等优势,更有利于大规模应用。
中图分类号:
冯咪, 张杰, 吕兴梅. 基于胆碱类离子液体的高纯甲壳素一步提取分离[J]. 化工学报, 2024, 75(11): 4286-4297.
Mi FENG, Jie ZHANG, Xingmei LYU. One-step extraction and separation of high purity chitin based on choline ionic liquid[J]. CIESC Journal, 2024, 75(11): 4286-4297.
| 离子液体 | 溶解率/% | 再生率/% |
|---|---|---|
| [Ch]OAc | 58.0 | <5.0 |
| [Emim]OAc | 63.0 | 19.0 |
| [Ch]Ms | 70.5 | <5.0 |
| [Emim]Ms | 38.8 | <5.0 |
表1 所用离子液体对虾壳的溶解率及再生率
Table 1 Dissolution rate and regeneration rate of shrimp shells by the used ionic liquids
| 离子液体 | 溶解率/% | 再生率/% |
|---|---|---|
| [Ch]OAc | 58.0 | <5.0 |
| [Emim]OAc | 63.0 | 19.0 |
| [Ch]Ms | 70.5 | <5.0 |
| [Emim]Ms | 38.8 | <5.0 |
| 离子液体 | 不溶物收率/% | 不溶物中甲壳素收率/% | 再生物收率/% | 再生物中甲壳素收率/% |
|---|---|---|---|---|
| [Ch]OAc | 42.0 | 73.7 | <5 | — |
| [Emim]OAc | 37.0 | 48.8 | 19 | 38.1 |
| [Ch]Ms | 29.5 | 63.9 | <5 | — |
| [Emim]Ms | 61.2 | 87.7 | <5 | — |
表2 所用离子液体处理虾壳不溶物、再生物及甲壳素收率
Table 2 The yield of precipitate, regenerated product and chitin using different ILs treatment
| 离子液体 | 不溶物收率/% | 不溶物中甲壳素收率/% | 再生物收率/% | 再生物中甲壳素收率/% |
|---|---|---|---|---|
| [Ch]OAc | 42.0 | 73.7 | <5 | — |
| [Emim]OAc | 37.0 | 48.8 | 19 | 38.1 |
| [Ch]Ms | 29.5 | 63.9 | <5 | — |
| [Emim]Ms | 61.2 | 87.7 | <5 | — |
| 提取体系 | 生物质原料 | 提取条件 | 甲壳素纯度/% | 甲壳素收率/% | 甲壳素分子量 | 甲壳素DA值/% |
|---|---|---|---|---|---|---|
[Amim]Br + 柠檬酸水溶液[ | 蟹壳 | 120℃ 24 h | >99 | 12.6 | 1.5×105 | 94 |
[DIPEA][Ac] + 柠檬酸水溶液[ | 虾壳 | 110℃ 36 h | >98 | 14.8 | 7.1×104 | 98.67 |
[DIPEA][P] + 柠檬酸水溶液[ | 虾壳 | 110℃ 30 h | >98 | 11.5 | 5.6×104 | 98.33 |
[DMBA][Ac] + 柠檬酸水溶液[ | 虾壳 | 110℃ 30 h | >98 | 13.7 | 6.3×104 | 98.99 |
| [NH3(CH2)2OH][OAc][ | 处理虾壳 | 90℃ 2 h | 46 | 81 | — | — |
| [NH3OH][OAc][ | 虾壳 | 100℃ 8 h | 76 | 96 | — | 83 |
| 处理虾壳 | 100℃ 8 h | 78 | 100 | — | 68 | |
| [DBNH][OAc]-AcOH[ | 虾壳 | 120℃ 30 h | 91 | 30 | 83 | |
| 硫酸镍水溶液+[Emim]Cl[ | 虾壳 | 130℃ 24 h + 150℃ 24 h | 96.5 | 72.5 | 1.3×105 | — |
| [Ch]Ms(本工作) | 虾壳 | 110℃ 7 h | 97.4 | 63.9 | 1.17×105 | 95.5 |
表3 不同离子液体提取甲壳素对比
Table 3 Comparison of chitin extraction using different ionic liquids
| 提取体系 | 生物质原料 | 提取条件 | 甲壳素纯度/% | 甲壳素收率/% | 甲壳素分子量 | 甲壳素DA值/% |
|---|---|---|---|---|---|---|
[Amim]Br + 柠檬酸水溶液[ | 蟹壳 | 120℃ 24 h | >99 | 12.6 | 1.5×105 | 94 |
[DIPEA][Ac] + 柠檬酸水溶液[ | 虾壳 | 110℃ 36 h | >98 | 14.8 | 7.1×104 | 98.67 |
[DIPEA][P] + 柠檬酸水溶液[ | 虾壳 | 110℃ 30 h | >98 | 11.5 | 5.6×104 | 98.33 |
[DMBA][Ac] + 柠檬酸水溶液[ | 虾壳 | 110℃ 30 h | >98 | 13.7 | 6.3×104 | 98.99 |
| [NH3(CH2)2OH][OAc][ | 处理虾壳 | 90℃ 2 h | 46 | 81 | — | — |
| [NH3OH][OAc][ | 虾壳 | 100℃ 8 h | 76 | 96 | — | 83 |
| 处理虾壳 | 100℃ 8 h | 78 | 100 | — | 68 | |
| [DBNH][OAc]-AcOH[ | 虾壳 | 120℃ 30 h | 91 | 30 | 83 | |
| 硫酸镍水溶液+[Emim]Cl[ | 虾壳 | 130℃ 24 h + 150℃ 24 h | 96.5 | 72.5 | 1.3×105 | — |
| [Ch]Ms(本工作) | 虾壳 | 110℃ 7 h | 97.4 | 63.9 | 1.17×105 | 95.5 |

图6 离子液体处理所得不溶物、再生物及甲壳素标样红外谱图a—甲壳素标样;b—[Ch]Ms处理虾壳所得不溶物;c—[Ch]OAc处理虾壳所得不溶物;d—[Emim]Ms处理虾壳所得不溶物;e—[Emim]OAc处理虾壳所得不溶物;f—[Emim]OAc处理虾壳所得再生物
Fig.6 The FT-IR spectra of obtained precipitate, regenerated product and chitin
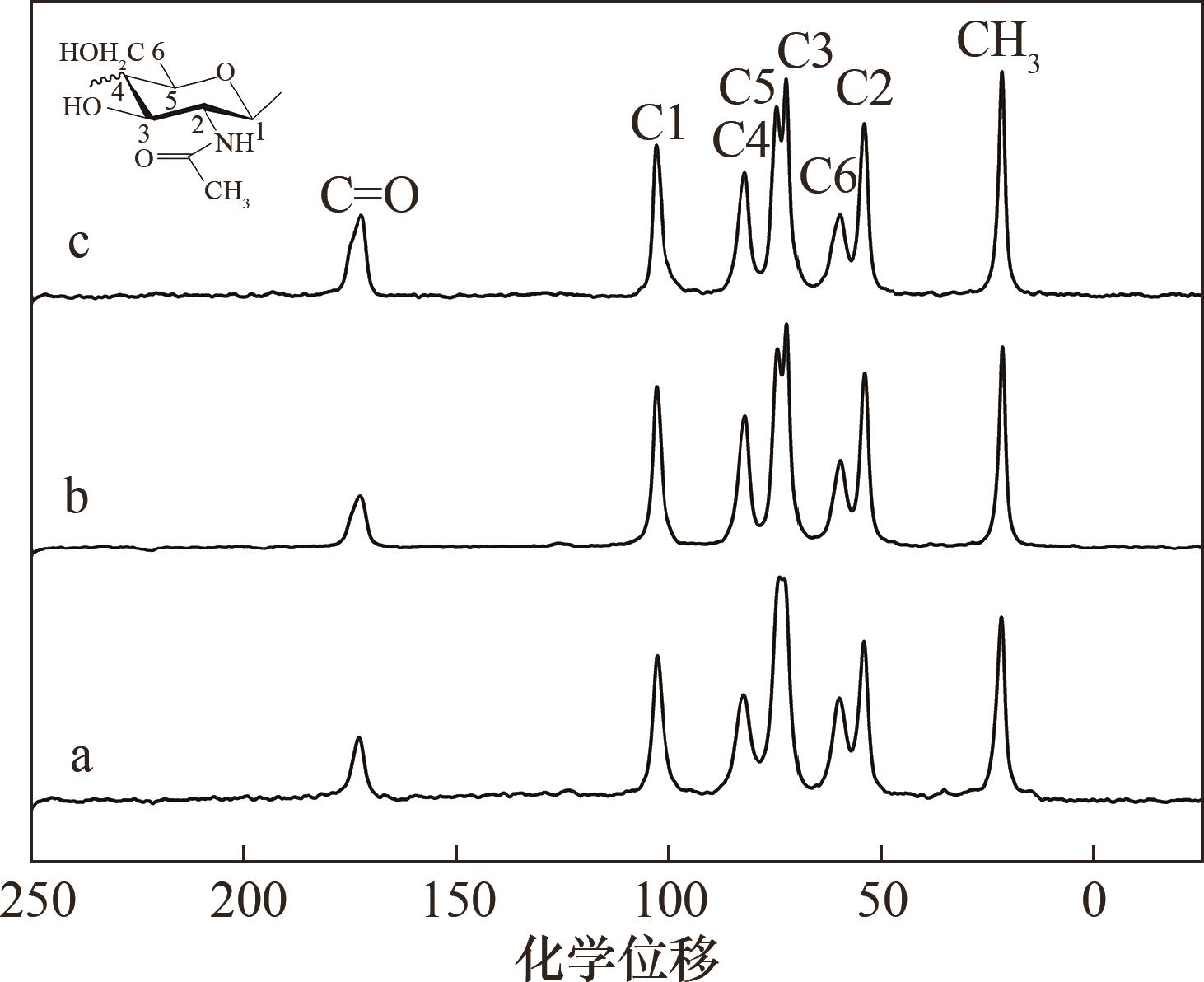
图7 离子液体处理所得不溶物甲壳素、再生物甲壳素及甲壳素标样固体13C NMR谱图a—[Emim]OAc处理所得再生物甲壳素;b—[Ch]Ms处理所得不溶物甲壳素;c—甲壳素标样
Fig.7 The solid 13C NMR spectra of precipitated chitin, regenerated chitin, and chitin
| 1 | Rinaudo M. Chitin and chitosan: properties and applications[J]. Progress in Polymer Science, 2006, 31(7): 603-632. |
| 2 | Zheng Y R, Zhang H, Wang Z W, et al. Chitin nanofibrils assisted 3D printing all-chitin hydrogels for wound dressing[J]. Carbohydrate Polymers, 2024, 334: 122028. |
| 3 | Naghdi T, Golmohammadi H, Yousefi H, et al. Chitin nanofiber paper toward optical (bio)sensing applications[J]. ACS Applied Materials & Interfaces, 2020, 12(13): 15538-15552. |
| 4 | Xing F, Chi Z, Yang R X, et al. Chitin-hydroxyapatite-collagen composite scaffolds for bone regeneration[J]. International Journal of Biological Macromolecules, 2021, 184: 170-180. |
| 5 | Lv J R, Lv X H, Ma M H, et al. Chitin and chitin-based biomaterials: a review of advances in processing and food applications[J]. Carbohydrate Polymers, 2023, 299: 120142. |
| 6 | Riseh R S, Vazvani M G, Vatankhah M, et al. Chitin-induced disease resistance in plants: a review[J]. International Journal of Biological Macromolecules, 2024, 266(Pt 1): 131105. |
| 7 | No H K, Meyers S P, Lee K S. Isolation and characterization of chitin from crawfish shell waste[J]. Journal of Agricultural and Food Chemistry, 1989, 37(3): 575-579. |
| 8 | Hamdi M, Hammami A, Hajji S, et al. Chitin extraction from blue crab (Portunus segnis) and shrimp (Penaeus kerathurus) shells using digestive alkaline proteases from P. segnis viscera [J]. International Journal of Biological Macromolecules, 2017, 101: 455-463. |
| 9 | Dhanabalan V, Martin Xavier K A, Eppen S, et al. Characterization of chitin extracted from enzymatically deproteinized acetes shell residue with varying degree of hydrolysis[J]. Carbohydrate Polymers, 2021, 253: 117203. |
| 10 | Zhang Q, Wang L Y, Liu S G, et al. Establishment of successive co-fermentation by Bacillus subtilis and Acetobacter pasteurianus for extracting chitin from shrimp shells[J]. Carbohydrate Polymers, 2021, 258: 117720. |
| 11 | Tan Y N, Lee P P, Chen W N. Dual extraction of crustacean and fungal chitosan from a single Mucor circinelloides fermentation[J]. Fermentation, 2020, 6(2): 40. |
| 12 | Setoguchi T, Kato T, Yamamoto K, et al. Facile production of chitin from crab shells using ionic liquid and citric acid[J]. International Journal of Biological Macromolecules, 2012, 50(3): 861-864. |
| 13 | Tolesa L D, Gupta B S, Lee M J. Chitin and chitosan production from shrimp shells using ammonium-based ionic liquids[J]. International Journal of Biological Macromolecules, 2019, 130: 818-826. |
| 14 | He J, Qiang Q, Bai L, et al. Acetalization strategy in biomass valorization: a review[J]. Industrial Chemistry & Materials, 2024, 2(1): 30-56. |
| 15 | Hülsey M J. Shell biorefinery: a comprehensive introduction[J]. Green Energy & Environment, 2018, 3(4): 318-327. |
| 16 | Magalhães F F, Pereira M M, de Cássia Superbi de Sousa R, et al. Tailoring the partitioning of proteins using ionic liquids as adjuvants in polymer-polymer aqueous biphasic systems[J]. Green Chemical Engineering, 2022, 3(4): 328-337. |
| 17 | Barber P S, Griggs C S, Gurau G, et al. Coagulation of chitin and cellulose from 1-ethyl-3-methylimidazolium acetate ionic-liquid solutions using carbon dioxide[J]. Angewandte Chemie (International Ed. in English), 2013, 52(47): 12350-12353. |
| 18 | Qin Y, Lu X M, Sun N, et al. Dissolution or extraction of crustacean shells using ionic liquids to obtain high molecular weight purified chitin and direct production of chitin films and fibers[J]. Green Chemistry, 2010, 12(6): 968-971. |
| 19 | Barber P S, Griggs C S, Bonner J R, et al. Electrospinning of chitin nanofibers directly from an ionic liquid extract of shrimp shells[J]. Green Chemistry, 2013, 15(3): 601-607. |
| 20 | Shamshina J L, Barber P S, Gurau G, et al. Pulping of crustacean waste using ionic liquids: to extract or not to extract[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(11): 6072-6081. |
| 21 | Feng M, He B, Chen X Y, et al. Separation of chitin from shrimp shells enabled by transition metal salt aqueous solution and ionic liquid[J]. Chinese Journal of Chemical Engineering, 2023, 53: 133-141. |
| 22 | 蒋挺大. 壳聚糖[M]. 2版. 北京: 化学工业出版社, 2007: 6-7. |
| Jiang T D. Chitosan[M]. 2nd ed. Beijing: Chemical Industry Press, 2007: 6-7. | |
| 23 | Feng M, Yan J P, He B, et al. Controllable conversion of shrimp shells into chitin or derived carbon material using acidic deep eutectic solvent[J]. International Journal of Biological Macromolecules, 2021, 193: 347-357. |
| 24 | Poirier M, Charlet G. Chitin fractionation and characterization in N,N-dimethylacetamide/lithium chloride solvent system[J]. Carbohydrate Polymers, 2002, 50(4): 363-370. |
| 25 | Feng M, Lu X M, Zhang J, et al. Direct conversion of shrimp shells to O-acylated chitin with antibacterial and anti-tumor effects by natural deep eutectic solvents[J]. Green Chemistry, 2019, 21(1): 87-98. |
| 26 | Sun N, Parthasarathi R, Socha A M, et al. Understanding pretreatment efficacy of four cholinium and imidazolium ionic liquids by chemistry and computation[J]. Green Chemistry, 2014, 16(5): 2546-2557. |
| 27 | Parviainen A, King A W T, Mutikainen I, et al. Predicting cellulose solvating capabilities of acid-base conjugate ionic liquids[J]. ChemSusChem, 2013, 6(11): 2161-2169. |
| 28 | Motlagh S R, Elgharbawy A A, Khezri R, et al. Ionic liquid-based microwave-assisted extraction of protein from Nannochloropsis sp. biomass[J]. Biomass Conversion and Biorefinery, 2023, 13(9): 8327-8338. |
| 29 | Uto T, Idenoue S, Yamamoto K, et al. Understanding dissolution process of chitin crystal in ionic liquids: theoretical study[J]. Physical Chemistry Chemical Physics, 2018, 20(31): 20669-20677. |
| 30 | Tao Q Q, Henriquez F N, Ding K, et al. One-pot chitin pulping using recyclable superbase-based protic ionic liquid[J]. Carbohydrate Polymers, 2024, 327: 121680. |
| 31 | Romano P, Fabritius H, Raabe D. The exoskeleton of the lobster Homarus americanus as an example of a smart anisotropic biological material[J]. Acta Biomaterialia, 2007, 3(3): 301-309. |
| 32 | Kim Y, Park R D. Progress in bioextraction processes of chitin from crustacean biowastes[J]. Journal of the Korean Society for Applied Biological Chemistry, 2015, 58(4): 545-554. |
| 33 | Politi Y, Arad T, Klein E, et al. Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase[J]. Science, 2004, 306(5699): 1161-1164. |
| 34 | Al‐Sawalmih A, Li C H, Siegel S, et al. Microtexture and chitin/calcite orientation relationship in the mineralized exoskeleton of the American lobster[J]. Advanced Functional Materials, 2008, 18(20): 3307-3314. |
| 35 | Nikolov S, Petrov M, Lymperakis L, et al. Revealing the design principles of high-performance biological composites using ab initio and multiscale simulations: the example of lobster cuticle[J]. Advanced Materials, 2010, 22(4): 519-526. |
| 36 | Ogawa Y, Lee C M, Nishiyama Y, et al. Absence of sum frequency generation in support of orthorhombic symmetry of α-chitin[J]. Macromolecules, 2016, 49(18): 7025-7031. |
| 37 | Yamaguchi Y, Nge T T, Takemura A, et al. Characterization of uniaxially aligned chitin film by 2D FT-IR spectroscopy[J]. Biomacromolecules, 2005, 6(4): 1941-1947. |
| 38 | Wu Y S, Sasaki T, Irie S, et al. A novel biomass-ionic liquid platform for the utilization of native chitin[J]. Polymer, 2008, 49(9): 2321-2327. |
| 39 | Cárdenas G, Cabrera G, Taboada E, et al. Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR[J]. Journal of Applied Polymer Science, 2004, 93(4): 1876-1885. |
| 40 | Sikorski P, Hori R, Wada M. Revisit of α-chitin crystal structure using high resolution X-ray diffraction data[J]. Biomacromolecules, 2009, 10(5): 1100-1105. |
| 41 | Xu J, McCarthy S P, Gross R A, et al. Chitosan film acylation and effects on biodegradability[J]. Macromolecules, 1996, 29(10): 3436-3440. |
| 42 | Ventura S P M, Neves C M S S, Freire M G, et al. Evaluation of anion influence on the formation and extraction capacity of ionic-liquid-based aqueous biphasic systems[J]. The Journal of Physical Chemistry B, 2009, 113(27): 9304-9310. |
| 43 | Schaefer J, Kramer K J, Garbow J R, et al. Aromatic cross-links in insect cuticle: detection by solid-state 13 C and 15 N NMR[J]. Science, 1987, 235(4793): 1200-1204. |
| 44 | Deng L L, Yue W, Zhang L H, et al. Biobased protic ionic liquids as sustainable solvents for wool keratin/cellulose simultaneous dissolution: solution properties and composited membrane preparation[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(6): 2158-216. |
| [1] | 李匡奚, 于佩潜, 王江云, 魏浩然, 郑志刚, 冯留海. 微气泡旋流气浮装置内流动分析与结构优化[J]. 化工学报, 2024, 75(S1): 223-234. |
| [2] | 谢慧慧, 姜佳鑫, 王鑫, 李正, 郭鑫, 吕欣然, 王凌云, 刘杨. 深共晶溶剂聚合物包覆膜传输分离铂、钯的研究[J]. 化工学报, 2024, 75(S1): 235-243. |
| [3] | 邱知, 谭明. 聚离子液体膜的制备及其在低钠高钾健康酱油中的应用[J]. 化工学报, 2024, 75(S1): 244-250. |
| [4] | 王新月, 徐小虎, 张海洋, 尹春华. 维生素A醋酸酯/环糊精包合及性质研究[J]. 化工学报, 2024, 75(S1): 321-328. |
| [5] | 刘律, 刘洁茹, 范亮亮, 赵亮. 基于层流效应的被动式颗粒分离微流控方法研究[J]. 化工学报, 2024, 75(S1): 67-75. |
| [6] | 吴学红, 韦新, 侯加文, 吕财, 刘勇, 刘鹤, 常志娟. 热解法制备碳纳米管及其在散热涂层中的应用研究[J]. 化工学报, 2024, 75(9): 3360-3368. |
| [7] | 唐昊, 胡定华, 李强, 张轩畅, 韩俊杰. 抗加速度双切线弧流道内气泡动力学行为数值与可视化研究[J]. 化工学报, 2024, 75(9): 3074-3082. |
| [8] | 李彦熹, 王晔春, 谢向东, 王进芝, 王江, 周煜, 潘盈秀, 丁文涛, 郭烈锦. 蜗壳式多通道气液旋流分离器结构优化及分离特性研究[J]. 化工学报, 2024, 75(8): 2875-2885. |
| [9] | 秦晓巧, 谭宏博, 温娜. 储能式低温空分系统热力学与经济性分析[J]. 化工学报, 2024, 75(7): 2409-2421. |
| [10] | 杜海燕, 朱凯, 游峰, 王金凤, 赵一帆, 张楠, 李英. 用于应变传感器的自愈合抗冻离子水凝胶[J]. 化工学报, 2024, 75(7): 2709-2722. |
| [11] | 周文轩, 刘珍, 张福建, 张忠强. 高通量-高截留率时间维度膜法水处理机理研究[J]. 化工学报, 2024, 75(7): 2583-2593. |
| [12] | 张香港, 常玉龙, 汪华林, 江霞. 废弃秸秆等生物质低能耗非相变秒级干燥[J]. 化工学报, 2024, 75(7): 2433-2445. |
| [13] | 罗小平, 侯云天, 范一杰. 逆流相分离结构微细通道流动沸腾传热与均温性[J]. 化工学报, 2024, 75(7): 2474-2485. |
| [14] | 张颂红, 赵欣怡, 楼小玲, 沈绍传, 贠军贤. 阳离子交换纳晶胶分离乳过氧化物酶的研究[J]. 化工学报, 2024, 75(7): 2574-2582. |
| [15] | 张广宇, 付然飞, 孙冰, 袁俊聪, 冯翔, 杨朝合, 徐伟. CO2-环氧丙烷合成碳酸丙烯酯:氢键供体效应研究[J]. 化工学报, 2024, 75(6): 2243-2251. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号