化工学报 ›› 2024, Vol. 75 ›› Issue (12): 4736-4748.DOI: 10.11949/0438-1157.20240635
梁宁1( ), 张守玉1(
), 张守玉1( ), 刘思梦1, 黄健添1, 吕邦勇1, 杨楚轲1, 胡南2, 吴玉新3
), 刘思梦1, 黄健添1, 吕邦勇1, 杨楚轲1, 胡南2, 吴玉新3
收稿日期:2024-06-07
修回日期:2024-07-17
出版日期:2024-12-25
发布日期:2025-01-03
通讯作者:
张守玉
作者简介:梁宁(1996—),女,硕士研究生,1677633263@qq.com
基金资助:
Ning LIANG1( ), Shouyu ZHANG1(
), Shouyu ZHANG1( ), Simeng LIU1, Jiantian HUANG1, Bangyong LYU1, Chuke YANG1, Nan HU2, Yuxin WU3
), Simeng LIU1, Jiantian HUANG1, Bangyong LYU1, Chuke YANG1, Nan HU2, Yuxin WU3
Received:2024-06-07
Revised:2024-07-17
Online:2024-12-25
Published:2025-01-03
Contact:
Shouyu ZHANG
摘要:
使用Materials Studio软件建立并优化纤维素-水模型体系,利用LAMMPS软件对其进行水热过程的分子动力学模拟,研究水热体系内纤维素水热产物的演变规律及其水热反应机理。结果表明:温度是影响纤维素水热产物分布的重要因素。随着水热温度的升高,纤维素分解加剧,体系内水热固相产物相对含量急剧下降,固相产物的芳香性和热值增高,亲水性降低;重质焦油、轻质焦油的相对含量均先增加后降低,焦油二次裂解为小分子反应加剧;随着水热温度的升高,体系内CO2和H2生成量均持续升高,甲酸生成量呈先升高后降低的趋势,甲醛生成量差异较小。模拟结果亦表明H2O分子主要以水合氢离子(H3O+)的形式参与纤维素水热反应,随着水热温度的升高,体系中水分子的电离反应与纤维素的脱水反应均增强。纤维素水热机理分析表明水热过程中纤维素的解聚包括分解为葡萄糖和脱水为左旋葡聚糖两条路径,而葡萄糖单体重排异构过程中会经历烯二醇3种过渡态结构,不同的结构通过脱水、逆羟醛缩合等反应分别生成呋喃类和醇醛类等主要中间产物。对纤维素水热反应机理的分析可为生物质水热转化提供理论指导。
中图分类号:
梁宁, 张守玉, 刘思梦, 黄健添, 吕邦勇, 杨楚轲, 胡南, 吴玉新. 基于ReaxFF力场的纤维素水热过程机理[J]. 化工学报, 2024, 75(12): 4736-4748.
Ning LIANG, Shouyu ZHANG, Simeng LIU, Jiantian HUANG, Bangyong LYU, Chuke YANG, Nan HU, Yuxin WU. Evolution mechanism of cellulose during hydrothermal process based on ReaxFF force field[J]. CIESC Journal, 2024, 75(12): 4736-4748.
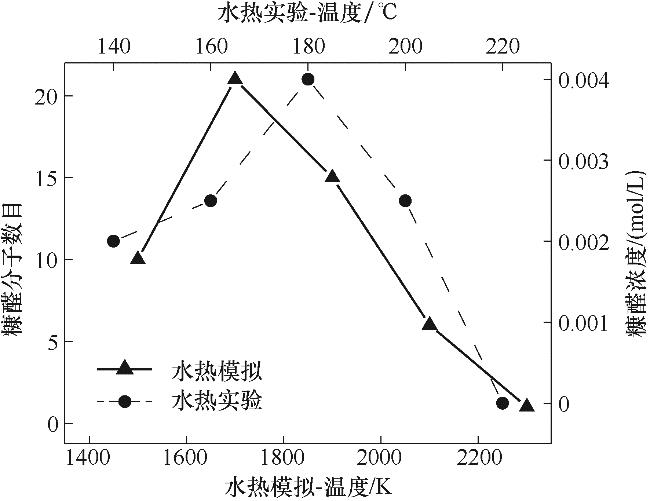
图2 纤维素水热过程糠醛生成量ReaxFF MD模拟与实验数据对比
Fig.2 Comparison between experimental and ReaxFF MD simulation data of furfural production from cellulose during hydrothermal process
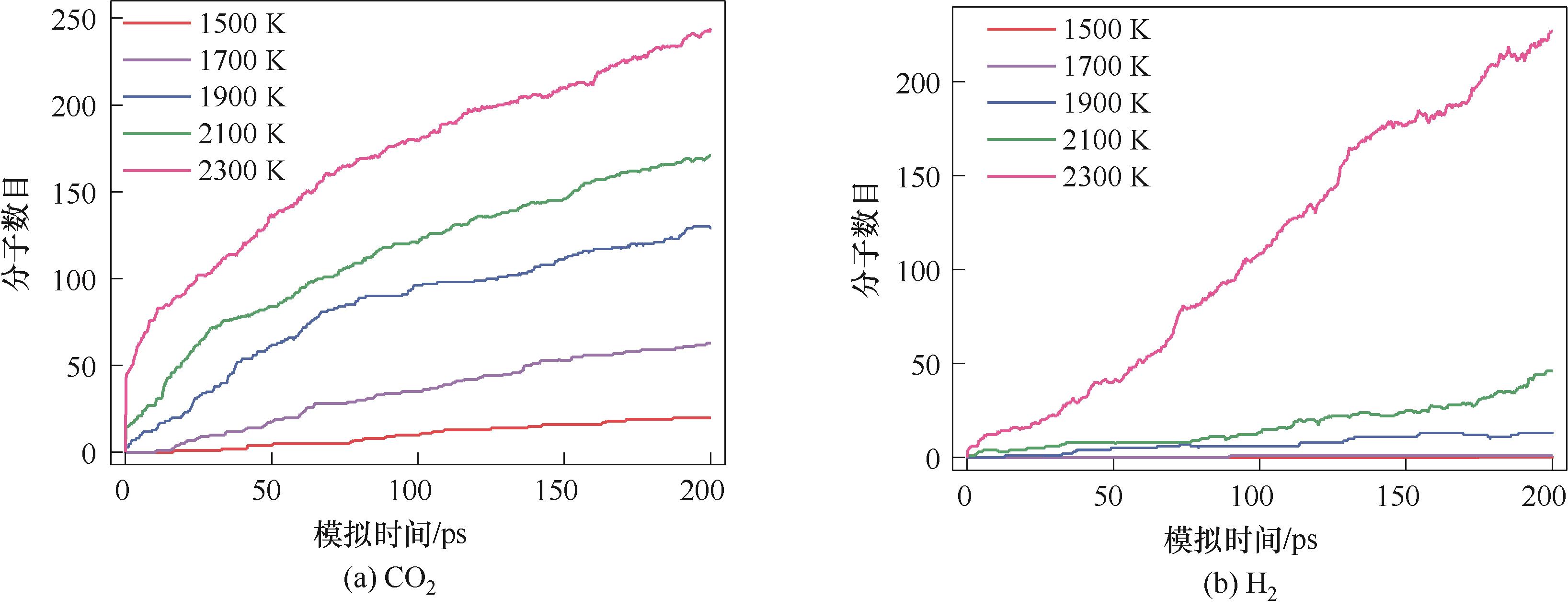
图6 1500~2300 K模拟温度下纤维素水热生成CO2和H2的分子数目
Fig.6 Molecular number of CO2 and H2 from cellulose hydrothermal process at simulated temperature of 1500—2300 K
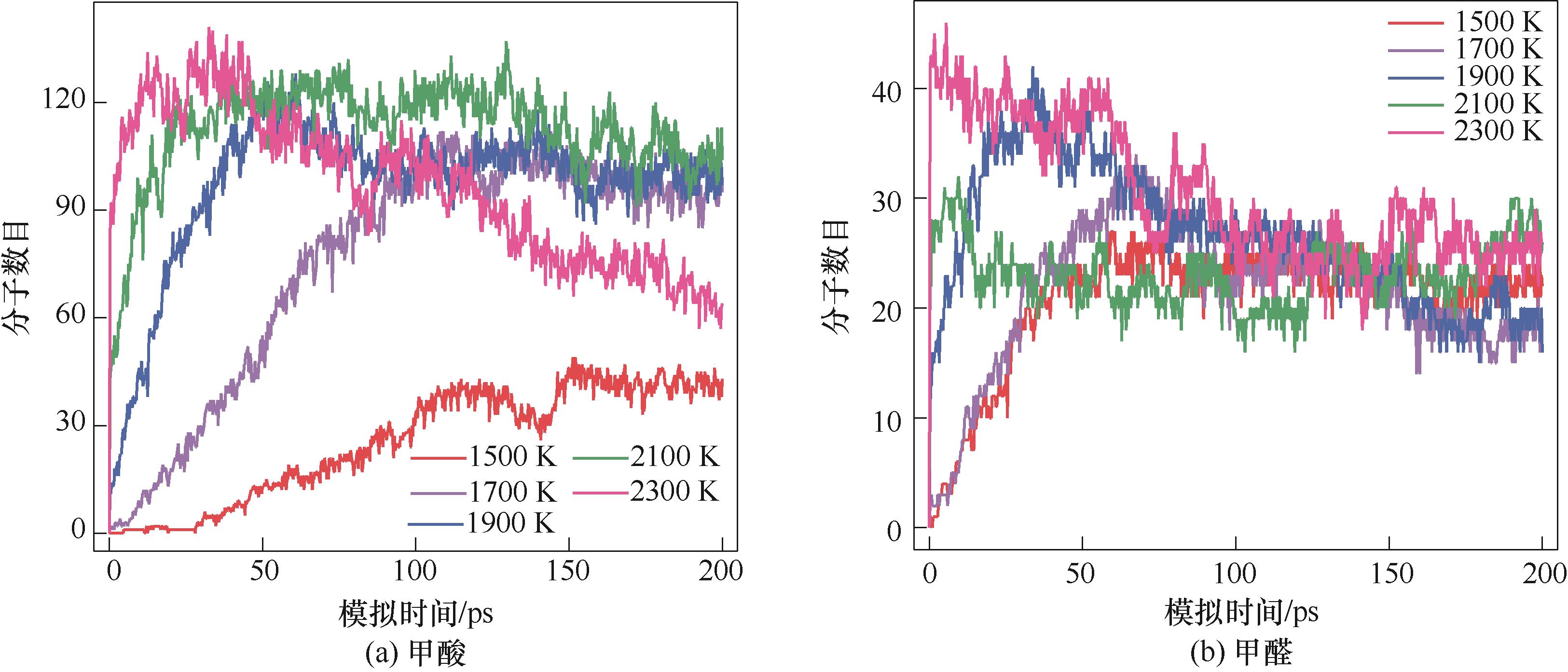
图7 1500~2300 K模拟温度下纤维素水热体系内甲酸、甲醛的分子数目
Fig.7 Molecular number of formic acid and formaldehyde from cellulose hydrothermal process at simulated temperature of 1500—2300 K
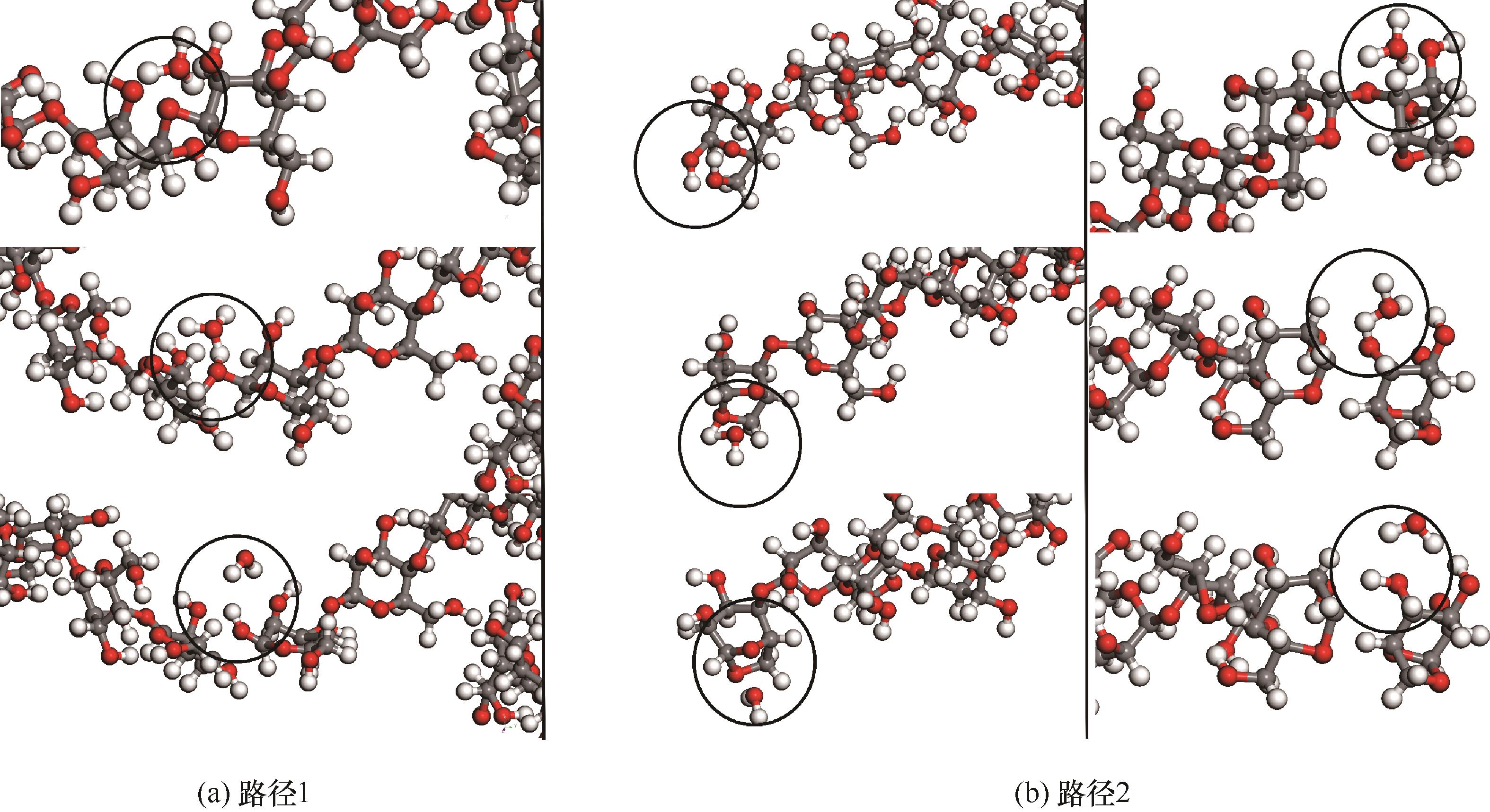
图9 2300 K模拟温度下纤维素分子水热反应路径(灰色:C;白色:H;红色:O)
Fig.9 Hydrothermal reaction path of cellulose molecules at simulated temperature of 2300 K (grey: C; white: H; red: O)
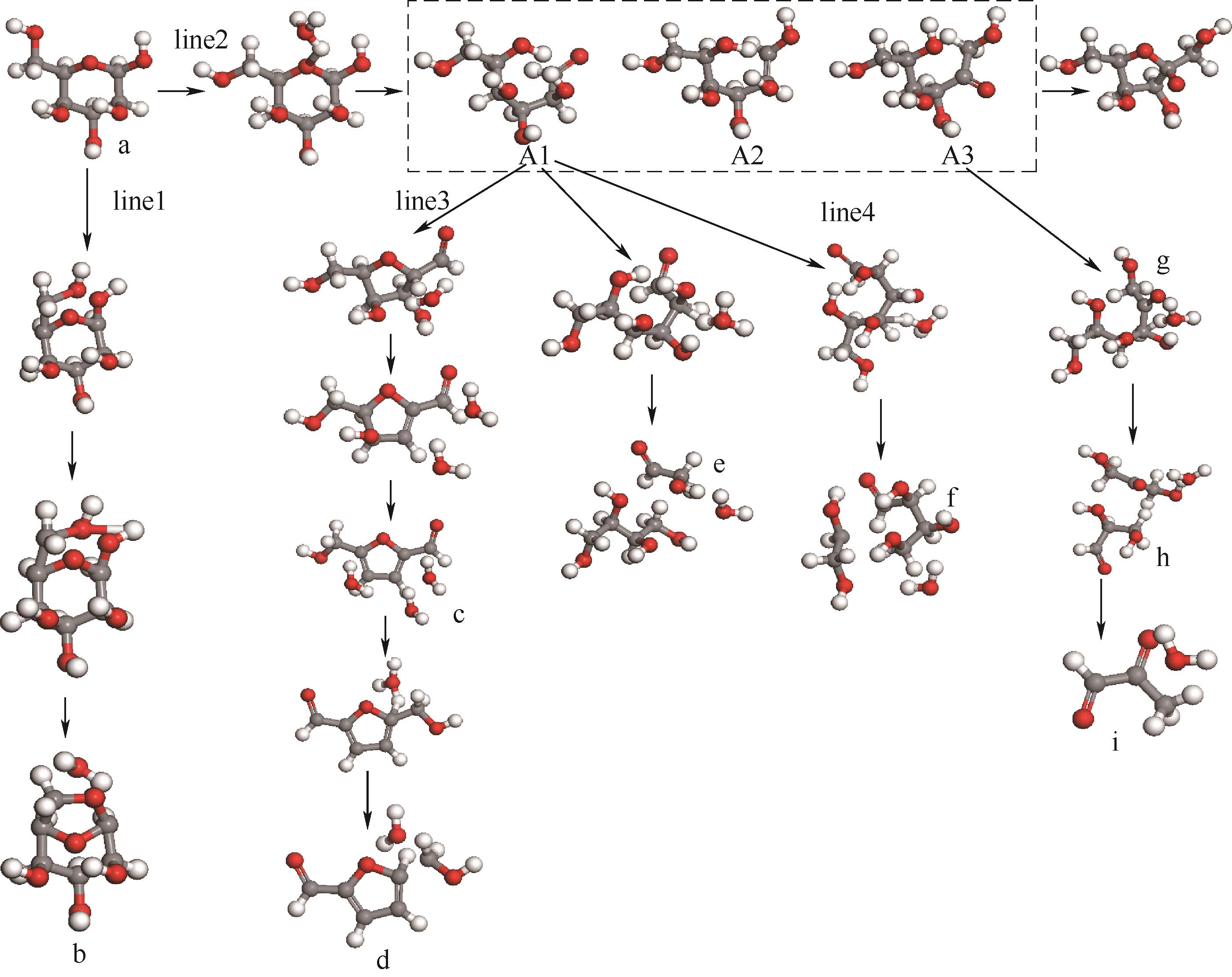
图10 2300 K模拟温度下葡萄糖分子的水热反应路径a—吡喃葡萄糖; b—左旋葡聚糖; c—5-羟甲基糠醛; d—糠醛; e—乙醇醛; f—赤藓糖; g—二羟基丙酮; h—甘油醛; i—丙酮醛
Fig.10 Hydrothermal reaction path of glucose molecules at simulated temperature of 2300 K
| 1 | 何笑, 刘晶晶, 李文瑶, 等. 玉米秸秆化学链热解过程铁基复合载氧体的载氧-催化性能[J]. 化工学报, 2023, 74(10): 4153-4163. |
| He X, Liu J J, Li W Y, et al. Oxygen-carrying and catalytic properties of iron-based composite oxygen carrier for chemical looping pyrolysis of corn stalk[J]. CIESC Journal, 2023, 74(10): 4153-4163. | |
| 2 | 孙梦圆, 张守玉, 王才威, 等. 棉秆水热及水热氧化过程水相产物分析研究[J]. 化工学报, 2020, 71(5): 2382-2388. |
| Sun M Y, Zhang S Y, Wang C W, et al. Aqueous products prepared by hydrothermal and hydrothermal oxidation processes of cotton stalk[J]. CIESC Journal, 2020, 71(5): 2382-2388. | |
| 3 | 杨济凡, 张守玉, 曹忠耀, 等. 水热氧化预处理对棉秆成型颗粒理化性质的影响[J]. 化工进展, 2022, 41(8): 4417-4424. |
| Yang J F, Zhang S Y, Cao Z Y, et al. Effect of hydrothermal oxidation pretreatment on the physicochemical properties of fuel pellets prepared from cotton stalks[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4417-4424. | |
| 4 | 王雪, 徐期勇, 张超. 木质纤维素类生物质水热炭化机理及水热炭应用进展[J]. 化工进展, 2023, 42(5): 2536-2545. |
| Wang X, Xu Q Y, Zhang C. Hydrothermal carbonization of the lignocellulosic biomass and application of the hydro-char[J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2536-2545. | |
| 5 | Xu J Q, Zhang S Y, Shi Y, et al. Upgrading the wood vinegar prepared from the pyrolysis of biomass wastes by hydrothermal pretreatment[J]. Energy, 2022, 244: 122631. |
| 6 | Kruse A, Dinjus E. Hot compressed water as reaction medium and reactant properties and synthesis reactions[J]. The Journal of Supercritical Fluids, 2007, 39(3): 362-380. |
| 7 | Bandura A V, Lvov S N. The ionization constant of water over wide ranges of temperature and density[J]. Journal of Physical and Chemical Reference Data, 2006, 35(1): 15-30. |
| 8 | Wang T F, Zhai Y B, Zhu Y, et al. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: process conditions, fundamentals, and physicochemical properties[J]. Renewable and Sustainable Energy Reviews, 2018, 90: 223-247. |
| 9 | Liu P, Le J W, Zhang D X, et al. Free radical reaction mechanism on improving tar yield and quality derived from lignite after hydrothermal treatment[J]. Fuel, 2017, 207: 244-252. |
| 10 | Zhang B, von Keitz M, Valentas K. Thermal effects on hydrothermal biomass liquefaction[J]. Applied Biochemistry and Biotechnology, 2008, 147(1/2/3): 143-150. |
| 11 | Onda A, Ochi T, Yanagisawa K. Hydrolysis of cellulose selectively into glucose over sulfonated activated-carbon catalyst under hydrothermal conditions[J]. Topics in Catalysis, 2009, 52(6): 801-807. |
| 12 | Yue R Y, An C J, Ye Z B, et al. A pH-responsive phosphoprotein surface washing fluid for cleaning oiled shoreline: performance evaluation, biotoxicity analysis, and molecular dynamic simulation[J]. Chemical Engineering Journal, 2022, 437: 135336. |
| 13 | van Duin A C T. Reactive molecular dynamics modeling and advanced power generation applications[C]// University Turbine Systems Research Meeting. 2010. |
| 14 | 韩君易, 李晓霞, 郭力, 等. ReaxFF MD模拟的物种和化学反应自动分类及可视化[J]. 计算机与应用化学, 2015, 32(5): 519-526. |
| Han J Y, Li X X, Guo L, et al. Automatic classification and visualization of species and reactions obtained from ReaxFF MD simulations[J]. Computers and Applied Chemistry, 2015, 32(5): 519-526. | |
| 15 | Liu X Y, Wang T, Chu J C, et al. Understanding lignin gasification in supercritical water using reactive molecular dynamics simulations[J]. Renewable Energy, 2020, 161: 858-866. |
| 16 | Arvelos S, Abrahão O, Eponina Hori C. ReaxFF molecular dynamics study on the pyrolysis process of cyclohexanone[J]. Journal of Analytical and Applied Pyrolysis, 2019, 141: 104620. |
| 17 | 张守玉, 黄健添, 郎森, 等. 生物质燃料颗粒热压成型过程分析[J]. 煤炭学报, 2024, 49(2): 1123-1137. |
| Zhang S Y, Huang J T, Lang S, et al. Analysis on hot briquetting mechanism of biomass fuel pellets[J]. Journal of China Coal Society, 2024, 49(2): 1123-1137. | |
| 18 | Sorensen M R, Voter A F. Temperature-accelerated dynamics for simulation of infrequent events[J]. The Journal of Chemical Physics, 2000, 112(21): 9599-9606. |
| 19 | Chenoweth K, van Duin A C T, Dasgupta S, et al. Initiation mechanisms and kinetics of pyrolysis and combustion of JP-10 hydrocarbon jet fuel[J]. The Journal of Physical Chemistry A, 2009, 113(9): 1740-1746. |
| 20 | Jiang C H, Liang W, Li K J, et al. A reactive molecular dynamics study of thermal pyrolysis behavior and mechanisms of lignin during the hydrothermal process: the function of the water molecules[J]. Bioresource Technology, 2023, 368: 128338. |
| 21 | Wang G W, Liu J W, Liang W, et al. Hydrothermal carbonization mechanism of agricultural waste under different conditions: an experimental and ReaxFF molecular dynamics study[J]. Journal of the Energy Institute, 2023, 110: 101353. |
| 22 | Ha D T, Tran K Q, Trinh T T. New insights into the hydrothermal carbonization process of sewage sludge: a reactive molecular dynamics study[J]. Fuel, 2024, 361: 130692. |
| 23 | Chen C, Zhao L L, Wu X, et al. Theoretical understanding of coal char oxidation and gasification using reactive molecular dynamics simulation[J]. Fuel, 2020, 260: 116300. |
| 24 | Pitman M C, van Duin A C T. Dynamics of confined reactive water in smectite clay-zeolite composites[J]. Journal of the American Chemical Society, 2012, 134(6): 3042-3053. |
| 25 | 韩修远, 张守玉, 徐嘉庆, 等. 水热过程中杉木屑组分的演变对木醋液的影响[J]. 化工学报, 2023, 74(10): 4311-4318. |
| Han X Y, Zhang S Y, Xu J Q, et al. Effect of the component evolution of Chinese fir sawdust on wood vinegar during hydrothermal process[J]. CIESC Journal, 2023, 74(10): 4311-4318. | |
| 26 | Borrero-López A M, Masson E, Celzard A, et al. Modelling the reactions of cellulose, hemicellulose and lignin submitted to hydrothermal treatment[J]. Industrial Crops and Products, 2018, 124: 919-930. |
| 27 | Fletcher T H, Kerstein A R, Pugmire R J, et al. Chemical percolation model for devolatilization. 3. Direct use of carbon-13NMR data to predict effects of coal type[J]. Energy & Fuels, 1992, 6(4): 414-431. |
| 28 | Zhang K, Li Y, Wang Z H, et al. Pyrolysis behavior of a typical Chinese sub-bituminous Zhundong coal from moderate to high temperatures[J]. Fuel, 2016, 185: 701-708. |
| 29 | Shi L, Liu Q Y, Guo X J, et al. Pyrolysis behavior and bonding information of coal—a TGA study[J]. Fuel Processing Technology, 2013, 108: 125-132. |
| 30 | Zhuang X Z, Zhan H, Song Y P, et al. Insights into the evolution of chemical structures in lignocellulose and non-lignocellulose biowastes during hydrothermal carbonization (HTC)[J]. Fuel, 2019, 236: 960-974. |
| 31 | Berge N D, Ro K S, Mao J D, et al. Hydrothermal carbonization of municipal waste streams[J]. Environmental Science & Technology, 2011, 45(13): 5696-5703. |
| 32 | Chen B L, Johnson E J, Chefetz B, et al. Sorption of polar and nonpolar aromatic organic contaminants by plant cuticular materials: role of polarity and accessibility[J]. Environmental Science & Technology, 2005, 39(16): 6138-6146. |
| 33 | Reza M T, Wirth B, Lüder U, et al. Behavior of selected hydrolyzed and dehydrated products during hydrothermal carbonization of biomass[J]. Bioresource Technology, 2014, 169: 352-361. |
| 34 | Zheng M, Li X X, Wang M J, et al. Dynamic profiles of tar products during Naomaohu coal pyrolysis revealed by large-scale reactive molecular dynamic simulation[J]. Fuel, 2019, 253: 910-920. |
| 35 | Guo S, Gan J Y, Zhao D, et al. Investigation of the hydrothermal carbonization process of furan compounds derived from cellulose using molecular dynamics[J]. Journal of Cleaner Production, 2024, 453: 142252. |
| 36 | Mäkelä M, Benavente V, Fullana A. Hydrothermal carbonization of lignocellulosic biomass: effect of process conditions on hydrochar properties[J]. Applied Energy, 2015, 155: 576-584. |
| 37 | Zheng M, Li X X, Liu J, et al. Initial chemical reaction simulation of coal pyrolysis via ReaxFF molecular dynamics[J]. Energy & Fuels, 2013, 27(6): 2942-2951. |
| 38 | 魏桂花. 纤维素催化氧化制甲酸的反应机理研究[D]. 唐山: 华北理工大学, 2023. |
| Wei G H. Study on reaction mechanism of catalytic oxidation of cellulose to formic acid[D]. Tangshan: North China University of Science and Technology, 2023. | |
| 39 | 汪利平. 纤维素水热降解制备5-羟甲基糠醛的实验研究[D]. 天津: 天津大学, 2006. |
| Wang L P. Experimental study on preparation of 5-hydroxymethylfurfural by hydrothermal degradation of cellulose[D]. Tianjin: Tianjin University, 2006. | |
| 40 | Martins-Vieira J C, Torres-Mayanga P C, Lachos-Perez D. Hydrothermal processing of lignocellulosic biomass: an overview of subcritical and supercritical water hydrolysis[J]. BioEnergy Research, 2023, 16(3): 1296-1317. |
| 41 | Ogihara Y, Smith R L, Inomata H, et al. Direct observation of cellulose dissolution in subcritical and supercritical water over a wide range of water densities (550—1000 kg/m3)[J]. Cellulose, 2005, 12(6): 595-606. |
| 42 | Guo S, Xu D D, Guo X, et al. Theoretical modeling of hydrochar precursor formation during the hydrothermal carbonization of sewage sludge[J]. Fuel Processing Technology, 2022, 231: 107212. |
| 43 | Guo S, Xiao W N, Zhao D, et al. Water-catalyzed conversion of glucose to small molecules during hydrothermal carbonization: a density functional theory study[J]. Sustainable Energy & Fuels, 2023, 7(5): 1322-1332. |
| 44 | Kabyemela B M, Adschiri T, Malaluan R, et al. Degradation kinetics of dihydroxyacetone and glyceraldehyde in subcritical and supercritical water[J]. Industrial & Engineering Chemistry Research, 1997, 36(6): 2025-2030. |
| 45 | Ehara K, Saka S. Decomposition behavior of cellulose in supercritical water, subcritical water, and their combined treatments[J]. Journal of Wood Science, 2005, 51(2): 148-153. |
| 46 | 张亚运. 木质纤维素热化学转化机理及裂解气体CO2和H2吸附分离的分子模拟研究[D]. 重庆: 重庆大学, 2017. |
| Zhang Y Y. Study on thermochemical transformation mechanism of lignocellulose and molecular simulation of adsorption and separation of pyrolysis gases CO2 and H2 [D]. Chongqing: Chongqing University, 2017. | |
| 47 | Sasaki M, Goto K, Tajima K, et al. Rapid and selective retro-aldol condensation of glucose to glycolaldehyde in supercritical water[J]. Green Chemistry, 2002, 4(3): 285-287. |
| [1] | 吴学红, 韦新, 侯加文, 吕财, 刘勇, 刘鹤, 常志娟. 热解法制备碳纳米管及其在散热涂层中的应用研究[J]. 化工学报, 2024, 75(9): 3360-3368. |
| [2] | 赵武灵, 满奕. 基于变分编码器的纳米纤维素分子结构预测模型框架研究[J]. 化工学报, 2024, 75(9): 3221-3230. |
| [3] | 张香港, 常玉龙, 汪华林, 江霞. 废弃秸秆等生物质低能耗非相变秒级干燥[J]. 化工学报, 2024, 75(7): 2433-2445. |
| [4] | 张祎琪, 谭雪松, 李吾环, 张权, 苗长林, 庄新姝. 温和条件下乙二醇苯醚高效分离回收甘蔗渣组分[J]. 化工学报, 2024, 75(6): 2274-2282. |
| [5] | 王文雅, 张玮, 楼小玲, 钟若菲, 陈冰冰, 贠军贤. 纳米纤维素嵌合型晶胶微球的多微管成形与模拟[J]. 化工学报, 2024, 75(5): 2060-2071. |
| [6] | 丁相斐, 丘晓琳, 朱喜成, 张佳伟, 陈锦华. pH响应性气体渗透CNC/PBAT复合膜的制备与性能[J]. 化工学报, 2024, 75(3): 1040-1051. |
| [7] | 董益斌, 熊敬超, 王敬宇, 汪守康, 王亚飞, 黄群星. 融合激光雷达料位测算的锅炉燃烧优化模型预测控制[J]. 化工学报, 2024, 75(3): 924-935. |
| [8] | 郭文显, 张燕, 张云, 邓才智, 石锦煜, 陈妹琼, 张敏, 程发良. 基于活化生物质碳气凝胶的高性能微生物燃料电池[J]. 化工学报, 2024, 75(12): 4793-4803. |
| [9] | 刘根, 孙仲顺, 张博, 张榕江, 吴志强, 杨伯伦. 机器学习驱动的生物质热解模型建立及挥发分化学链重整制氢工艺优化[J]. 化工学报, 2024, 75(11): 4333-4347. |
| [10] | 刘启顺, 褚德育, 马金晶, 尹恒. 几丁质生物质制备含氮生物基化学品进展及挑战[J]. 化工学报, 2024, 75(11): 4065-4081. |
| [11] | 冯咪, 张杰, 吕兴梅. 基于胆碱类离子液体的高纯甲壳素一步提取分离[J]. 化工学报, 2024, 75(11): 4286-4297. |
| [12] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [13] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [14] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| [15] | 吴文涛, 褚良永, 张玲洁, 谭伟民, 沈丽明, 暴宁钟. 腰果酚生物基自愈合微胶囊的高效制备工艺研究[J]. 化工学报, 2023, 74(7): 3103-3115. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号