化工学报 ›› 2023, Vol. 74 ›› Issue (9): 3708-3715.DOI: 10.11949/0438-1157.20230255
郑佳丽1( ), 李志会1,2(
), 李志会1,2( ), 赵新强1, 王延吉1(
), 赵新强1, 王延吉1( )
)
收稿日期:2023-03-17
修回日期:2023-07-24
出版日期:2023-09-25
发布日期:2023-11-20
通讯作者:
李志会,王延吉
作者简介:郑佳丽(1998—),女,博士研究生,2547124405@qq.com
基金资助:
Jiali ZHENG1( ), Zhihui LI1,2(
), Zhihui LI1,2( ), Xinqiang ZHAO1, Yanji WANG1(
), Xinqiang ZHAO1, Yanji WANG1( )
)
Received:2023-03-17
Revised:2023-07-24
Online:2023-09-25
Published:2023-11-20
Contact:
Zhihui LI, Yanji WANG
摘要:
2-氰基呋喃(CF)是一种具有广泛潜在应用的重要生物基衍生物,是合成糠胺、糠酸等产品的关键原料,但目前对于合成2-氰基呋喃的研究甚少。本研究采用离子液体为催化剂,探究了糠醛与离子液体型羟胺盐反应制备2-氰基呋喃的动力学过程。考察了反应温度和反应时间对产物收率的影响,建立了反应动力学模型,对动力学参数进行了有效性验证。实验结果显示:当反应温度为120℃,反应时间为2 h时,糠醛转化率和2-氰基呋喃收率均为100%。通过计算得到了活化能、指前因子及动力学方程,对比了计算值和实验值,发现平均误差为0.34%,从而验证了该反应动力学方程的准确性,为2-氰基呋喃的后续应用提供了理论指导。
中图分类号:
郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715.
Jiali ZHENG, Zhihui LI, Xinqiang ZHAO, Yanji WANG. Kinetics of ionic liquid catalyzed synthesis of 2-cyanofuran[J]. CIESC Journal, 2023, 74(9): 3708-3715.
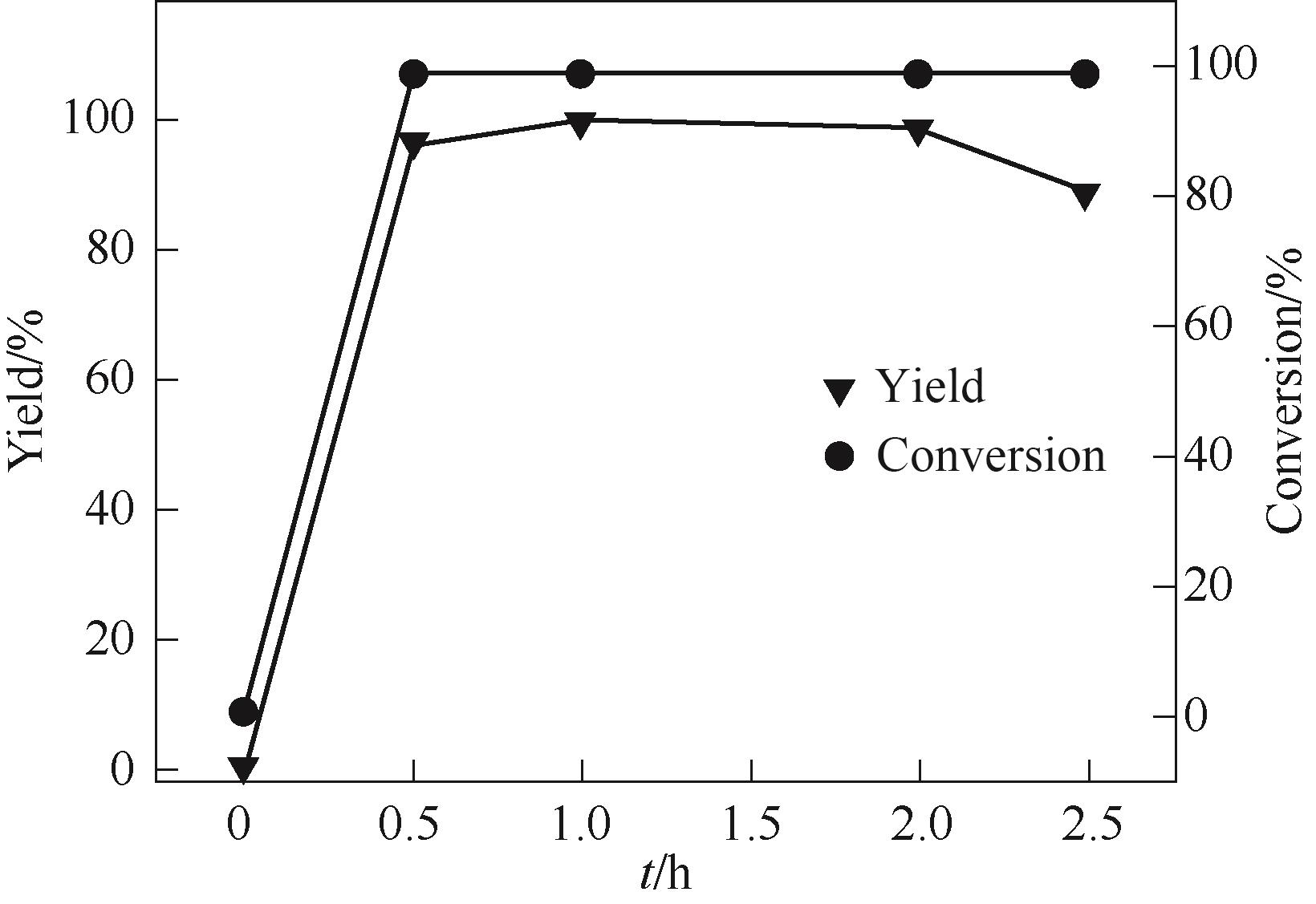
图5 反应时间对2-氰基呋喃合成的影响reaction conditions: FF 3.6 mmol, n (FF)∶n[(NH2OH)2·[HSO3-b-mim]·HSO4] = 1∶1.5, paraxylene 8 ml, [HSO3-b-mim]·HSO4 4 ml, 120℃
Fig.5 Effect of reaction time on synthesis of 2-cyanofuran
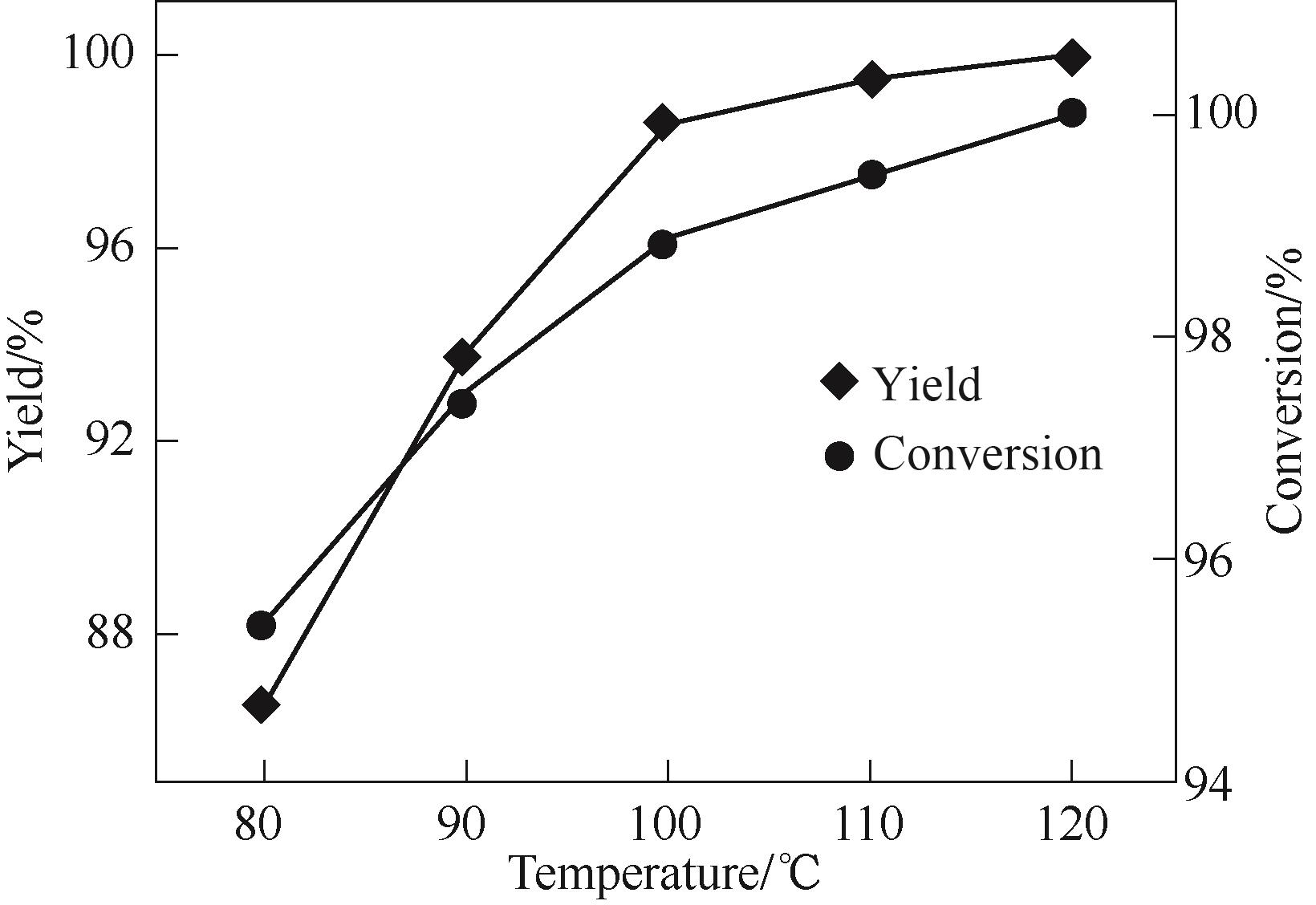
图6 反应温度对2-氰基呋喃合成的影响reaction conditions: FF 3.6 mmol, n (FF)∶n[(NH2OH)2·[HSO3-b-mim]·HSO4] = 1∶1.5, 2 h, paraxylene 8 ml, [HSO3-b-mim]·HSO4 4 ml
Fig.6 Effect of reaction temperature on synthesis of 2-cyanofuran
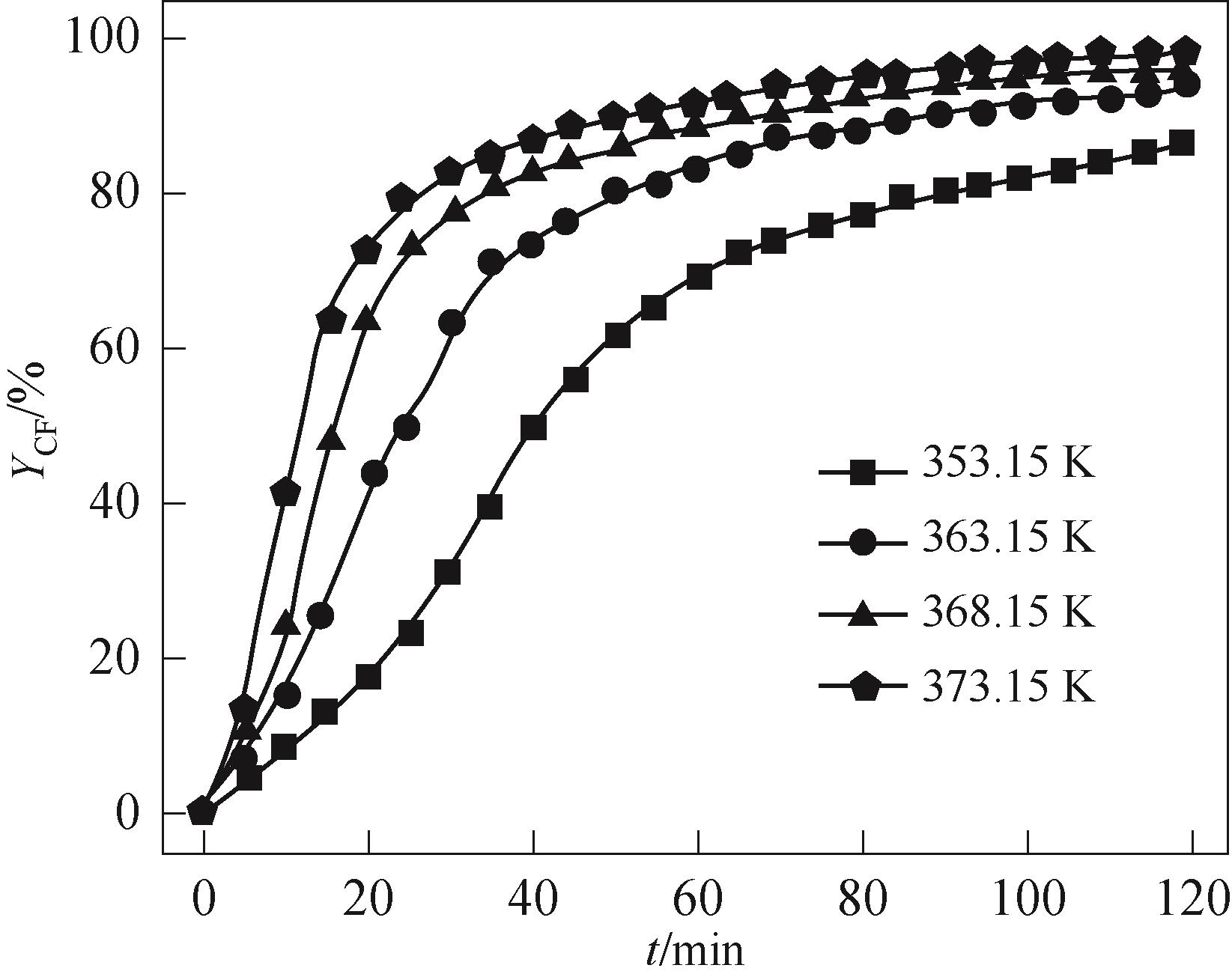
图7 不同温度下的YCF-t曲线reaction conditions: FF 3.6 mmol, n (FF)∶n[(NH2OH)2·[HSO3-b-mim]·HSO4] = 1∶1.5, paraxylene 8 ml, [HSO3-b-mim]·HSO4 4 ml
Fig.7 YCF-t curves at different temperatures

图8 不同温度下的XFF-t曲线reaction conditions: FF 3.6 mmol, n (FF)∶n[(NH2OH)2·[HSO3-b-mim]·HSO4] = 1∶1.5, paraxylene 8 ml, [HSO3-b-mim]·HSO4 4 ml
Fig.8 XFF-t curves at different temperatures
| 温度/K | 反应时间/min | 反应转化率/% | 积分值Ii/(L/mol) |
|---|---|---|---|
| 353.15 | 5 | 16.67 | 0.42 |
| 15 | 36.06 | 1.06 | |
| 25 | 50.49 | 1.73 | |
| 35 | 67.44 | 2.89 | |
| 45 | 75.40 | 3.71 | |
| 65 | 89.20 | 5.46 | |
| 90 | 93.23 | 7.74 | |
| 95 | 94.26 | 8.27 | |
| 363.15 | 5 | 16.30 | 0.41 |
| 15 | 48.87 | 1.64 | |
| 25 | 68.93 | 3.03 | |
| 40 | 80.99 | 4.49 | |
| 50 | 87.43 | 5.77 | |
| 60 | 93.50 | 7.03 | |
| 80 | 95.72 | 9.22 | |
| 85 | 96.09 | 9.52 | |
| 90 | 96.31 | 9.70 | |
| 368.15 | 5 | 24.56 | 0.65 |
| 10 | 44.68 | 1.44 | |
| 15 | 62.23 | 2.47 | |
| 30 | 89.41 | 4.20 | |
| 40 | 94.78 | 5.72 | |
| 50 | 96.18 | 6.40 | |
| 55 | 97.00 | 6.94 | |
| 70 | 97.18 | 9.70 | |
| 80 | 97.65 | 11.18 | |
| 85 | 97.82 | 11.44 | |
| 90 | 97.96 | 11.65 | |
| 373.15 | 5 | 53.48 | 1.51 |
| 10 | 65.37 | 1.81 | |
| 25 | 91.08 | 4.57 | |
| 40 | 96.06 | 6.33 | |
| 45 | 96.40 | 6.52 | |
| 60 | 97.64 | 9.12 | |
| 70 | 97.70 | 11.26 | |
| 75 | 97.90 | 11.56 | |
| 80 | 98.09 | 11.88 | |
| 85 | 98.26 | 12.19 |
表1 不同温度体系下不同时刻反应转化率及积分值Ii
Table 1 Conversion and Ii at different time under different temperature systems
| 温度/K | 反应时间/min | 反应转化率/% | 积分值Ii/(L/mol) |
|---|---|---|---|
| 353.15 | 5 | 16.67 | 0.42 |
| 15 | 36.06 | 1.06 | |
| 25 | 50.49 | 1.73 | |
| 35 | 67.44 | 2.89 | |
| 45 | 75.40 | 3.71 | |
| 65 | 89.20 | 5.46 | |
| 90 | 93.23 | 7.74 | |
| 95 | 94.26 | 8.27 | |
| 363.15 | 5 | 16.30 | 0.41 |
| 15 | 48.87 | 1.64 | |
| 25 | 68.93 | 3.03 | |
| 40 | 80.99 | 4.49 | |
| 50 | 87.43 | 5.77 | |
| 60 | 93.50 | 7.03 | |
| 80 | 95.72 | 9.22 | |
| 85 | 96.09 | 9.52 | |
| 90 | 96.31 | 9.70 | |
| 368.15 | 5 | 24.56 | 0.65 |
| 10 | 44.68 | 1.44 | |
| 15 | 62.23 | 2.47 | |
| 30 | 89.41 | 4.20 | |
| 40 | 94.78 | 5.72 | |
| 50 | 96.18 | 6.40 | |
| 55 | 97.00 | 6.94 | |
| 70 | 97.18 | 9.70 | |
| 80 | 97.65 | 11.18 | |
| 85 | 97.82 | 11.44 | |
| 90 | 97.96 | 11.65 | |
| 373.15 | 5 | 53.48 | 1.51 |
| 10 | 65.37 | 1.81 | |
| 25 | 91.08 | 4.57 | |
| 40 | 96.06 | 6.33 | |
| 45 | 96.40 | 6.52 | |
| 60 | 97.64 | 9.12 | |
| 70 | 97.70 | 11.26 | |
| 75 | 97.90 | 11.56 | |
| 80 | 98.09 | 11.88 | |
| 85 | 98.26 | 12.19 |
| 温度T/K | 拟合方程 | R2 |
|---|---|---|
| 353.15 | y=0.086x | 0.999 |
| 363.15 | y=0.112x | 0.999 |
| 368.15 | y=0.134x | 0.998 |
| 373.15 | y=0.151x | 0.995 |
表2 线性拟合方程
Table 2 Linear fitting equation
| 温度T/K | 拟合方程 | R2 |
|---|---|---|
| 353.15 | y=0.086x | 0.999 |
| 363.15 | y=0.112x | 0.999 |
| 368.15 | y=0.134x | 0.998 |
| 373.15 | y=0.151x | 0.995 |
| 温度T/K | T-1/K-1 | k/(L/(min∙mol)) | lnk |
|---|---|---|---|
| 353.15 | 0.00283 | 0.086 | -2.453 |
| 363.15 | 0.00275 | 0.112 | -2.189 |
| 368.15 | 0.00272 | 0.134 | -2.010 |
| 373.15 | 0.00268 | 0.151 | -1.890 |
表3 不同温度体系下的lnk值
Table 3 lnk value at different temperature systems
| 温度T/K | T-1/K-1 | k/(L/(min∙mol)) | lnk |
|---|---|---|---|
| 353.15 | 0.00283 | 0.086 | -2.453 |
| 363.15 | 0.00275 | 0.112 | -2.189 |
| 368.15 | 0.00272 | 0.134 | -2.010 |
| 373.15 | 0.00268 | 0.151 | -1.890 |
| t/min | Xcal/% | Xexp/% | δ/% |
|---|---|---|---|
| 70 | 98.98 | 99.56 | 0.64 |
| 80 | 99.40 | 99.55 | 0.15 |
| 90 | 99.67 | 99.53 | -0.14 |
| 100 | 99.81 | 99.51 | -0.30 |
| 110 | 99.89 | 99.51 | -0.38 |
| 120 | 99.94 | 99.50 | -0.44 |
表4 110℃下糠醛转化率计算值与实验值的比较
Table 4 Comparison of calculated value and measured value of furfural conversion at 110℃
| t/min | Xcal/% | Xexp/% | δ/% |
|---|---|---|---|
| 70 | 98.98 | 99.56 | 0.64 |
| 80 | 99.40 | 99.55 | 0.15 |
| 90 | 99.67 | 99.53 | -0.14 |
| 100 | 99.81 | 99.51 | -0.30 |
| 110 | 99.89 | 99.51 | -0.38 |
| 120 | 99.94 | 99.50 | -0.44 |
| 1 | 姚倩, 徐禄江, 张颖. 催化快速热解生物质制备高附加值化学品研究进展[J]. 林产化学与工业, 2015, 35(4): 138-144. |
| Yao Q, Xu L J, Zhang Y. Production of high value-added chemicals by catalytic fast pyrolysis of biomass[J]. Chemistry and Industry of Forest Products, 2015, 35(4): 138-144. | |
| 2 | Isikgor F H, Becer C R. Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers[J]. Polymer Chemistry, 2015, 6(25): 4497-4559. |
| 3 | 石元春. 我国生物质能源发展综述[J]. 智慧电力, 2017, 45(7): 1-5, 42. |
| Shi Y C. Overview of biomass energy development in China[J]. Smart Power, 2017, 45(7): 1-5, 42. | |
| 4 | Fan L L, Ruan R, Li J, et al. Aromatics production from fast co-pyrolysis of lignin and waste cooking oil catalyzed by HZSM-5 zeolite[J]. Applied Energy, 2020, 263: 114629. |
| 5 | 郭海军, 张海荣, 丁帅, 等. 木质纤维素多元醇液化及液化产物提质的研究进展[J]. 化工学报, 2021, 72(6): 3228-3238. |
| Guo H J, Zhang H R, Ding S, et al. Research progress on lignocellulose liquefaction in polyhydric alcohol and upgrading of liquefaction product[J]. CIESC Journal, 2021, 72(6): 3228-3238. | |
| 6 | He Y F, Bie Y W, Lehtonen J, et al. Hydrodeoxygenation of guaiacol as a model compound of lignin-derived pyrolysis bio-oil over zirconia-supported Rh catalyst: process optimization and reaction kinetics[J]. Fuel, 2019, 239: 1015-1027. |
| 7 | 刘长松. 碳中和的科学内涵、建设路径与政策措施[J]. 阅江学刊, 2021, 13(2): 48-60, 121. |
| Liu C S. Scientific connotation, construction path and policy measures of carbon neutrality[J]. Yuejiang Academic Journal, 2021, 13(2): 48-60, 121. | |
| 8 | 舟丹. 天然气在实现碳达峰、碳中和目标中的作用[J]. 中外能源, 2021, 26(4): 68. |
| Zhou D. The role of natural gas in achieving carbon peaking and carbon neutrality[J]. Sino-Global Energy, 2021, 26(4): 68. | |
| 9 | 柴麒敏. 积极推进"碳达峰"行动与"碳中和"国家建设[J]. 中国机关后勤, 2021 (4): 34-35. |
| Chai Q M. Actively promote the "carbon peak" action and "carbon neutral" national construction[J]. Chinese Government General Services, 2021(4): 34-35. | |
| 10 | Yao Q, Xu L J, Han Z, et al. Production of indoles via thermo-catalytic conversion and ammonization of bio-derived furfural[J]. Chemical Engineering Journal, 2015, 280: 74-81. |
| 11 | 欧阳洪生, 肖竹钱, 蒋成君, 等. 生物质基平台化合物糠醛的研究进展[J]. 应用化工, 2014, 43(10): 1903-1907. |
| Ouyang H S, Xiao Z Q, Jiang C J, et al. Advances in bio-based platform chemical-furfural[J]. Applied Chemical Industry, 2014, 43(10): 1903-1907. | |
| 12 | 张军, 胡升, 顾菁, 等. 甲醇体系电镀污泥衍生磁性多金属材料催化糠醛加氢转化[J]. 化工学报, 2022, 73(7): 2996-3006. |
| Zhang J, Hu S, Gu J, et al. Catalytic hydrogenation of furfural over magnetic polymetallic materials derived from electroplating sludge in methanol[J]. CIESC Journal, 2022, 73(7): 2996-3006. | |
| 13 | Mariscal R, Maireles-Torres P, Ojeda M, et al. Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels[J]. Energy & Environmental Science, 2016, 9(4): 1144-1189. |
| 14 | Peleteiro S, Rivas S, Alonso J L, et al. Furfural production using ionic liquids: a review[J]. Bioresource Technology, 2016, 202: 181-191. |
| 15 | Korepp C, Santner H J, Fujii T, et al. 2-Cyanofuran—a novel vinylene electrolyte additive for PC-based electrolytes in lithium-ion batteries[J]. Journal of Power Sources, 2006, 158(1): 578-582. |
| 16 | Jennings T J. Process for preparing furonitrile: US3260731[P]. 1966-07-12. |
| 17 | 黄志荣, 贾玉香, 胡伟峰, 等. 一种2-腈基呋喃的制备方法: 100999509[P]. 2007-07-18. |
| Huang Z R, Jia Y X, Hu W F, et al. The invention relates to a preparation method of 2-nitrile-furan: 100999509[P]. 2007-07-18. | |
| 18 | Li Z H, Yang Q S, Qi X D, et al. A novel hydroxylamine ionic liquid salt resulting from the stabilization of NH2OH by a SO3H-functionalized ionic liquid[J]. Chemical Communications, 2015, 51(10): 1930-1932. |
| 19 | Zhang S X, Zheng J L, Li Z H, et al. A green catalytic reaction system for the synthesis 5-amino-1-pentanol with furfural and ionic liquid hydroxylamine salt as the initial raw material[J]. Molecular Catalysis, 2023, 538: 112995. |
| 20 | 徐浩, 李洋, 夏成康, 等. 吡啶硫酸氢盐离子液体催化甘油与乙酸酯化反应动力学[J]. 化工学报, 2020, 71(11): 5178-5187. |
| Xu H, Li Y, Xia C K, et al. Kinetics of esterification of glycerol with acetic acid catalyzed by pyridine bisulfate ionic liquid[J]. CIESC Journal, 2020, 71(11): 5178-5187. | |
| 21 | 孙晓波, 黄强, 任珂, 等. 以磷钨酸为催化剂的丁二酸二丁酯的合成及其动力学研究[J]. 高校化学工程学报, 2007, 21(4): 627-632. |
| Sun X B, Huang Q, Ren K, et al. Study on synthesis of dibutyl succinate catalyzed by phosphtungstic acid and its reaction kinetics[J]. Journal of Chemical Engineering of Chinese Universities, 2007, 21(4): 627-632. | |
| 22 | Li Z H, Yang Q S, Gao L Y, et al. Reactivity of hydroxylamine ionic liquid salts in the direct synthesis of caprolactam from cyclohexanone under mild conditions[J]. RSC Advances, 2016, 6(87): 83619-83625. |
| 23 | Xu Y Y, Li Z H, Gao L Y, et al. An integrated process for the synthesis of solid hydroxylamine salt with ammonia and hydrogen peroxide as raw materials[J]. Industrial & Engineering Chemistry Research, 2015, 54(3): 1068-1073. |
| 24 | 李志会. 环境友好离子液体型羟胺盐的设计、制备及其在清洁合成反应中的应用[D]. 天津: 河北工业大学, 2017. |
| Li Z H. Design, preparation and application of environmentally friendly ionic liquid hydroxylamine salt in clean synthesis reaction[D]. Tianjin: Hebei University of Technology, 2017. | |
| 25 | Zheng J L, Li Z H, Zhang D S, et al. Thermal decomposition behavior, thermal stability and thermal explosion risk evaluation of a novel green hydroxylamine ionic liquid salt[J]. Journal of Molecular Liquids, 2022, 348: 118407. |
| 26 | Levinthal M L, Willer R L, Park D J, et al. Process for making high purity hydroxylammonium nitrate: US5266290[P]. 1993-11-30. |
| 27 | 王延吉, 李志会, 张东升, 等. 一种离子液体型羟胺盐的制备方法: 103539742A[P]. 2014-01-29. |
| Wang Y J, Li Z H, Zhang D S, et al. The invention relates to a preparation method of ionic liquid hydroxylamine salt: 103539742A[P]. 2014-01-29. | |
| 28 | Li Z H, Wang T T, Qi X D, et al. Green synthesis of benzonitrile using ionic liquid with multiple roles as the recycling agent[J]. RSC Advances, 2019, 9(31): 17631-17638. |
| 29 | Gao X, Li Z H, Zhang D S, et al. Synthesis and kinetics of 2, 5-dicyanofuran in the presence of hydroxylamine ionic liquid salts[J]. Chinese Journal of Chemical Engineering, 2023, 53: 310-316. |
| 30 | 衣思敏, 马亚丽, 刘伟强, 等. 微晶菱镁矿蒸氨及水化动力学研究[J]. 化工学报, 2023, 74(4): 1578-1586. |
| Yi S M, Ma Y L, Liu W Q, et al. Study on ammonia evaporation and hydration kinetics of microcrystalline magnesite[J]. CIESC Journal, 2023, 74(4): 1578-1586. | |
| 31 | 胡晗, 杨亮, 李春晓, 等. 天然烟浸滤液水合物法储甲烷动力学研究[J]. 化工学报, 2023, 74(3): 1313-1321. |
| Hu H, Yang L, Li C X, et al. Kinetics of methane storage in the natural tobacco leaching filtrate in the hydrate form[J]. CIESC Journal, 2023, 74(3): 1313-1321. |
| [1] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [2] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [3] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [4] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [5] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [6] | 肖明堃, 杨光, 黄永华, 吴静怡. 浸没孔液氧气泡动力学数值研究[J]. 化工学报, 2023, 74(S1): 87-95. |
| [7] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [8] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [9] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [10] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [11] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [12] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [13] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [14] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [15] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号