化工学报 ›› 2025, Vol. 76 ›› Issue (5): 2279-2293.DOI: 10.11949/0438-1157.20241210
唐磊( ), 王振菲, 李聪利, 杨佳辉, 郑浩, 石琪(
), 王振菲, 李聪利, 杨佳辉, 郑浩, 石琪( ), 董晋湘
), 董晋湘
收稿日期:2024-10-31
修回日期:2024-11-30
出版日期:2025-05-25
发布日期:2025-06-13
通讯作者:
石琪
作者简介:唐磊(1997—),男,硕士研究生,1193580514@qq.com
基金资助:
Lei TANG( ), Zhenfei WANG, Congli LI, Jiahui YANG, Hao ZHENG, Qi SHI(
), Zhenfei WANG, Congli LI, Jiahui YANG, Hao ZHENG, Qi SHI( ), Jinxiang DONG
), Jinxiang DONG
Received:2024-10-31
Revised:2024-11-30
Online:2025-05-25
Published:2025-06-13
Contact:
Qi SHI
摘要:
Co-MOF-74和Mg-MOF-74分别代表具有强和弱CO结合位点的材料,其常温常压下的CO吸附容量不能评估其在CO/N2变压吸附过程的适用性。通过不同操作条件下的单组分静态和双组分动态穿透实验,研究Co-MOF-74和Mg-MOF-74的CO工作吸附容量、再生性和吸附热(Qst)及其对应的操作温度和吸-脱附压力。结果表明,当CO/N2组成为50%/50%时,Co-MOF-74的最佳操作条件为100℃、3.0~0.2 bar(吸附-解吸总压),CO工作吸附容量和再生性分别为2.85 mmol·g-1和83.82%;而Mg-MOF-74为25℃、2.0~0.2 bar,CO工作吸附容量和再生性分别为1.63 mmol·g-1和79.51%。Co-MOF-74在100℃下的Qst(34.57 kJ·mol-1)与Mg-MOF-74在25℃下的Qst(35.45 kJ·mol-1)相近,表明具有不同结合位点的吸附剂对CO的Qst在35.00 kJ·mol-1左右所对应的温度为最佳操作温度。研究结果可以为具有不同CO结合位点的吸附剂的变压吸附工艺设计提供参考。
中图分类号:
唐磊, 王振菲, 李聪利, 杨佳辉, 郑浩, 石琪, 董晋湘. Co-MOF-74和Mg-MOF-74的CO工作吸附容量及操作条件[J]. 化工学报, 2025, 76(5): 2279-2293.
Lei TANG, Zhenfei WANG, Congli LI, Jiahui YANG, Hao ZHENG, Qi SHI, Jinxiang DONG. CO working capacity and operating conditions of Co-MOF-74 and Mg-MOF-74[J]. CIESC Journal, 2025, 76(5): 2279-2293.
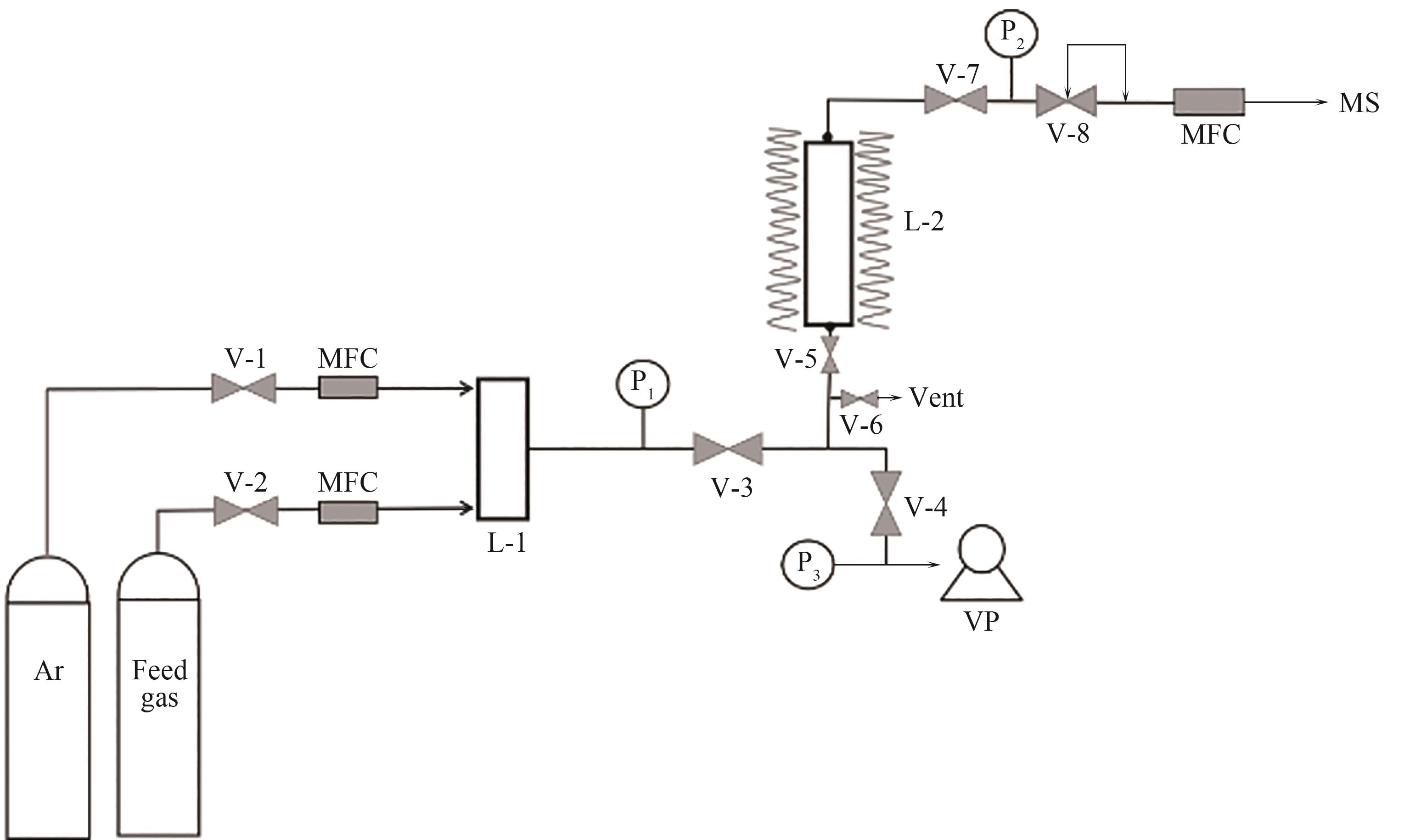
图1 测试CO/N2穿透曲线的装置示意图V-1,V-2,V-3,V-4,V-5,V-6,V-7—球阀;V-8—背压调节器;MFC—质量流量控制器;L-1—混合罐;L-2—吸附柱;VP—真空泵;MS—质谱分析仪;P1—吸附柱前压力表;P2—吸附柱后压力表;P3—真空泵前压力表;Vent—排空
Fig.1 Diagram of device for testing CO/N2 breakthrough curve
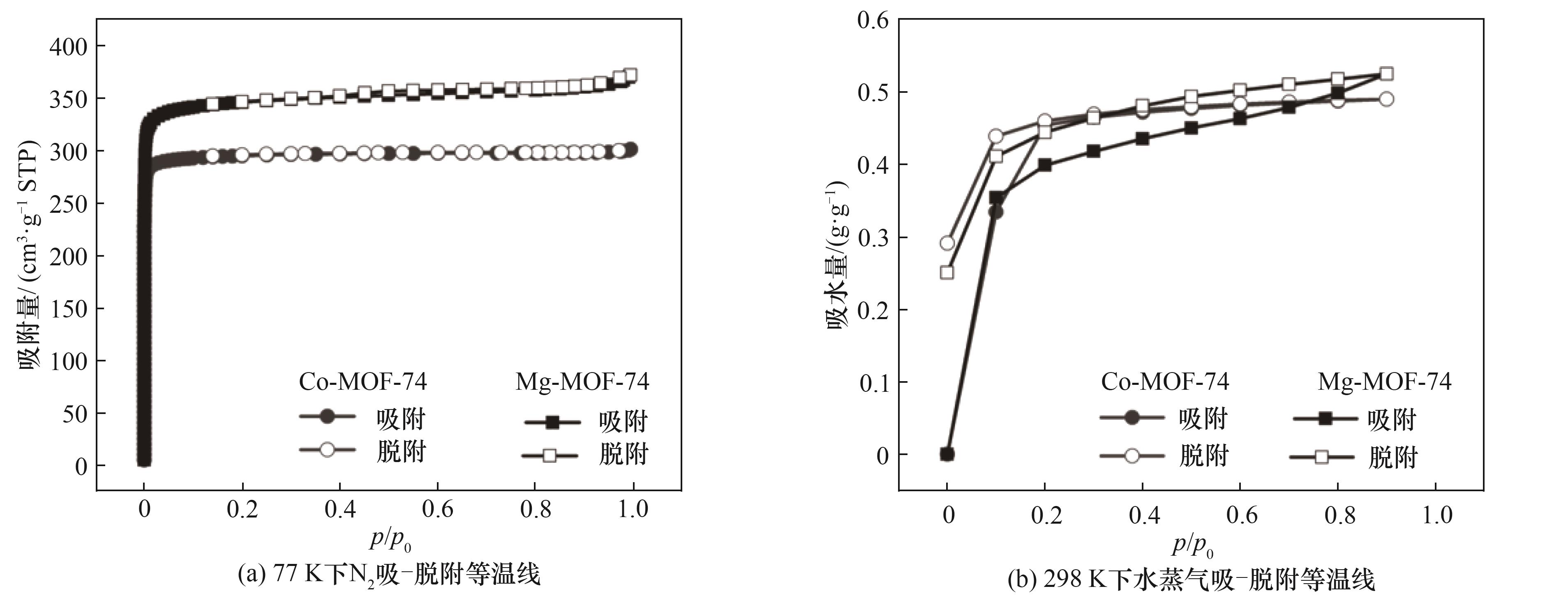
图5 Co-MOF-74和Mg-MOF-74样品的N2吸-脱附等温线(77 K)和水蒸气吸-脱附等温线(298 K)
Fig.5 N2 adsorption-desorption isotherms (77 K) and water vapor adsorption-desorption isotherms (298 K) of Co-MOF-74 and Mg-MOF-74
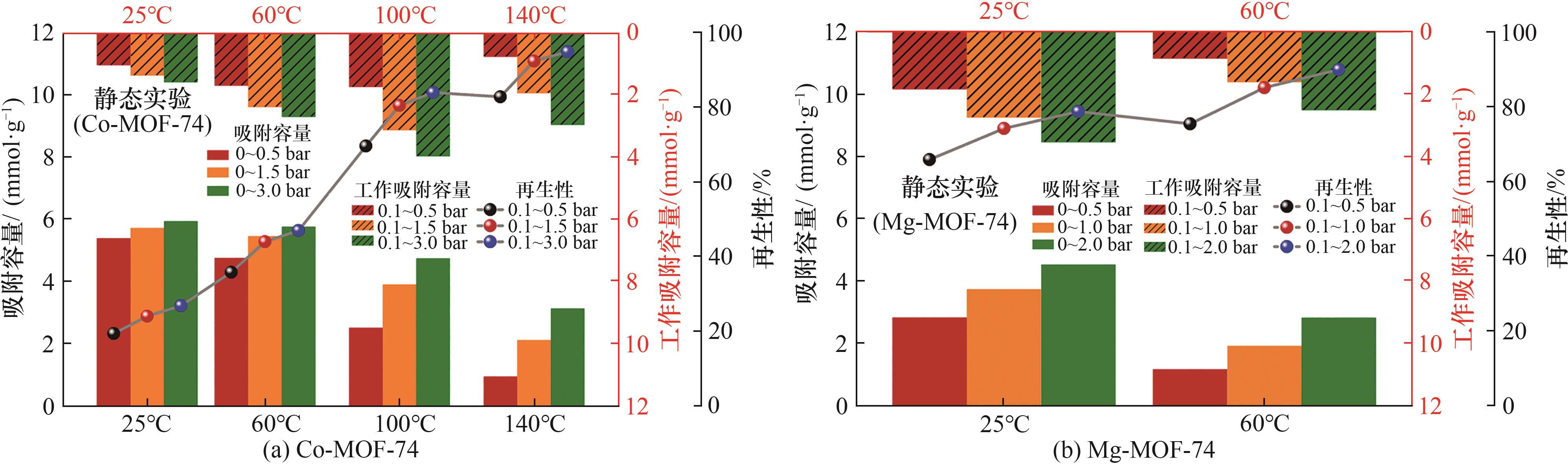
图8 根据两种样品CO吸附等温线计算出的CO吸附容量、工作吸附容量和再生性
Fig.8 CO adsorption capacity, working capacity and regenerability of two samples were calculated based on CO adsorption isotherms
| 样品 | T/℃ | 吸附容量 | 工作吸附容量 | R/% | ||||
|---|---|---|---|---|---|---|---|---|
| p/bar | nCO/(mmol·g-1) | p/bar | ΔnCO/(mmol·g-1) | |||||
| 解吸 | 吸附 | 解吸 | 吸附 | |||||
| Co-MOF-74 | 25 | 0 | 0.5 | 5.38 | 0.1 | 0.5 | 1.04 | 19.33 |
| 60 | 0 | 0.5 | 4.75 | 0.1 | 0.5 | 1.70 | 35.79 | |
| 100 | 0 | 0.5 | 2.50 | 0.1 | 0.5 | 1.74 | 69.60 | |
| 140 | 0 | 0.5 | 0.93 | 0.1 | 0.5 | 0.77 | 82.80 | |
| 25 | 0 | 1.5 | 5.71 | 0.1 | 1.5 | 1.37 | 23.99 | |
| 60 | 0 | 1.5 | 5.44 | 0.1 | 1.5 | 2.39 | 43.93 | |
| 100 | 0 | 1.5 | 3.89 | 0.1 | 1.5 | 3.13 | 80.46 | |
| 140 | 0 | 1.5 | 2.10 | 0.1 | 1.5 | 1.94 | 92.38 | |
| 25 | 0 | 3.0 | 5.93 | 0.1 | 3.0 | 1.59 | 26.81 | |
| 60 | 0 | 3.0 | 5.75 | 0.1 | 3.0 | 2.70 | 46.96 | |
| 100 | 0 | 3.0 | 4.73 | 0.1 | 3.0 | 3.97 | 83.93 | |
| 140 | 0 | 3.0 | 3.12 | 0.1 | 3.0 | 2.96 | 94.87 | |
| Mg-MOF-74 | 25 | 0 | 0.5 | 2.83 | 0.1 | 0.5 | 1.86 | 65.72 |
| 60 | 0 | 0.5 | 1.17 | 0.1 | 0.5 | 0.88 | 75.21 | |
| 25 | 0 | 1.0 | 3.73 | 0.1 | 1.0 | 2.76 | 73.99 | |
| 60 | 0 | 1.0 | 1.92 | 0.1 | 1.0 | 1.63 | 84.90 | |
| 25 | 0 | 2.0 | 4.52 | 0.1 | 2.0 | 3.55 | 78.54 | |
| 60 | 0 | 2.0 | 2.82 | 0.1 | 2.0 | 2.53 | 89.72 | |
表1 Co-MOF-74和Mg-MOF-74的CO静态吸附容量、工作吸附容量和再生性总结
Table 1 Summary of static adsorption capacity, working capacity and regenerability of CO for Co-MOF-74 and Mg-MOF-74
| 样品 | T/℃ | 吸附容量 | 工作吸附容量 | R/% | ||||
|---|---|---|---|---|---|---|---|---|
| p/bar | nCO/(mmol·g-1) | p/bar | ΔnCO/(mmol·g-1) | |||||
| 解吸 | 吸附 | 解吸 | 吸附 | |||||
| Co-MOF-74 | 25 | 0 | 0.5 | 5.38 | 0.1 | 0.5 | 1.04 | 19.33 |
| 60 | 0 | 0.5 | 4.75 | 0.1 | 0.5 | 1.70 | 35.79 | |
| 100 | 0 | 0.5 | 2.50 | 0.1 | 0.5 | 1.74 | 69.60 | |
| 140 | 0 | 0.5 | 0.93 | 0.1 | 0.5 | 0.77 | 82.80 | |
| 25 | 0 | 1.5 | 5.71 | 0.1 | 1.5 | 1.37 | 23.99 | |
| 60 | 0 | 1.5 | 5.44 | 0.1 | 1.5 | 2.39 | 43.93 | |
| 100 | 0 | 1.5 | 3.89 | 0.1 | 1.5 | 3.13 | 80.46 | |
| 140 | 0 | 1.5 | 2.10 | 0.1 | 1.5 | 1.94 | 92.38 | |
| 25 | 0 | 3.0 | 5.93 | 0.1 | 3.0 | 1.59 | 26.81 | |
| 60 | 0 | 3.0 | 5.75 | 0.1 | 3.0 | 2.70 | 46.96 | |
| 100 | 0 | 3.0 | 4.73 | 0.1 | 3.0 | 3.97 | 83.93 | |
| 140 | 0 | 3.0 | 3.12 | 0.1 | 3.0 | 2.96 | 94.87 | |
| Mg-MOF-74 | 25 | 0 | 0.5 | 2.83 | 0.1 | 0.5 | 1.86 | 65.72 |
| 60 | 0 | 0.5 | 1.17 | 0.1 | 0.5 | 0.88 | 75.21 | |
| 25 | 0 | 1.0 | 3.73 | 0.1 | 1.0 | 2.76 | 73.99 | |
| 60 | 0 | 1.0 | 1.92 | 0.1 | 1.0 | 1.63 | 84.90 | |
| 25 | 0 | 2.0 | 4.52 | 0.1 | 2.0 | 3.55 | 78.54 | |
| 60 | 0 | 2.0 | 2.82 | 0.1 | 2.0 | 2.53 | 89.72 | |
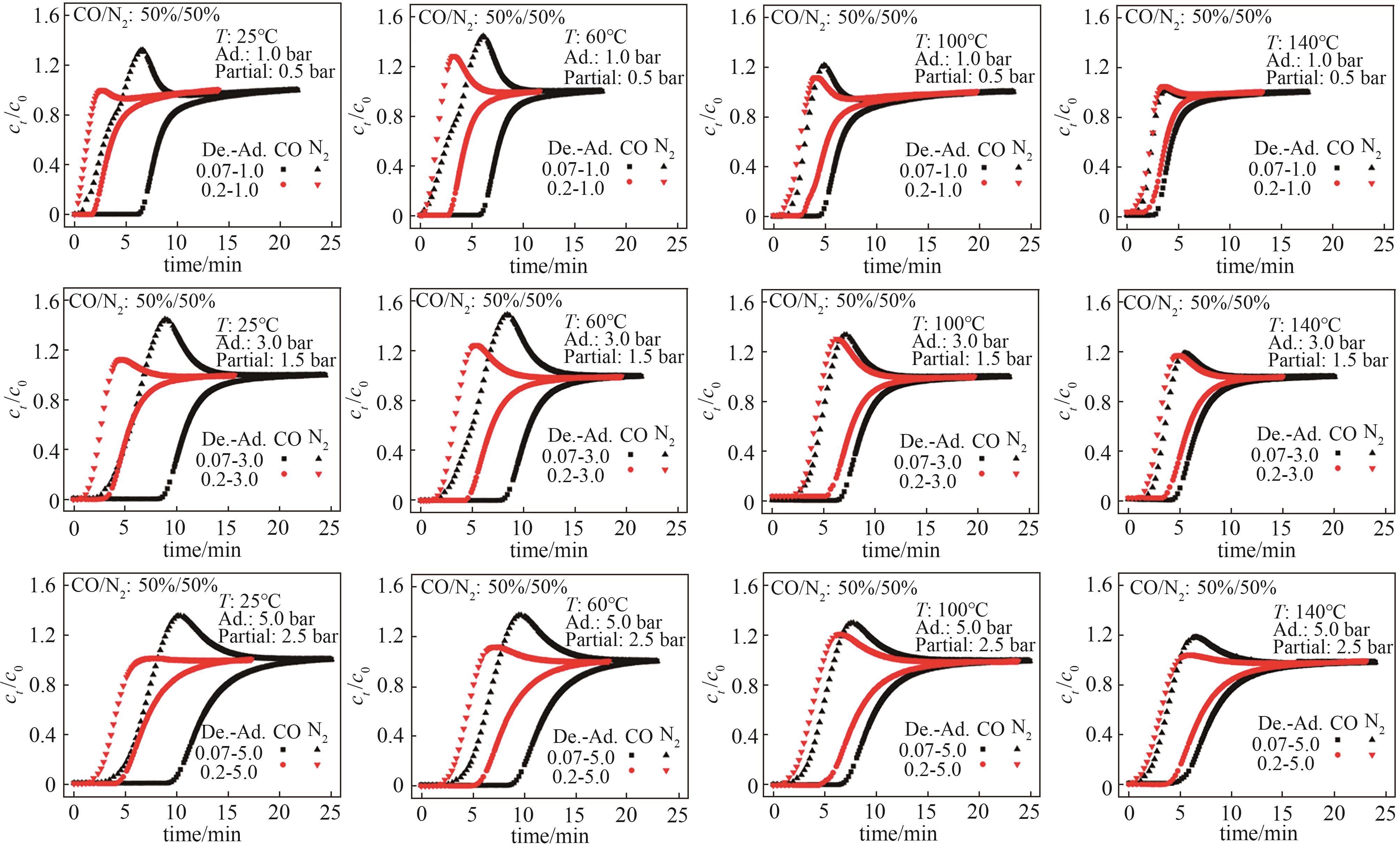
图10 Co-MOF-74的CO/N2(体积比50%/50%)穿透曲线T—操作温度;Partial—CO吸附分压;Ad.—吸附总压;De.—解吸总压
Fig.10 CO/N2 (50%/50%, vol) breakthrough curves of Co-MOF-74T—operating temperature; Partial—CO adsorption partial pressure; Ad.—total adsorption pressure; De.—total desorption pressure
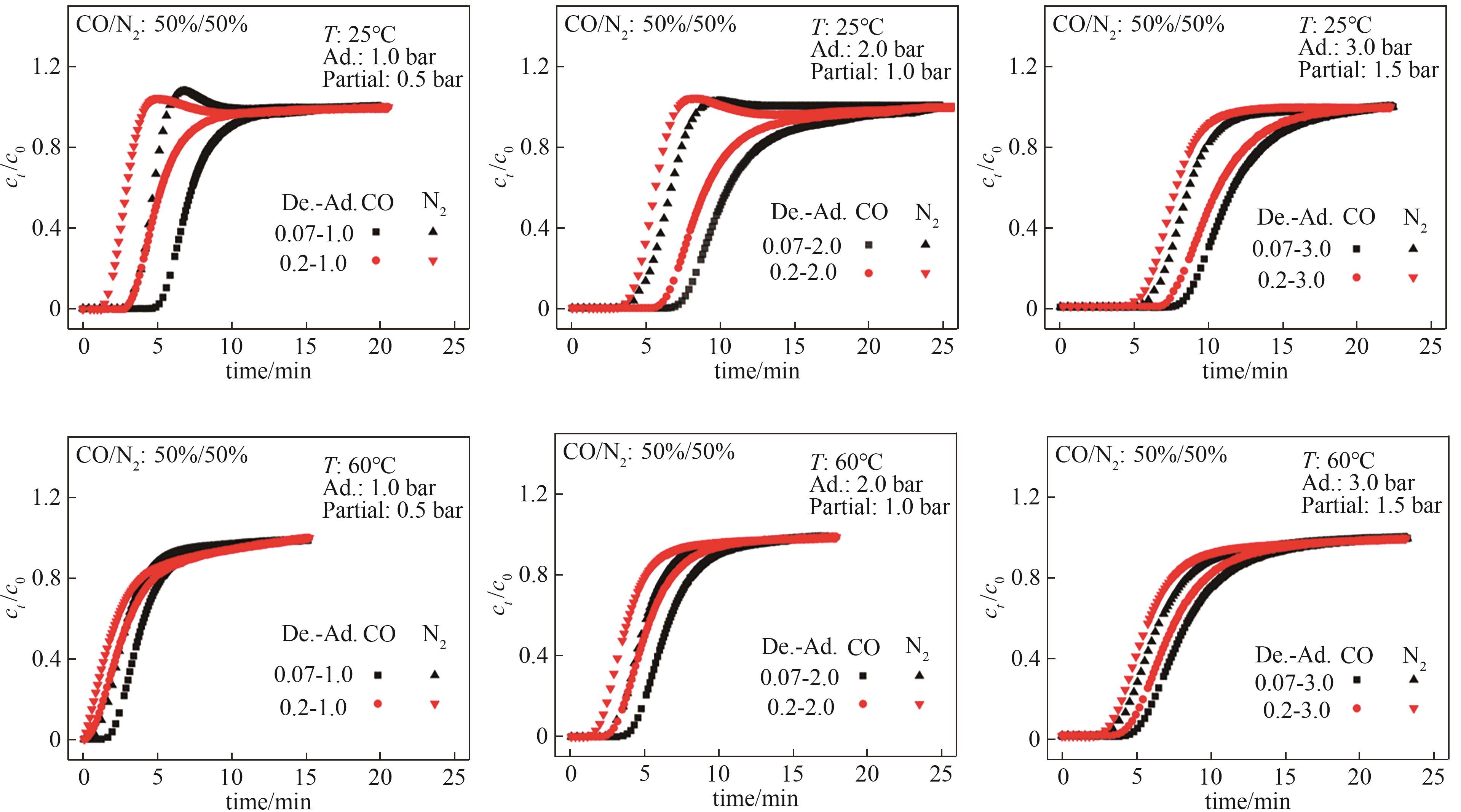
图11 Mg-MOF-74的CO/N2(体积比50%/50%)穿透曲线T—操作温度;Partial—CO吸附分压;Ad.—吸附总压;De.—解吸总压
Fig.11 CO/N2 (50%/50%, vol) breakthrough curves of Mg-MOF-74T—operating temperature; Partial—CO adsorption partial pressure; Ad.—total adsorption pressure; De.—total desorption pressure
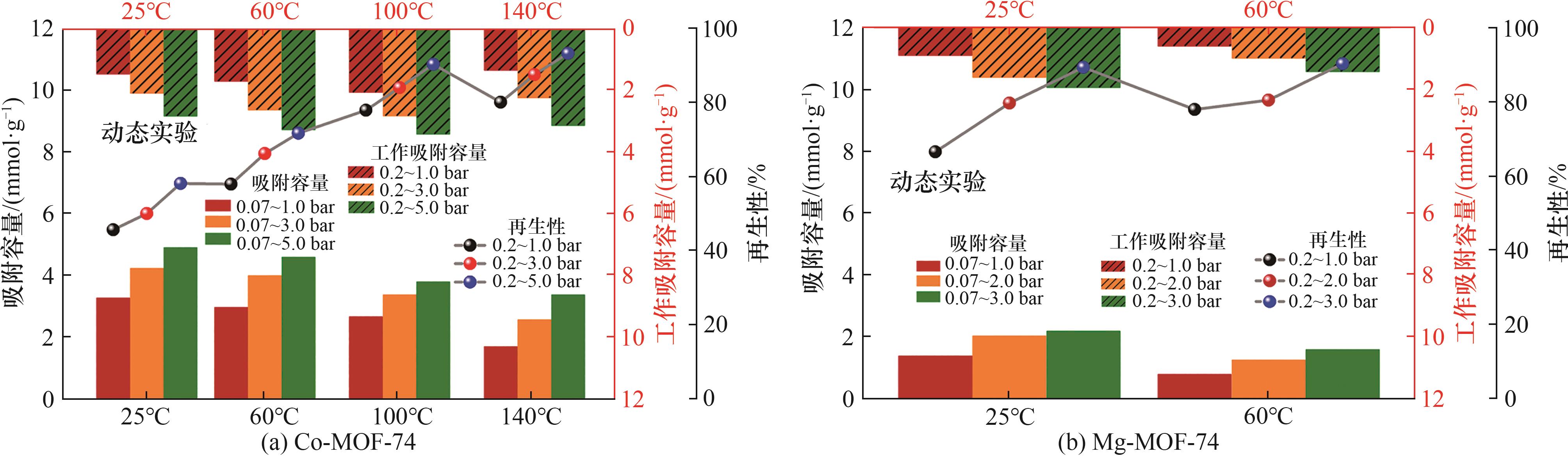
图12 根据两种样品CO/N2穿透曲线计算出的CO吸附容量、工作吸附容量和再生性
Fig.12 CO adsorption capacity, working capacity and regenerability of two samples were calculated based on CO/N2 breakthrough curves
| 样品 | 进样组成(CO/N2) | T/℃ | 吸附容量 | 工作吸附容量 | R/% | ||||
|---|---|---|---|---|---|---|---|---|---|
| p/bar | nCO/(mmol·g-1) | p/bar | ΔnCO/(mmol·g-1) | ||||||
| 解吸 | 吸附 | 解吸 | 吸附 | ||||||
| Co-MOF-74 | 50%/50% | 25 | 0.07① 0② | 1.0① 0.5② | 3.30 | 0.2① 0.1② | 1.0① 0.5② | 1.51 | 45.76 |
| 60 | 3.00 | 1.74 | 58.00 | ||||||
| 100 | 2.70 | 2.10 | 77.78 | ||||||
| 140 | 1.74 | 1.39 | 79.88 | ||||||
| 25 | 0.07① 0② | 3.0① 1.5② | 4.25 | 0.2① 0.1② | 3.0① 1.5② | 2.13 | 50.12 | ||
| 60 | 4.02 | 2.66 | 66.17 | ||||||
| 100 | 3.40 | 2.85 | 83.82 | ||||||
| 140 | 2.60 | 2.27 | 87.31 | ||||||
| 25 | 0.07① 0② | 5.0① 2.5② | 4.92 | 0.2① 0.1② | 5.0① 2.5② | 2.86 | 58.13 | ||
| 60 | 4.61 | 3.30 | 71.58 | ||||||
| 100 | 3.82 | 3.44 | 90.05 | ||||||
| 140 | 3.08 | 2.82 | 91.56 | ||||||
| Mg-MOF-74 | 50%/50% | 25 | 0.07① | 1.0① | 1.40 | 0.2① | 1.0① | 0.93 | 66.43 |
| 60 | 0② | 0.5② | 0.81 | 0.1② | 0.5② | 0.63 | 77.77 | ||
| 25 | 0.07① | 2.0① | 2.05 | 0.2① | 2.0① | 1.63 | 79.51 | ||
| 60 | 0② | 1.0② | 1.27 | 0.1② | 1.0② | 1.02 | 80.31 | ||
| 25 | 0.07① | 3.0① | 2.20 | 0.2① | 3.0① | 1.96 | 89.09 | ||
| 60 | 0② | 1.5② | 1.61 | 0.1② | 1.5② | 1.45 | 90.06 | ||
表2 Co-MOF-74和Mg-MOF-74的CO动态吸附容量、工作吸附容量和再生性总结
Table 2 Summary of dynamic adsorption capacity, working capacity and regenerability of CO for Co-MOF-74 and Mg-MOF-74
| 样品 | 进样组成(CO/N2) | T/℃ | 吸附容量 | 工作吸附容量 | R/% | ||||
|---|---|---|---|---|---|---|---|---|---|
| p/bar | nCO/(mmol·g-1) | p/bar | ΔnCO/(mmol·g-1) | ||||||
| 解吸 | 吸附 | 解吸 | 吸附 | ||||||
| Co-MOF-74 | 50%/50% | 25 | 0.07① 0② | 1.0① 0.5② | 3.30 | 0.2① 0.1② | 1.0① 0.5② | 1.51 | 45.76 |
| 60 | 3.00 | 1.74 | 58.00 | ||||||
| 100 | 2.70 | 2.10 | 77.78 | ||||||
| 140 | 1.74 | 1.39 | 79.88 | ||||||
| 25 | 0.07① 0② | 3.0① 1.5② | 4.25 | 0.2① 0.1② | 3.0① 1.5② | 2.13 | 50.12 | ||
| 60 | 4.02 | 2.66 | 66.17 | ||||||
| 100 | 3.40 | 2.85 | 83.82 | ||||||
| 140 | 2.60 | 2.27 | 87.31 | ||||||
| 25 | 0.07① 0② | 5.0① 2.5② | 4.92 | 0.2① 0.1② | 5.0① 2.5② | 2.86 | 58.13 | ||
| 60 | 4.61 | 3.30 | 71.58 | ||||||
| 100 | 3.82 | 3.44 | 90.05 | ||||||
| 140 | 3.08 | 2.82 | 91.56 | ||||||
| Mg-MOF-74 | 50%/50% | 25 | 0.07① | 1.0① | 1.40 | 0.2① | 1.0① | 0.93 | 66.43 |
| 60 | 0② | 0.5② | 0.81 | 0.1② | 0.5② | 0.63 | 77.77 | ||
| 25 | 0.07① | 2.0① | 2.05 | 0.2① | 2.0① | 1.63 | 79.51 | ||
| 60 | 0② | 1.0② | 1.27 | 0.1② | 1.0② | 1.02 | 80.31 | ||
| 25 | 0.07① | 3.0① | 2.20 | 0.2① | 3.0① | 1.96 | 89.09 | ||
| 60 | 0② | 1.5② | 1.61 | 0.1② | 1.5② | 1.45 | 90.06 | ||
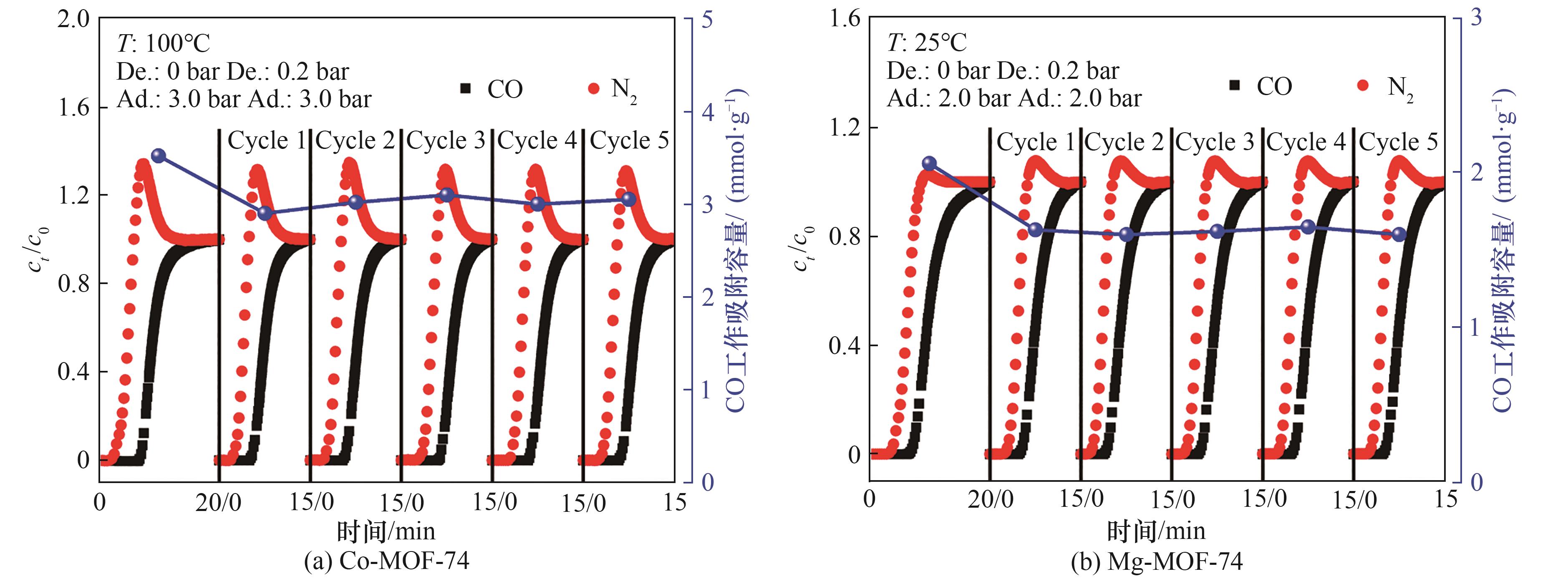
图13 两种样品的工作吸附容量循环性能T—操作温度;Ad.—吸附总压;De.—解吸总压
Fig.13 Working capacity cycling performance of two samplesT—operating temperature; Ad.—total adsorption pressure; De.—total desorption pressure
| 1 | Martinelli M, Gnanamani M K, LeViness S, et al. An overview of Fischer-Tropsch synthesis: XtL processes, catalysts and reactors[J]. Applied Catalysis A: General, 2020, 608: 117740. |
| 2 | Liu Y T, Deng D H, Bao X H. Catalysis for selected C1 chemistry[J]. Chem, 2020, 6(10): 2497-2514. |
| 3 | 霍猛, 彭晓婉, 赵金, 等. 基于COSMO-RS的离子液体吸收CO的溶剂筛选及H2/CO分离实验[J]. 化工学报, 2022, 73(12): 5305-5313. |
| Huo M, Peng X W, Zhao J, et al. COSMO-RS based solvent screening and H2/CO separation experiments for CO absorption by ionic liquids[J]. CIESC Journal, 2022, 73(12): 5305-5313. | |
| 4 | Ramírez-Santos Á A, Castel C, Favre E. A review of gas separation technologies within emission reduction programs in the iron and steel sector: current application and development perspectives[J]. Separation and Purification Technology, 2018, 194: 425-442. |
| 5 | Mondal P, Dang G S, Garg M O. Syngas production through gasification and cleanup for downstream applications—recent developments[J]. Fuel Processing Technology, 2011, 92(8): 1395-1410. |
| 6 | Flores-Granobles M, Saeys M. Dynamic pressure-swing chemical looping process for the recovery of CO from blast furnace gas[J]. Energy Conversion and Management, 2022, 258: 115515. |
| 7 | Lee H H, Lee J C, Joo Y J, et al. Dynamic modeling of Shell entrained flow gasifier in an integrated gasification combined cycle process[J]. Applied Energy, 2014, 131: 425-440. |
| 8 | Li Y X, Li S S, Xue D M, et al. Incorporation of Cu(Ⅱ) and its selective reduction to Cu(Ⅰ) within confined spaces: efficient active sites for CO adsorption[J]. Journal of Materials Chemistry A, 2018, 6(19): 8930-8939. |
| 9 | Fakhraei Ghazvini M, Vahedi M, Najafi Nobar S, et al. Investigation of the MOF adsorbents and the gas adsorptive separation mechanisms[J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104790. |
| 10 | Oh H, Beum H T, Yoon Y S, et al. Experiment and modeling of adsorption of CO from blast furnace gas onto CuCl/boehmite[J]. Industrial & Engineering Chemistry Research, 2020, 59(26): 12176-12185. |
| 11 | 蔺彩虹, 王丽, 吴瑜, 等. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| Lin C H, Wang L, Wu Y, et al. Effect of alkali cations in zeolites on adsorption and separation of CO2/N2O[J]. CIESC Journal, 2023, 74(5): 2013-2021. | |
| 12 | Oh H, Tae Beum H, Lee S Y, et al. Bed configurations in CO vacuum pressure swing adsorption process for basic oxygen furnace gas utilization: experiment, simulation, and techno-economic analysis[J]. Chemical Engineering Journal, 2023, 454: 140432. |
| 13 | He Y B, Xiang S C, Chen B L. A microporous hydrogen-bonded organic framework for highly selective C2H2/C2H4 separation at ambient temperature[J]. Journal of the American Chemical Society, 2011, 133(37): 14570-14573. |
| 14 | Ko K J, Kim H, Cho Y H, et al. Overview of carbon monoxide adsorption performance of pristine and modified adsorbents[J]. Journal of Chemical & Engineering Data, 2022, 67(7): 1599-1616. |
| 15 | Lopes F V S, Grande C A, Rodrigues A E. Activated carbon for hydrogen purification by pressure swing adsorption: multicomponent breakthrough curves and PSA performance[J]. Chemical Engineering Science, 2011, 66(3): 303-317. |
| 16 | Huang H Y, Padin J, Yang R T. Comparison of π-complexations of ethylene and carbon monoxide with Cu+ and Ag+ [J]. Industrial & Engineering Chemistry Research, 1999, 38(7): 2720-2725. |
| 17 | Feyzbar-Khalkhali-Nejad F, Hassani E, Rashti A, et al. Adsorption-based CO removal: principles and materials[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105317. |
| 18 | Peng J J, Xian S K, Xiao J, et al. A supported Cu(Ⅰ)@MIL-100(Fe) adsorbent with high CO adsorption capacity and CO/N2 selectivity[J]. Chemical Engineering Journal, 2015, 270: 282-289. |
| 19 | 梁晓武. Co-MOF-74的合成及CO/N2吸附分离性能的研究[D]. 太原: 太原理工大学, 2021. |
| Liang X W. Synthesis of Co-MOF-74 and study on adsorption and separation performance of CO/N2 [D]. Taiyuan: Taiyuan University of Technology, 2021. | |
| 20 | Evans A, Cummings M S, Luebke R, et al. Screening metal-organic frameworks for dynamic CO/N2 separation using complementary adsorption measurement techniques[J]. Industrial & Engineering Chemistry Research, 2019, 58(39): 18336-18344. |
| 21 | Bloch E D, Hudson M R, Mason J A, et al. Reversible CO binding enables tunable CO/H₂ and CO/N₂ separations in metal-organic frameworks with exposed divalent metal cations[J]. Journal of the American Chemical Society, 2014, 136(30): 10752-10761. |
| 22 | Evans A, Luebke R, Petit C. The use of metal-organic frameworks for CO purification[J]. Journal of Materials Chemistry A, 2018, 6(23): 10570-10594. |
| 23 | Kim H, Sohail M, Yim K, et al. Effective CO2 and CO separation using[M2(DOBDC)] (M = Mg, Co, Ni) with unsaturated metal sites and excavation of their adsorption sites[J]. ACS Applied Materials & Interfaces, 2019, 11(7): 7014-7021. |
| 24 | Pandey I, Lin L C, Chen C C, et al. Understanding carbon monoxide binding and interactions in M-MOF-74 (M = Mg, Mn, Ni, Zn)[J]. Langmuir, 2023, 39(50): 18187-18197. |
| 25 | Cheng M, Wang S H, Zhang Z Y, et al. High-throughput virtual screening of metal-organic frameworks for xenon recovery from exhaled anesthetic gas mixture[J]. Chemical Engineering Journal, 2023, 451: 138218. |
| 26 | Raganati F, Chirone R, Ammendola P. CO2 capture by temperature swing adsorption: working capacity As affected by temperature and CO2 partial pressure[J]. Industrial & Engineering Chemistry Research, 2020, 59(8): 3593-3605. |
| 27 | Wang Z F, Li C L, Tang L, et al. The CO working capacity of Ni-MOF-74 and corresponding operating conditions for CO/N2 adsorption separation[J]. Industrial & Engineering Chemistry Research, 2024, 63(31): 13776-13786. |
| 28 | Rowsell J L C, Yaghi O M. Effects of functionalization, catenation, and variation of the metal oxide and organic linking units on the low-pressure hydrogen adsorption properties of metal-organic frameworks[J]. Journal of the American Chemical Society, 2006, 128(4): 1304-1315. |
| 29 | Caskey S R, Wong-Foy A G, Matzger A J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores[J]. Journal of the American Chemical Society, 2008, 130(33): 10870-10871. |
| 30 | Xue C L, Hao W M, Cheng W P, et al. CO adsorption performance of CuCl/activated carbon by simultaneous Reduction-Dispersion of mixed Cu(Ⅱ) salts[J]. Materials, 2019, 12(10): 1605. |
| 31 | Oliveira M L M, Miranda A A L, Barbosa C M B M, et al. Adsorption of thiophene and toluene on NaY zeolites exchanged with Ag(Ⅰ), Ni(Ⅱ) and Zn(Ⅱ)[J]. Fuel, 2009, 88(10): 1885-1892. |
| 32 | Cessford N F, Seaton N A, Düren T. Evaluation of ideal adsorbed solution theory as a tool for the design of metal-organic framework materials[J]. Industrial & Engineering Chemistry Research, 2012, 51(13): 4911-4921. |
| 33 | Bae Y S, Lee C Y, Kim K C, et al. High propene/propane selectivity in isostructural metal-organic frameworks with high densities of open metal sites[J]. Angewandte Chemie (International Ed), 2012, 51(8): 1857-1860. |
| 34 | Choma J, Stachurska K, Marszewski M, et al. Equilibrium isotherms and isosteric heat for CO2 adsorption on nanoporous carbons from polymers[J]. Adsorption, 2016, 22(4): 581-588. |
| 35 | Tao L R, You Y Y, Liu X J. Numerical studies of CO separation and enrichment from blast furnace gas by using a CuCl/Y fixed bed[J]. Ironmaking & Steelmaking, 2021, 48(10): 1187-1199. |
| 36 | Basdogan Y, Sezginel K B, Keskin S. Identifying highly selective metal organic frameworks for CH4/H2 separations using computational tools[J]. Industrial & Engineering Chemistry Research, 2015, 54(34): 8479-8491. |
| 37 | Bae Y S, Snurr R Q. Development and evaluation of porous materials for carbon dioxide separation and capture[J]. Angewandte Chemie International Edition, 2011, 50(49): 11586-11596. |
| 38 | Tong M M, Yang Q Y, Xiao Y L, et al. Revealing the structure-property relationship of covalent organic frameworks for CO₂ capture from postcombustion gas: a multi-scale computational study[J]. Physical Chemistry Chemical Physics, 2014, 16(29): 15189-15198. |
| 39 | Jiang H X, Wang Q Y, Wang H Q, et al. MOF-74 as an efficient catalyst for the low-temperature selective catalytic reduction of NO x with NH3 [J]. ACS Applied Materials & Interfaces, 2016, 8(40): 26817-26826. |
| 40 | Sun H, Ren D N, Kong R Q, et al. Tuning 1-hexene/n-hexane adsorption on MOF-74 via constructing Co-Mg bimetallic frameworks[J]. Microporous and Mesoporous Materials, 2019, 284: 151-160. |
| 41 | Wu Y Q, Chen Z A, Li B, et al. Highly selective adsorption of CO over N2 on CuCl-loaded SAPO-34 adsorbent[J]. Journal of Energy Chemistry, 2019, 36: 122-128. |
| 42 | Álvarez-Gutiérrez N, Gil M V, Rubiera F, et al. Adsorption performance indicators for the CO2/CH4 separation: application to biomass-based activated carbons[J]. Fuel Processing Technology, 2016, 142: 361-369. |
| [1] | 齐昊, 王玉杰, 李申辉, 邹琦, 刘轶群, 赵之平. 双金属Co/Zn-ZIFs中C3H6和C3H8吸附和扩散行为分子模拟研究[J]. 化工学报, 2025, 76(5): 2313-2326. |
| [2] | 陶春珲, 李印辉, 傅钰, 段然, 赵泽一, 唐羽丰, 张罡, 马和平. 不同吸附剂对低浓度Kr气的选择性吸附与纯化[J]. 化工学报, 2025, 76(5): 2358-2366. |
| [3] | 张越, 刘佳鑫, 马敬, 刘毅. 金属有机骨架膜应用于海水提铀研究进展[J]. 化工学报, 2025, 76(5): 2087-2100. |
| [4] | 胡嘉朗, 姜明源, 金律铭, 张永刚, 胡鹏, 纪红兵. 机器学习辅助MOFs高通量计算筛选及气体分离研究进展[J]. 化工学报, 2025, 76(5): 1973-1996. |
| [5] | 徐智超, 俞镇东, 吴昊峰, 吴沛文, 武洪翔, 巢艳红, 朱文帅, 刘植昌, 徐春明. 富酸位13X分子筛的制备及其超深度吸附脱除生物柴油中硫醇[J]. 化工学报, 2025, 76(5): 2198-2208. |
| [6] | 马瑞洁, 黄子轩, 关雪倩, 陈光进, 刘蓓. ZIF-8/DMPU浆液分离C2H6/ CH4混合气研究[J]. 化工学报, 2025, 76(5): 2262-2269. |
| [7] | 郭彭涛, 王婷, 薛波, 应允攀, 刘大欢. 用于CH4/N2分离的多吸附位点超微孔MOF[J]. 化工学报, 2025, 76(5): 2304-2312. |
| [8] | 李艳, 雷美丽, 李鑫钢. 基于分离性能的顺序式模拟移动床结构调控策略[J]. 化工学报, 2025, 76(5): 2219-2229. |
| [9] | 巴雅琪, 吴涛, 邸安頔, 陆安慧. 多孔炭材料用于低碳烃分离的研究进展[J]. 化工学报, 2025, 76(5): 2136-2157. |
| [10] | 谈朋, 李雪梅, 刘晓勤, 孙林兵. 基于柔性MOFs的磁响应复合材料及其丙烯吸附性能研究[J]. 化工学报, 2025, 76(5): 2230-2240. |
| [11] | 晋伊浩, 罗俊欣, 胡章茂, 王唯, 殷谦. 亲水改性硫酸镁/膨胀蛭石复合材料的吸附储热性能[J]. 化工学报, 2025, 76(4): 1852-1862. |
| [12] | 朱峰, 赵跃, 马凤翔, 刘伟. 改性UIO-66对SF6/N2混合气体及其分解产物的吸附特性[J]. 化工学报, 2025, 76(4): 1604-1616. |
| [13] | 蔡天姿, 张海丰, 林海丹, 张子龙, 周鹏宇, 王柏林, 李小年. 硼掺杂氮基石墨烯检测变压器油中溶解气体CO和CO2的密度泛函理论研究[J]. 化工学报, 2025, 76(4): 1841-1851. |
| [14] | 陶智能, 邱彤, 王保国. 阴离子交换膜电解水制氢稳态建模[J]. 化工学报, 2025, 76(4): 1711-1721. |
| [15] | 赵俊德, 周爱国, 陈彦霖, 郑家乐, 葛天舒. 吸附法CO2直接空气捕集技术能耗现状[J]. 化工学报, 2025, 76(4): 1375-1390. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号