化工学报 ›› 2025, Vol. 76 ›› Issue (5): 1927-1942.DOI: 10.11949/0438-1157.20241207
• 综述与专论 • 上一篇
收稿日期:2024-10-30
修回日期:2025-01-10
出版日期:2025-05-25
发布日期:2025-06-13
通讯作者:
潘建明,邢卫红
作者简介:杨雅南(2001—),女,硕士研究生,2222312083@stmail.ujs.edu.cn
基金资助:
Yanan YANG( ), Shengran CHANG, Songlin XUE, Jianming PAN(
), Shengran CHANG, Songlin XUE, Jianming PAN( ), Weihong XING(
), Weihong XING( )
)
Received:2024-10-30
Revised:2025-01-10
Online:2025-05-25
Published:2025-06-13
Contact:
Jianming PAN, Weihong XING
摘要:
铀、锂的富集和分离对绿色能源和工业升级具有重要意义。海水中铀、锂的储量是陆地的数千倍,但浓度极低,从海水中高效提取铀、锂,已成为化学、材料、能源与催化领域的研究前沿之一,基于光电驱动的还原吸附分离技术发展迅速。介绍了光电驱动促进海水中铀和锂提取的研究进展,重点介绍了典型光电驱动提取材料与过程,总结了光、电驱动海水中分离锂和铀的性能及优缺点。基于光电驱动的新策略对锂和铀提取展现出了更快的吸附动力和更强的捕获能力,分析总结了海水提铀和锂中的能量消耗及成本效益,并展望了光电驱动促进海水中铀和锂提取研究的发展趋势,旨在为设计开发新型海水提铀和锂的光电提取材料提供启示。
中图分类号:
杨雅南, 常胜然, 薛松林, 潘建明, 邢卫红. 基于光、电驱动促进海水中铀和锂提取的研究进展[J]. 化工学报, 2025, 76(5): 1927-1942.
Yanan YANG, Shengran CHANG, Songlin XUE, Jianming PAN, Weihong XING. Progress of research on photo- and electric-driven to promote uranium and lithium extraction from seawater[J]. CIESC Journal, 2025, 76(5): 1927-1942.
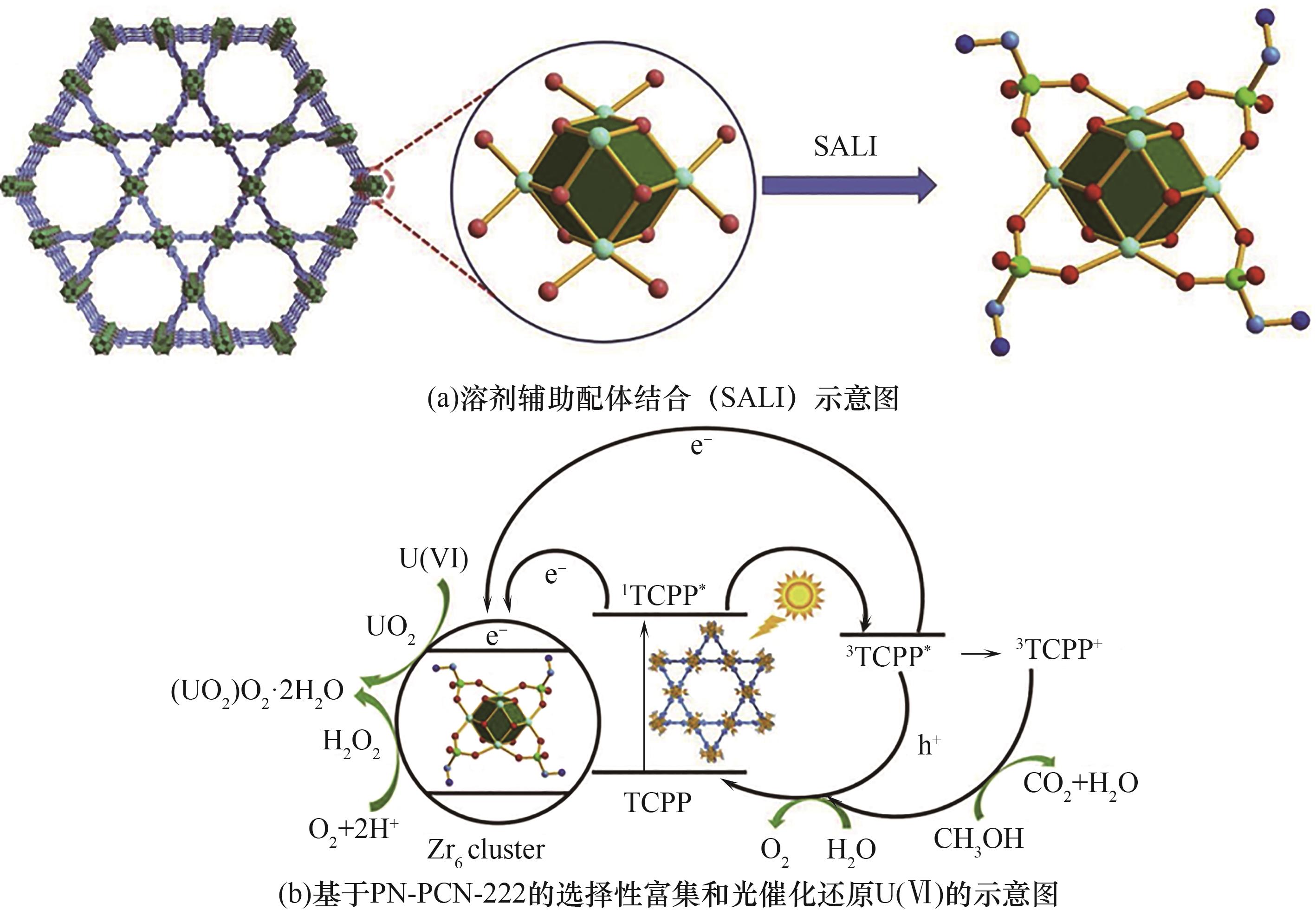
图2 溶剂辅助配体结合提铀MOF示意图和PN-PCN-222光催化还原铀的机理[47]
Fig.2 Schematic diagram of solvent-assisted ligand-bound uranium extraction MOF and mechanistic diagram of photocatalytic reduction of uranium by PN-PCN-222[47]
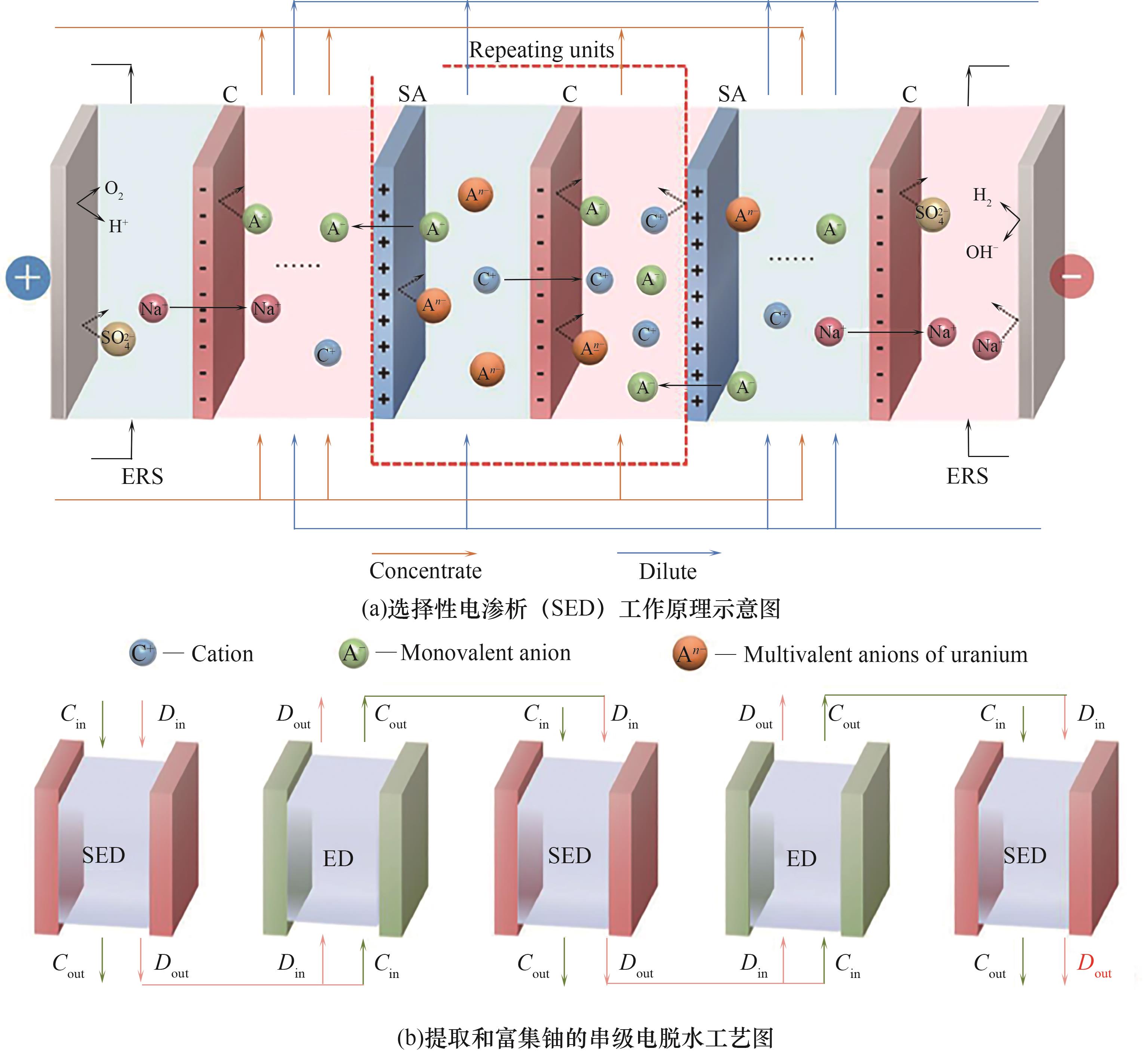
图6 电渗析中提取和富集铀的串联脱水工艺[73]Cout—浓缩液出水;Cin—浓缩液进水;Dout—稀释后的流出液;Din—稀释液进水
Fig.6 A schematic diagram of the series dehydration process for uranium extraction and enrichment in electrodialysis[73]Cout—concentrate outlet; Cin—concentrate inlet; Dout—diluted effluent; Din—diluent inlet
| 类型 | 分类 | 材料 | 铀吸附量/(mg·g-1) | 铀还原效率/ 铀富集效率 | 溶液形式 |
|---|---|---|---|---|---|
| 基于光驱动 | 元素掺杂 | Sn-In2S3微球[ | 约112.83 | — | — |
| g-C3N4(S/P/B)[ | — | — | — | ||
| 界面修饰 | PN-PCN-222[ | 1289.3 | 96.7% | 模拟海水 | |
| TTh-COF-AO[ | 10.24 | — | 自然海水 | ||
TpBD-X[ (X = —OH、—NH、—OCH、—NO、—SOH) | — | 73.11% (max) | 模拟海水 | ||
| 缺陷工程及异质结引入 | ZnFe2O4/g-C3N4 Z-scheme异质结[ | 1892.4 | 94.62% | 模拟海水 | |
| AO-C3N4[ | 850 μg·g-1 | 94.2% | 自然海水 | ||
| CN550[ | 1057.3 (明黄色变至完全透明) | — | 模拟海水/ 自然海水 | ||
| 基于电驱动 | 电容去离子 | WO3/C复合电极[ | 449.9 | 71% | 模拟海水 |
In-N x -C-R[ (R为偕胺肟基团) | 12.7 | >90% | 自然海水 | ||
| 电沉积 | Fe-N x -C-R[ | 1.2 | — | 自然海水 | |
| MPSOF[ | 1.21 | — | 自然海水 | ||
| TFPM-PDAN-AO[ | 12.8 (20 d) | 98.2% | 自然海水 | ||
| 电渗析 | CJMC-5阳离子交换膜[ | — | >80% | 模拟海水 | |
| PAO交换膜[ | — | 30%(与未掺杂PAO相比) | 模拟海水 |
表1 基于光电驱动海水提铀的提取性能
Table 1 Recent advances based on photo/electric-driven methods for uranium extraction from seawater
| 类型 | 分类 | 材料 | 铀吸附量/(mg·g-1) | 铀还原效率/ 铀富集效率 | 溶液形式 |
|---|---|---|---|---|---|
| 基于光驱动 | 元素掺杂 | Sn-In2S3微球[ | 约112.83 | — | — |
| g-C3N4(S/P/B)[ | — | — | — | ||
| 界面修饰 | PN-PCN-222[ | 1289.3 | 96.7% | 模拟海水 | |
| TTh-COF-AO[ | 10.24 | — | 自然海水 | ||
TpBD-X[ (X = —OH、—NH、—OCH、—NO、—SOH) | — | 73.11% (max) | 模拟海水 | ||
| 缺陷工程及异质结引入 | ZnFe2O4/g-C3N4 Z-scheme异质结[ | 1892.4 | 94.62% | 模拟海水 | |
| AO-C3N4[ | 850 μg·g-1 | 94.2% | 自然海水 | ||
| CN550[ | 1057.3 (明黄色变至完全透明) | — | 模拟海水/ 自然海水 | ||
| 基于电驱动 | 电容去离子 | WO3/C复合电极[ | 449.9 | 71% | 模拟海水 |
In-N x -C-R[ (R为偕胺肟基团) | 12.7 | >90% | 自然海水 | ||
| 电沉积 | Fe-N x -C-R[ | 1.2 | — | 自然海水 | |
| MPSOF[ | 1.21 | — | 自然海水 | ||
| TFPM-PDAN-AO[ | 12.8 (20 d) | 98.2% | 自然海水 | ||
| 电渗析 | CJMC-5阳离子交换膜[ | — | >80% | 模拟海水 | |
| PAO交换膜[ | — | 30%(与未掺杂PAO相比) | 模拟海水 |
| 方法 | 优点 | 不足 | 研究重点 |
|---|---|---|---|
| 电容去离子 | 能耗低;操作简便;电极使用寿命长; 无其他化学试剂的引入 | 电极易堵塞;电解质系统复杂 | 提高电极材料的比表面积和电荷存储能力; 有效回收和处理吸附的铀离子 |
| 电沉积 | 可直接获取固体形式的铀;出售固体铀可降低运营成本;无须复杂的化学处理 | 存在副反应,会产生二次废物 | 提高电沉积效率和选择性;防止其他离子的共沉积 |
| 电渗析 | 引入离子交换膜;可实现连续操作 | 膜易被污染或堵塞;维修需要投入较高成本 | 提高离子交换膜的耐久性以降低成本; 提高能源效率 |
表2 三种基于电驱动技术海水提铀的比较
Table 2 Comparison of three types of electrically driven seawater-based uranium extraction
| 方法 | 优点 | 不足 | 研究重点 |
|---|---|---|---|
| 电容去离子 | 能耗低;操作简便;电极使用寿命长; 无其他化学试剂的引入 | 电极易堵塞;电解质系统复杂 | 提高电极材料的比表面积和电荷存储能力; 有效回收和处理吸附的铀离子 |
| 电沉积 | 可直接获取固体形式的铀;出售固体铀可降低运营成本;无须复杂的化学处理 | 存在副反应,会产生二次废物 | 提高电沉积效率和选择性;防止其他离子的共沉积 |
| 电渗析 | 引入离子交换膜;可实现连续操作 | 膜易被污染或堵塞;维修需要投入较高成本 | 提高离子交换膜的耐久性以降低成本; 提高能源效率 |
| 类型 | 分类 | 材料 | 锂吸附量/(mg·g-1) | 锂吸附效率 | 溶液形式 |
|---|---|---|---|---|---|
| 基于光驱动 | 光电化学 | InGaP/GaAs/Ge光电极[ | 783.56 | — | 自然海水 |
| 光热 | PIP[ | 1289.3 | 308.6 mg·m-2·d-1 | 自然海水 | |
| 改性复合材料 | 聚丙烯/聚乙烯芯鞘纤维毛毡[ | 约520 mg·m-2 | 9.368 mg·g-1·h-1 | 自然海水 | |
| 基于电驱动 | 电渗析 | LLTO膜[ | 9013.43 | 71% | 模拟海水 |
| M-GA/PEI膜[ | 12.7 | — | 自然海水 | ||
| 15CE/PEI-PDA-CR671膜[ | — | 90% | 模拟海水 | ||
| 电化学插层 | TiO2包覆LiFePO4电极[ | — | 94.3% ± 4.0% | 模拟海水 | |
| pD包覆LiFePO4电极[ | — | — | 模拟海水 | ||
| MnO2包覆LiFePO4电极[ | 20.6 | — | 自然海水 |
表3 海水提锂基于光电驱动的研究进展
Table 3 Recent advances based on photo/electric-driven methods for lithium extraction from seawater
| 类型 | 分类 | 材料 | 锂吸附量/(mg·g-1) | 锂吸附效率 | 溶液形式 |
|---|---|---|---|---|---|
| 基于光驱动 | 光电化学 | InGaP/GaAs/Ge光电极[ | 783.56 | — | 自然海水 |
| 光热 | PIP[ | 1289.3 | 308.6 mg·m-2·d-1 | 自然海水 | |
| 改性复合材料 | 聚丙烯/聚乙烯芯鞘纤维毛毡[ | 约520 mg·m-2 | 9.368 mg·g-1·h-1 | 自然海水 | |
| 基于电驱动 | 电渗析 | LLTO膜[ | 9013.43 | 71% | 模拟海水 |
| M-GA/PEI膜[ | 12.7 | — | 自然海水 | ||
| 15CE/PEI-PDA-CR671膜[ | — | 90% | 模拟海水 | ||
| 电化学插层 | TiO2包覆LiFePO4电极[ | — | 94.3% ± 4.0% | 模拟海水 | |
| pD包覆LiFePO4电极[ | — | — | 模拟海水 | ||
| MnO2包覆LiFePO4电极[ | 20.6 | — | 自然海水 |
| 类型 | 材料 | 外加电压/电流 | 能耗/成本估算 |
|---|---|---|---|
| 基于电驱动提铀 | WO3/C复合电极[ | 1.2 V(恒定电压) | — |
In-N x -C-R[ (R为偕胺肟基团) | -5~0 V(交流电压) | 约806 USD·g-1 | |
| MPSOF[ | -3.5 V(交流电压) | — | |
| TFPM-PDAN-AO[ | -6~-1 V(交流电压) | — | |
| CJMC-5阳离子交换膜[ | 4 mA·cm-2(恒定电流) | 101.78 USD·m-3 | |
| Fe@PDACN[ | ≤-1.5 V(交流电压) | 3.22×10-2 USD·kg-1 | |
| 基于电驱动提锂 | LLTO膜[ | 0.6 V(恒定电压) | 46011.16 J |
| M-GA/PEI膜[ | — | 第一级 0.029~0.039 kWh·mol-1 第二级 0.011~0.014 kWh·mol-1 | |
| 15CE/PEI-PDA-CR671膜[ | 15.9 mA·cm-2 (恒定电流) | — | |
| TiO2包覆LiFePO4电极[ | 3.4 V (恒定电压) | — | |
| pD包覆LiFePO4电极[ | 1.3 mA (恒定电流) | — | |
| MnO2包覆LiFePO4电极[ | 0.5 mA (恒定电流) | 7.2 Wh |
表4 基于电驱动海水提锂和铀的能耗和成本估算
Table 4 Energy and cost estimation of lithium and uranium extraction based on electrically-driven from seawater
| 类型 | 材料 | 外加电压/电流 | 能耗/成本估算 |
|---|---|---|---|
| 基于电驱动提铀 | WO3/C复合电极[ | 1.2 V(恒定电压) | — |
In-N x -C-R[ (R为偕胺肟基团) | -5~0 V(交流电压) | 约806 USD·g-1 | |
| MPSOF[ | -3.5 V(交流电压) | — | |
| TFPM-PDAN-AO[ | -6~-1 V(交流电压) | — | |
| CJMC-5阳离子交换膜[ | 4 mA·cm-2(恒定电流) | 101.78 USD·m-3 | |
| Fe@PDACN[ | ≤-1.5 V(交流电压) | 3.22×10-2 USD·kg-1 | |
| 基于电驱动提锂 | LLTO膜[ | 0.6 V(恒定电压) | 46011.16 J |
| M-GA/PEI膜[ | — | 第一级 0.029~0.039 kWh·mol-1 第二级 0.011~0.014 kWh·mol-1 | |
| 15CE/PEI-PDA-CR671膜[ | 15.9 mA·cm-2 (恒定电流) | — | |
| TiO2包覆LiFePO4电极[ | 3.4 V (恒定电压) | — | |
| pD包覆LiFePO4电极[ | 1.3 mA (恒定电流) | — | |
| MnO2包覆LiFePO4电极[ | 0.5 mA (恒定电流) | 7.2 Wh |
| 1 | Tian G X, Teat S J, Rao L F. Thermodynamic studies of U(Ⅵ) complexation with glutardiamidoxime for sequestration of uranium from seawater[J]. Dalton Transaction, 2013, 42(16): 5690-5696. |
| 2 | Zeng M, Liu Y X, Ouyang S J, et al. Nuclear energy in the post-fukushima era: research on the developments of the Chinese and worldwide nuclear power industries[J]. Renewable and Sustainable Energy Reviews, 2016, 58: 147-156. |
| 3 | Yang H, Liu Y F, Chen Z S, et al. Emerging technologies for uranium extraction from seawater[J]. Science China Chemistry, 2022, 65(12): 2335-2337. |
| 4 | Kim J, Tsouris C, Oyola Y, et al. Uptake of uranium from seawater by amidoxime-based polymeric adsorbent: field experiments, modeling, and updated economic assessment[J]. Industrial & Engineering Chemistry Research, 2014, 53(14): 6076-6083. |
| 5 | Abney C W, Mayes R T, Saito T, et al. Materials for the recovery of uranium from seawater[J]. Chemical Reviews, 2017, 117(23): 13935-14013. |
| 6 | Asiabi H, Yamini Y, Shamsayei M. Highly efficient capture and recovery of uranium by reusable layered double hydroxide intercalated with 2-mercaptoethanesulfonate[J]. Chemical Engineering Journal, 2018, 337: 609-615. |
| 7 | Chen L, Bai Z L, Zhu L, et al. Ultrafast and efficient extraction of uranium from seawater using an amidoxime appended metal-organic framework[J]. ACS Applied Materials & Interfaces, 2017, 9(38): 32446-32451. |
| 8 | Guo L C, Yang Y T, Gong L L, et al. Innovative conversion strategy for wastewater with one-pot uranium extraction and valuable chemical production by a smart COF photocatalyst[J]. Advanced Functional Materials, 2024, 34(29): 2400588. |
| 9 | Hu E M, Chen Q, Gao Q, et al. Cyano-functionalized graphitic carbon nitride with adsorption and photoreduction isosite achieving efficient uranium extraction from seawater[J]. Advanced Functional Materials, 2024, 34(19): 2312215. |
| 10 | Jiao R R, Zhou H Y, Wang D, et al. Uranium electrolytic deposition with regenerable Se2- active sites at low cell voltage[J]. Chemical Engineering Journal, 2024, 499: 156227. |
| 11 | Gao W, Long Y T, Qing Y F, et al. A novel strategy for efficient uranium extraction and energy storage: uranium extraction cell[J]. Separation and Purification Technology, 2024, 339: 126723. |
| 12 | Yu Q H, Yuan Y H, Feng L J, et al. Spidroin-inspired, high-strength, loofah-shaped protein fiber for capturing uranium from seawater[J]. Angewandte Chemie International Edition, 2020, 59(37): 15997-16001. |
| 13 | Song Y P, Zhu C J, Sun Q, et al. Nanospace decoration with uranyl-specific "hooks" for selective uranium extraction from seawater with ultrahigh enrichment index[J]. ACS Central Science, 2021, 7(10): 1650-1656. |
| 14 | Li Y, Zheng Y J, Ahamd Z, et al. Strategies for designing highly efficient adsorbents to capture uranium from seawater[J]. Coordination Chemistry Reviews, 2023, 491: 215234. |
| 15 | Liu S Q, Tao B B, Zuo B, et al. Function-oriented design principles for adsorbent materials of uranium extraction from seawater[J]. Chemical Engineering Journal, 2024, 500: 156783. |
| 16 | Wu Y, Xie Y H, Liu X L, et al. Functional nanomaterials for selective uranium recovery from seawater: material design, extraction properties and mechanisms[J]. Coordination Chemistry Reviews, 2023, 483: 215097. |
| 17 | Li W D, Erickson E M, Manthiram A. High-nickel layered oxide cathodes for lithium-based automotive batteries[J]. Nature Energy, 2020, 5: 26-34. |
| 18 | Zhao Y, Wu M Y, Guo Y, et al. Metal-organic framework based membranes for selective separation of target ions[J]. Journal of Membrane Science, 2021, 634: 119407. |
| 19 | Wang R H, Zhang Y H, Sun K W, et al. Emerging green technologies for recovery and reuse of spent lithium-ion batteries-a review[J]. Journal of Materials Chemistry A, 2022, 10(33): 17053-17076. |
| 20 | 李彦乐, 刘宜林, 霍俊杰, 等. 层状结构铝系吸附剂在盐湖提锂领域的研究[J]. 化工学报, 2023, 74(12): 4777-4791. |
| Li Y L, Liu Y L, Huo J J, et al. Research progress of aluminum adsorbents in lithium extraction from salt lakes[J]. CIESC Journal, 2023, 74(12): 4777-4791. | |
| 21 | Paranthaman M P, Li L, Luo J Q, et al. Recovery of lithium from geothermal brine with lithium-aluminum layered double hydroxide chloride sorbents[J]. Environmental Science & Technology, 2017, 51(22): 13481-13486. |
| 22 | Yu J T, Zhu J, Luo G L, et al. 3D-printed titanium-based ionic sieve monolithic adsorbent for selective lithium recovery from salt lakes[J]. Desalination, 2023, 560: 116651. |
| 23 | Chen S Q, Chen Z S, Wei Z W, et al. Titanium-based ion sieve with enhanced post-separation ability for high performance lithium recovery from geothermal water[J]. Chemical Engineering Journal, 2021, 410: 128320. |
| 24 | Roobavannan S, Choo Y, Truong D Q, et al. Seawater lithium mining by zeolitic imidazolate framework encapsulated manganese oxide ion sieve nanomaterial[J]. Chemical Engineering Journal, 2023, 474: 145957. |
| 25 | Tang L, Huang S D, Wang Y, et al. Highly efficient, stable, and recyclable hydrogen manganese oxide/cellulose film for the extraction of lithium from seawater[J]. ACS Applied Materials & Interfaces, 2020, 12(8): 9775-9781. |
| 26 | Peng Q, Wang R Y, Zhao Z L, et al. Extreme Li-Mg selectivity via precise ion size differentiation of polyamide membrane[J]. Nature Communications, 2024, 15(1): 2505. |
| 27 | Steven Kurniawan Y, Rao Sathuluri R, Ohto K, et al. A rapid and efficient lithium-ion recovery from seawater with tripropyl-monoacetic acid calix[4]arene derivative employing droplet-based microreactor system[J]. Separation and Purification Technology, 2019, 211: 925-934. |
| 28 | Harvianto G R, Kim S H, Ju C S. Solvent extraction and stripping of lithium ion from aqueous solution and its application to seawater[J]. Rare Metals, 2016, 35(12): 948-953. |
| 29 | Paredes C, Rodríguez de San Miguel E. Selective lithium extraction and concentration from diluted alkaline aqueous media by a polymer inclusion membrane and application to seawater[J]. Desalination, 2020, 487: 114500. |
| 30 | 石成龙, 贾永忠, 景燕. 离子液体-磷酸三丁酯体系分离盐湖卤水镁锂[J]. 化工学报, 2015, 66(S1): 253-259. |
| Shi C L, Jia Y Z, Jing Y. Lithium and magnesium separation from salt lake brine by ionic liquids containing tributyl phosphate[J]. CIESC Journal, 2015, 66(S1): 253-259. | |
| 31 | Abdulazeez I, Baig N, Salhi B, et al. Electrochemical behavior of novel electroactive LaTi4Mn3O12/polyaniline composite for Li+-ion recovery from brine with high selectivity[J]. Separation and Purification Technology, 2023, 309: 122997. |
| 32 | Yang S X, Zhang F, Ding H P, et al. Lithium metal extraction from seawater[J]. Joule, 2018, 2(9): 1648-1651. |
| 33 | Kim J S, Lee Y H, Choi S, et al. An electrochemical cell for selective lithium capture from seawater[J]. Environmental Science & Technology, 2015, 49(16): 9415-9422. |
| 34 | Ma H Y, Xia Y, Wang Z Y, et al. Dual-channel-ion conductor membrane for low-energy lithium extraction[J]. Environmental Science & Technology, 2023, 57(45): 17246-17255. |
| 35 | Hu H S, Xiong L, Shi Z X, et al. Study on lithium extraction from natural brine without additional energy consumption by photocatalytic technology[J]. Sustainable Materials and Technologies, 2024, 41: e01108. |
| 36 | Zhang S X, Wei X, Cao X, et al. Solar-driven membrane separation for direct lithium extraction from artificial salt-lake brine[J]. Nature Communications, 2024, 15(1): 238. |
| 37 | Song Y, Fang S Q, Xu N, et al. Solar transpiration-powered lithium extraction and storage[J]. Science, 2024, 385(6716): 1444-1449. |
| 38 | Lim Y J, Goh K, Goto A, et al. Uranium and lithium extraction from seawater: challenges and opportunities for a sustainable energy future[J]. Journal of Materials Chemistry A, 2023, 11(42): 22551-22589. |
| 39 | Yu S J, Tang H, Zhang D, et al. MXenes as emerging nanomaterials in water purification and environmental remediation[J]. Science of the Total Environment, 2022, 811: 152280. |
| 40 | Zhang Y F, Zhu M Y, Zhang S, et al. Highly efficient removal of U(Ⅵ) by the photoreduction of SnO2/CdCO3/CdS nanocomposite under visible light irradiation[J]. Applied Catalysis B: Environmental, 2020, 279: 119390. |
| 41 | Sarsfield M J, Helliwell M. Extending the chemistry of the uranyl ion: Lewis acid coordination to a U=O oxygen[J]. Journal of the American Chemical Society, 2004, 126(4): 1036-1037. |
| 42 | Amadelli R, Maldotti A, Sostero S, et al. Photodeposition of uranium oxides onto TiO2 from aqueous uranyl solutions[J]. Journal of the Chemical Society, Faraday Transactions, 1991, 87(19): 3267-3273. |
| 43 | Li P, Wang J J, Wang Y, et al. Photoconversion of U(Ⅵ) by TiO2: an efficient strategy for seawater uranium extraction[J]. Chemical Engineering Journal, 2019, 365: 231-241. |
| 44 | Lu C H, Zhang P, Jiang S J, et al. Photocatalytic reduction elimination of U O 2 2 + pollutant under visible light with metal-free sulfur doped g-C3N4 photocatalyst[J]. Applied Catalysis B: Environmental, 2017, 200: 378-385. |
| 45 | Lu C H, Chen R Y, Wu X, et al. Boron doped g-C3N4 with enhanced photocatalytic U O 2 2 + reduction performance[J]. Applied Surface Science, 2016, 360: 1016-1022. |
| 46 | Wu X, Jiang S J, Song S Q, et al. Constructing effective photocatalytic purification system with P-introduced g-C3N4 for elimination of U O 2 2 + [J]. Applied Surface Science, 2018, 430: 371-379. |
| 47 | Li H, Zhai F W, Gui D X, et al. Powerful uranium extraction strategy with combined ligand complexation and photocatalytic reduction by postsynthetically modified photoactive metal-organic frameworks[J]. Applied Catalysis B: Environmental, 2019, 254: 47-54. |
| 48 | Yu F T, Li C Y, Li W R, et al. π-skeleton tailoring of olefin-linked covalent organic frameworks achieving low exciton binding energy for photo-enhanced uranium extraction from seawater[J]. Advanced Functional Materials, 2024, 34(1): 2307230. |
| 49 | Zhong X, Ling Q, Kuang P L, et al. Modified side-chain COFs construct built-in electric fields with low exciton binding energy for photo-reduced uranium[J]. Chemical Engineering Journal, 2024, 483: 149339. |
| 50 | Chen T, Li M X, Zhou L, et al. Harmonizing the energy band between adsorbent and semiconductor enables efficient uranium extraction[J]. Chemical Engineering Journal, 2021, 420: 127645. |
| 51 | Chen T, Liu B, Li M X, et al. Efficient uranium reduction of bacterial cellulose-MoS2 heterojunction via the synergistically effect of Schottky junction and S-vacancies engineering[J]. Chemical Engineering Journal, 2021, 406: 126791. |
| 52 | Liu X N, Du P H, Pan W Y, et al. Immobilization of uranium(Ⅵ) by niobate/titanate nanoflakes heterojunction through combined adsorption and solar-light-driven photocatalytic reduction[J]. Applied Catalysis B: Environmental, 2018, 231: 11-22. |
| 53 | Huang X S, Xiao J T, Mei P, et al. The synthesis of Z-scheme MoS2/g-C3N4 heterojunction for enhanced visible-light-driven photoreduction of uranium[J]. Catalysis Letters, 2022, 152(7): 1981-1989. |
| 54 | Dai Z R, Zhen Y, Sun Y S, et al. ZnFe2O4/g-C3N4 S-scheme photocatalyst with enhanced adsorption and photocatalytic activity for uranium(Ⅵ) removal[J]. Chemical Engineering Journal, 2021, 415: 129002. |
| 55 | Devi L G, Kavitha R. A review on non metal ion doped titania for the photocatalytic degradation of organic pollutants under UV/solar light: role of photogenerated charge carrier dynamics in enhancing the activity[J]. Applied Catalysis B: Environmental, 2013, 140/141: 559-587. |
| 56 | Feng J N, Yang Z Q, He S, et al. Photocatalytic reduction of uranium(Ⅵ) under visible light with Sn-doped In2S3 microspheres[J]. Chemosphere, 2018, 212: 114-123. |
| 57 | Chen T, Yu K F, Dong C X, et al. Advanced photocatalysts for uranium extraction: elaborate design and future perspectives[J]. Coordination Chemistry Reviews, 2022, 467: 214615. |
| 58 | Zhang P, Tong Y W, Liu Y, et al. Heteroatom dopants promote two-electron O2 reduction for photocatalytic production of H2O2 on polymeric carbon nitride[J]. Angewandte Chemie-International Edition, 2020, 59(37): 16209-16217. |
| 59 | Chen L, Chen C, Yang Z, et al. Simultaneously tuning band structure and oxygen reduction pathway toward high-efficient photocatalytic hydrogen peroxide production using cyano-rich graphitic carbon nitride[J]. Advanced Functional Materials, 2021, 31(46): 2105731. |
| 60 | Zhao D M, Dong C L, Wang B, et al. Synergy of dopants and defects in graphitic carbon nitride with exceptionally modulated band structures for efficient photocatalytic oxygen evolution[J]. Advanced Materials, 2019, 31(43): 1903545. |
| 61 | Yu K F, Li Y, Cao X, et al. In-situ constructing amidoxime groups on metal-free g-C3N4 to enhance chemisorption, light absorption, and carrier separation for efficient photo-assisted uranium(Ⅵ) extraction[J]. Journal of Hazardous Materials, 2023, 460: 132356. |
| 62 | Liu S, Wang Z, Lu Y X, et al. Sunlight-induced uranium extraction with triazine-based carbon nitride as both photocatalyst and adsorbent[J]. Applied Catalysis B: Environmental, 2021, 282: 119523. |
| 63 | Raj S K, Carrier A J, Youden B C, et al. Electrochemical techniques for uranium extraction from water[J]. Chemical Engineering Journal, 2024, 492: 152341. |
| 64 | Jung C H, Lee H Y, Moon J K, et al. Electrosorption of uranium ions on activated carbon fibers[J]. Journal of Radioanalytical and Nuclear Chemistry, 2011, 287(3): 833-839. |
| 65 | Ismail A F, Yim M S. Investigation of activated carbon adsorbent electrode for electrosorption-based uranium extraction from seawater[J]. Nuclear Engineering and Technology, 2015, 47(5): 579-587. |
| 66 | Zhou J, Zhou H J, Zhang Y Z, et al. Pseudocapacitive deionization of uranium(Ⅵ) with WO3/C electrode[J]. Chemical Engineering Journal, 2020, 398: 125460. |
| 67 | Liu X L, Xie Y H, Hao M J, et al. Highly efficient electrocatalytic uranium extraction from seawater over an amidoxime-functionalized In-N-C catalyst[J]. Advanced Science, 2022, 9(23): 2201735. |
| 68 | Wang W W, Liu S J, Zhou Y M, et al. Extraction of Sr2+ from aqueous solutions using an asymmetric pulsed current-assisted electrochemical method[J]. Separation and Purification Technology, 2021, 276: 119235. |
| 69 | Yang H, Liu X L, Hao M J, et al. Functionalized iron-nitrogen-carbon electrocatalyst provides a reversible electron transfer platform for efficient uranium extraction from seawater[J]. Advanced Materials, 2021, 33(51): 2106621. |
| 70 | Wang C Y, Xu M Y, Wang W W, et al. A supramolecular organic framework-mediated electrochemical strategy achieves highly selective and continuous uranium extraction[J]. Advanced Functional Materials, 2024, 34(41): 2402130. |
| 71 | Zhang C R, Qi J X, Cui W R, et al. A novel 3D sp2 carbon-linked covalent organic framework as a platform for efficient electro-extraction of uranium[J]. Science China Chemistry, 2023, 66(2): 562-569. |
| 72 | Zaheri A, Moheb A, Keshtkar A, et al. Uranium separation from wastewater by electrodialysis[J]. Iranian Journal of Environmental Health Science & Engineering, 2010, 7: 423-430. |
| 73 | Li R R, Wang H Y, Yan J Y, et al. A cascade electro-dehydration process for simultaneous extraction and enrichment of uranium from simulated seawater[J]. Water Research, 2023, 240: 120079. |
| 74 | Li T H, Ye H, Jin J M, et al. Electric-field strengthening uranium extraction from seawater assisted by nanofiltration membranes with sieving-adsorption properties[J]. Journal of Membrane Science, 2024, 693: 122334. |
| 75 | Li Z X, Li Z, Huang H, et al. Green lithium: photoelectrochemical extraction[J]. PhotoniX, 2023, 4(1): 23. |
| 76 | Li H N, Zhang C, Xin J H, et al. Design of photothermal "ion pumps" for achieving energy-efficient, augmented, and durable lithium extraction from seawater[J]. ACS Nano, 2024, 18(3): 2434-2445. |
| 77 | Chen X, Yu W H, Zhang Y, et al. Solar-driven lithium extraction by a floating felt[J]. Advanced Functional Materials, 2024, 34(28): 2316178. |
| 78 | Wang M L, Zhang T Y, Meng Z X, et al. Self-intercepting interference of hydrogen-bond induced flexible hybrid film to facilitate lithium extraction[J]. Chemical Engineering Journal, 2023, 458: 141403. |
| 79 | Li Z, Chen I C, Cao L, et al. Lithium extraction from brine through a decoupled and membrane-free electrochemical cell design[J]. Science, 2024, 385(6716): 1438-1444. |
| 80 | Bai L, Xu R B, Wu W J, et al. Insights into adsorbent materials for lithium extraction by capacitive deionization: reconceptualizing the role of materials informatics[J]. Journal of Materials Chemistry A, 2024, 12(18): 10676-10685. |
| 81 | Li Z, Li C Y, Liu X W, et al. Continuous electrical pumping membrane process for seawater lithium mining[J]. Energy & Environmental Science, 2021, 14(5): 3152-3159. |
| 82 | Wang W G, Hong G H, Zhang Y Q, et al. Designing an energy-efficient multi-stage selective electrodialysis process based on high-performance materials for lithium extraction[J]. Journal of Membrane Science, 2023, 675: 121534. |
| 83 | Yin X C, Xu P, Wang H Y. Modification of cation exchange membranes for enhanced extraction of lithium from magnesium and sodium brine solutions via selective electrodialysis[J]. Journal of Membrane Science, 2024, 701: 122705. |
| 84 | Liu C, Li Y B, Lin D C, et al. Lithium extraction from seawater through pulsed electrochemical intercalation[J]. Joule, 2020, 4(7): 1459-1469. |
| 85 | Yu J Z, Fang D L, Zhang H, et al. Ocean mining: a fluidic electrochemical route for lithium extraction from seawater[J]. ACS Materials Letters, 2020, 2(12): 1662-1668. |
| 86 | Liu D F, Sun S Y, Yu J G. A new high-efficiency process for Li+ recovery from solutions based on LiMn2O4/λ-MnO2 materials[J]. Chemical Engineering Journal, 2019, 377: 119825. |
| 87 | Wang W W, Xu M Y, Wu H T, et al. Precise electrocatalysis on Fe-porphyrin conjugated networks achieves energy-efficient extraction of uranium[J]. Advanced Science, 2024, 11(44): e2409084. |
| 88 | Xie Y, Liu Z Y, Geng Y Y, et al. Uranium extraction from seawater: material design, emerging technologies and marine engineering[J]. Chemical Society Reviews, 2023, 52(1): 97-162. |
| [1] | 何燎, 李俊, 高梦舒, 刘东阳, 张宇豪, 赵亮, 高金森, 徐春明. 石油烃中芳烃分离技术研究进展[J]. 化工学报, 2025, 76(5): 1909-1926. |
| [2] | 李坤, 黄锐, 丛君, 马海涛, 常龙娇, 罗绍华. NCM622正极材料结构形态和储锂特性的同步演变[J]. 化工学报, 2025, 76(4): 1831-1840. |
| [3] | 石孟琪, 王欢, 王守娟, 席跃宾, 孔凡功. 木质素基炭材料的制备及其在锂硫电池中的研究进展[J]. 化工学报, 2025, 76(4): 1463-1483. |
| [4] | 吴迪, 刘世朋, 王文伟, 姜久春, 杨晓光. 机械压力对锂金属电池性能影响的研究进展[J]. 化工学报, 2025, 76(4): 1422-1431. |
| [5] | 周印洁, 吉思蓓, 何松阳, 吉旭, 贺革. 机器学习辅助高通量筛选金属有机骨架用于富碳天然气中分离CO2[J]. 化工学报, 2025, 76(3): 1093-1101. |
| [6] | 马钟琛, 魏子杰, 朱明涛, 叶恒棣, 郭学益, 谭磊. 一步氧化法制备锰酸锂正极材料用电池级四氧化三锰[J]. 化工学报, 2025, 76(3): 1363-1374. |
| [7] | 孟祥军, 杨林睿, 彭立培, 杨献奎, 花莹曦, 张人仁, 郑凯天, 许春建. 三氟化氮精馏分离流程的设计与控制[J]. 化工学报, 2025, 76(2): 707-717. |
| [8] | 崔家馨, 殷梦凡, 郑涛, 刘晗, 张睿, 刘植昌, 刘海燕, 徐春明, 孟祥海. 铝铜双金属离子液体在1-己烯/正己烷分离中的应用[J]. 化工学报, 2025, 76(2): 686-694. |
| [9] | 贾晶宇, 孔德齐, 沈圆辉, 张东辉, 李文彬, 唐忠利. 合成氨反应器尾气变压吸附氨分离工艺的模拟与分析[J]. 化工学报, 2025, 76(2): 718-730. |
| [10] | 李舒月, 王欢, 周少强, 毛志宏, 张永民, 王军武, 吴秀花. 重质颗粒流态化研究现状与展望[J]. 化工学报, 2025, 76(2): 466-483. |
| [11] | 杨林睿, 刘鉴漪, 李玲, 何永超, 郑凯天, 任建坡, 许春建. 苯/环己烷/环己烯萃取精馏过程的流程设计与节能[J]. 化工学报, 2025, 76(2): 731-743. |
| [12] | 尤潇楠, 范小强, 杨遥, 王靖岱, 阳永荣. 超临界乙烯和高压聚乙烯混合物的减压分离过程建模方法[J]. 化工学报, 2025, 76(2): 695-706. |
| [13] | 李匡奚, 于佩潜, 王江云, 魏浩然, 郑志刚, 冯留海. 微气泡旋流气浮装置内流动分析与结构优化[J]. 化工学报, 2024, 75(S1): 223-234. |
| [14] | 谢慧慧, 姜佳鑫, 王鑫, 李正, 郭鑫, 吕欣然, 王凌云, 刘杨. 深共晶溶剂聚合物包覆膜传输分离铂、钯的研究[J]. 化工学报, 2024, 75(S1): 235-243. |
| [15] | 邱知, 谭明. 聚离子液体膜的制备及其在低钠高钾健康酱油中的应用[J]. 化工学报, 2024, 75(S1): 244-250. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号