化工学报 ›› 2025, Vol. 76 ›› Issue (2): 654-666.DOI: 10.11949/0438-1157.20240266
• 催化、动力学与反应器 • 上一篇
收稿日期:2024-05-05
修回日期:2024-06-11
出版日期:2025-03-25
发布日期:2025-03-10
通讯作者:
周静红
作者简介:何传超(1995—),男,硕士研究生,1982950218@qq.com
基金资助:
Chuanchao HE( ), Jinghong ZHOU(
), Jinghong ZHOU( ), Yueqiang CAO, Yao SHI, Xinggui ZHOU
), Yueqiang CAO, Yao SHI, Xinggui ZHOU
Received:2024-05-05
Revised:2024-06-11
Online:2025-03-25
Published:2025-03-10
Contact:
Jinghong ZHOU
摘要:
草酸二甲酯(DMO)选择性加氢制乙醇酸甲酯(MG)过程中由于目标产物为串联加氢反应的中间产物,催化剂颗粒尺度以及床层尺度的传递过程对于催化性能具有显著影响,因此颗粒催化剂的结构设计对于其工业应用极为重要。首先通过实验获取了Ag/SiO2催化DMO加氢制MG的动力学方程,在此基础上构建了床层-颗粒双尺度耦合的二维反应器模型,从床层尺度与颗粒尺度描述DMO加氢过程的传递与反应过程,考察了颗粒催化剂内活性组分的宏观分布对其在单管反应器内催化性能的影响。模拟计算结果表明,采用均匀型催化剂的反应器中存在着严重的MG过度加氢问题,采用蛋壳型催化剂则能够有效抑制过度加氢产物乙二醇的生成,提升MG的收率,且能够减少活性组分用量,显著提高DMO加氢制MG工艺的经济效益。研究结果可为DMO加氢制MG工业催化剂的结构设计和制备提供基础知识和指导。
中图分类号:
何传超, 周静红, 曹约强, 施尧, 周兴贵. Ag/SiO2催化草酸酯加氢制乙醇酸甲酯的床层-颗粒双尺度耦合模拟研究[J]. 化工学报, 2025, 76(2): 654-666.
Chuanchao HE, Jinghong ZHOU, Yueqiang CAO, Yao SHI, Xinggui ZHOU. Bed-particle dual scale coupled simulation on Ag/SiO2 catalyzed hydrogenation of oxalate to methyl glycolate[J]. CIESC Journal, 2025, 76(2): 654-666.
| ζ | 浓度边界条件 | 温度边界条件 |
|---|---|---|
| 0 | ||
| 1 |
表1 颗粒模型的边界条件
Table 1 Boundary conditions applied to the particle model
| ζ | 浓度边界条件 | 温度边界条件 |
|---|---|---|
| 0 | ||
| 1 |
| 参数 | 浓度边界条件 | 温度边界条件 | 速度边界条件 |
|---|---|---|---|
| z=0,∀r | cf,i =cf,i0 | T=T0 | u=u0 |
| z=Ht,∀r | P=P0 | ||
| r=0,∀z | |||
| r=R,∀z | T=Tw | u=0 |
表2 床层模型的边界条件
Table 2 Boundary conditions applied to the bed model
| 参数 | 浓度边界条件 | 温度边界条件 | 速度边界条件 |
|---|---|---|---|
| z=0,∀r | cf,i =cf,i0 | T=T0 | u=u0 |
| z=Ht,∀r | P=P0 | ||
| r=0,∀z | |||
| r=R,∀z | T=Tw | u=0 |
| 参数 | 数值 |
|---|---|
| 颗粒直径(dpe)/mm | 2 |
| 孔径(dpore)/nm | 6 |
| 孔隙率(εpe) | 0.6 |
| 曲折因子(τ) | 1.6667 |
| 密度(ρpe)/(kg/m3) | 880 |
| 热导率(λpe)/(W/(m∙K)) | 0.574 |
| 比热容(Cp,pe)/(J/(kg∙K)) | 1000 |
表3 颗粒催化剂的基本性质
Table 3 Properties of the catalyst pellets
| 参数 | 数值 |
|---|---|
| 颗粒直径(dpe)/mm | 2 |
| 孔径(dpore)/nm | 6 |
| 孔隙率(εpe) | 0.6 |
| 曲折因子(τ) | 1.6667 |
| 密度(ρpe)/(kg/m3) | 880 |
| 热导率(λpe)/(W/(m∙K)) | 0.574 |
| 比热容(Cp,pe)/(J/(kg∙K)) | 1000 |
| 参数 | 数值 |
|---|---|
| 反应器内径(dbed)/mm | 32 |
| 床层高度(Ht)/m | 1 |
| 床层空隙率(εbed) | 0.45 |
| 进口温度(T0)/K | 493.15 |
| 反应器壁温(Tw)/K | 493.15 |
| 进口压力(P0)/MPa | 2 |
| DMO进口摩尔分数(yDMO) | 7.4532×10-3 |
| H2进口摩尔分数( | 7.4532×10-1 |
| 甲醇(ME)进口摩尔分数(yME) | 2.4723×10-1 |
| 质量空速(WHSV)/h-1 | 0.5 |
表4 床层性质与操作参数
Table 4 The bed properties and operating parameters
| 参数 | 数值 |
|---|---|
| 反应器内径(dbed)/mm | 32 |
| 床层高度(Ht)/m | 1 |
| 床层空隙率(εbed) | 0.45 |
| 进口温度(T0)/K | 493.15 |
| 反应器壁温(Tw)/K | 493.15 |
| 进口压力(P0)/MPa | 2 |
| DMO进口摩尔分数(yDMO) | 7.4532×10-3 |
| H2进口摩尔分数( | 7.4532×10-1 |
| 甲醇(ME)进口摩尔分数(yME) | 2.4723×10-1 |
| 质量空速(WHSV)/h-1 | 0.5 |
| 分子 | 扩散体积/(cm3/mol) |
|---|---|
| DMO | 101.9 |
| H2 | 6.12 |
| MG | 79.89 |
| 乙二醇(EG) | 57.88 |
| 甲醇(ME) | 31.15 |
表5 各物质的扩散体积
Table 5 Diffusion volume of each substance
| 分子 | 扩散体积/(cm3/mol) |
|---|---|
| DMO | 101.9 |
| H2 | 6.12 |
| MG | 79.89 |
| 乙二醇(EG) | 57.88 |
| 甲醇(ME) | 31.15 |
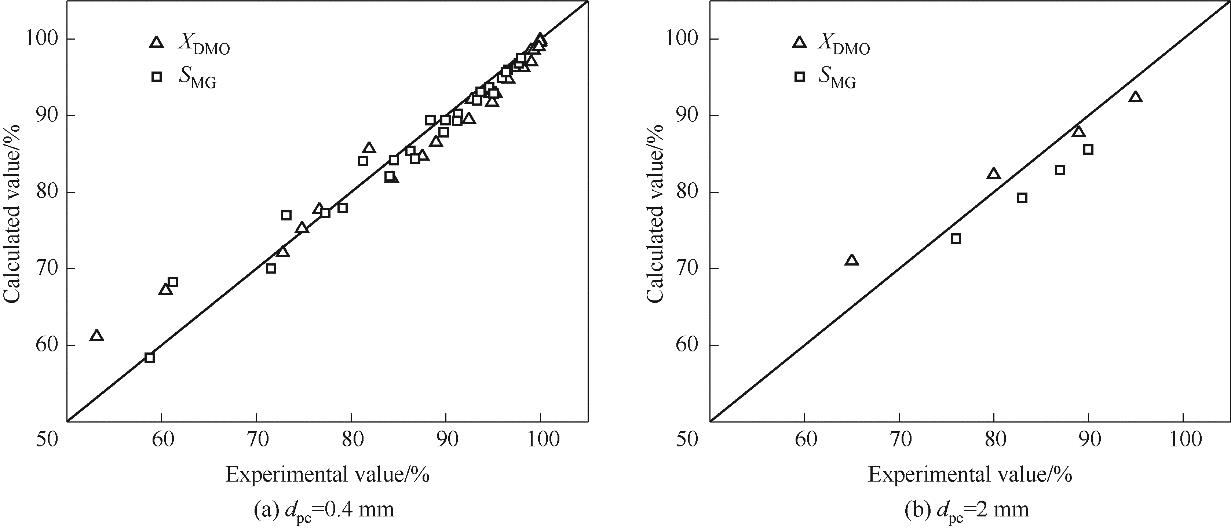
图3 0.4及2 mm颗粒催化剂在管式反应器内DMO加氢反应双尺度模型计算值与实验值的比较
Fig.3 Comparison of experimental and simulated values of DMO conversion and MG selectivity for catalyst pellets of 0.4 and 2 mm
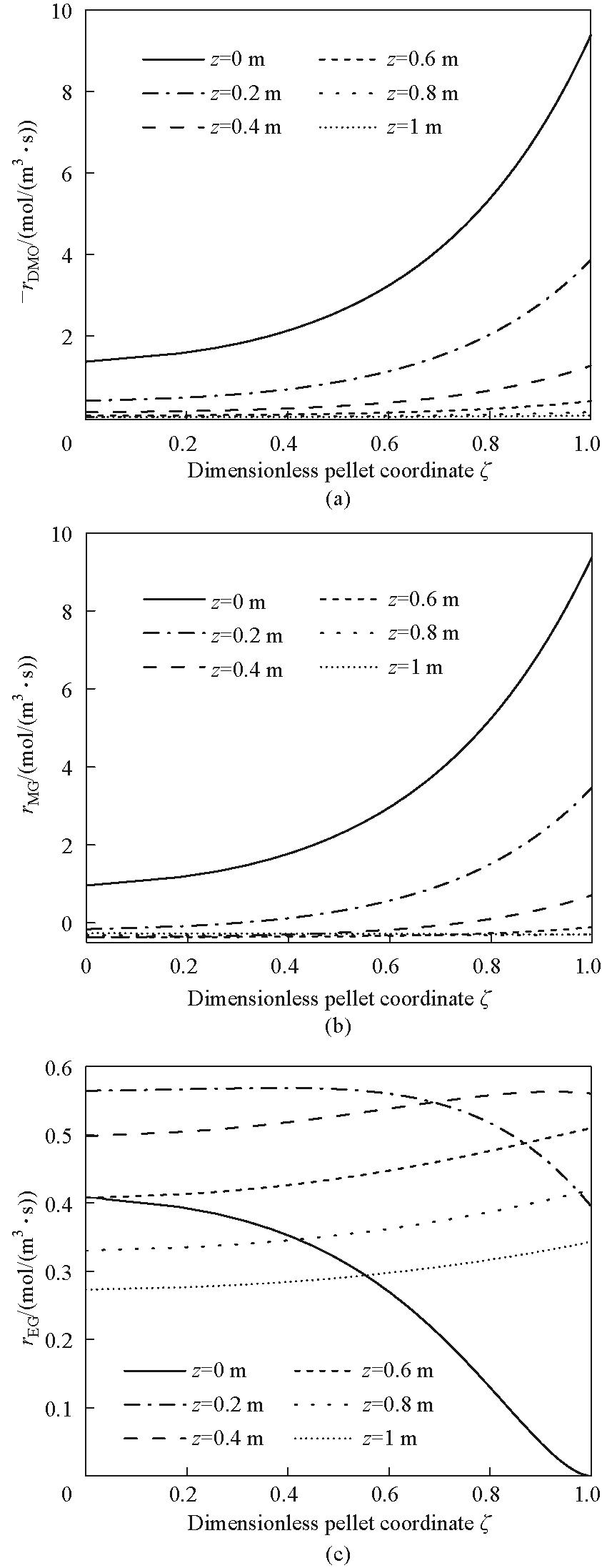
图5 反应管中心床层轴向不同位置颗粒内部DMO消耗速率、MG生成速率及EG生成速率
Fig.5 The internal DMO consumption rate, MG generation rate, and EG generation rate of the tube-centered particles at different axial positions along the bed height

图7 不同相对蛋壳厚度的催化剂对床层中MG选择性与DMO转化率的影响
Fig.7 The effect of relative eggshell thickness of pellet catalysts on MG selectivity and DMO conversion along the bed height
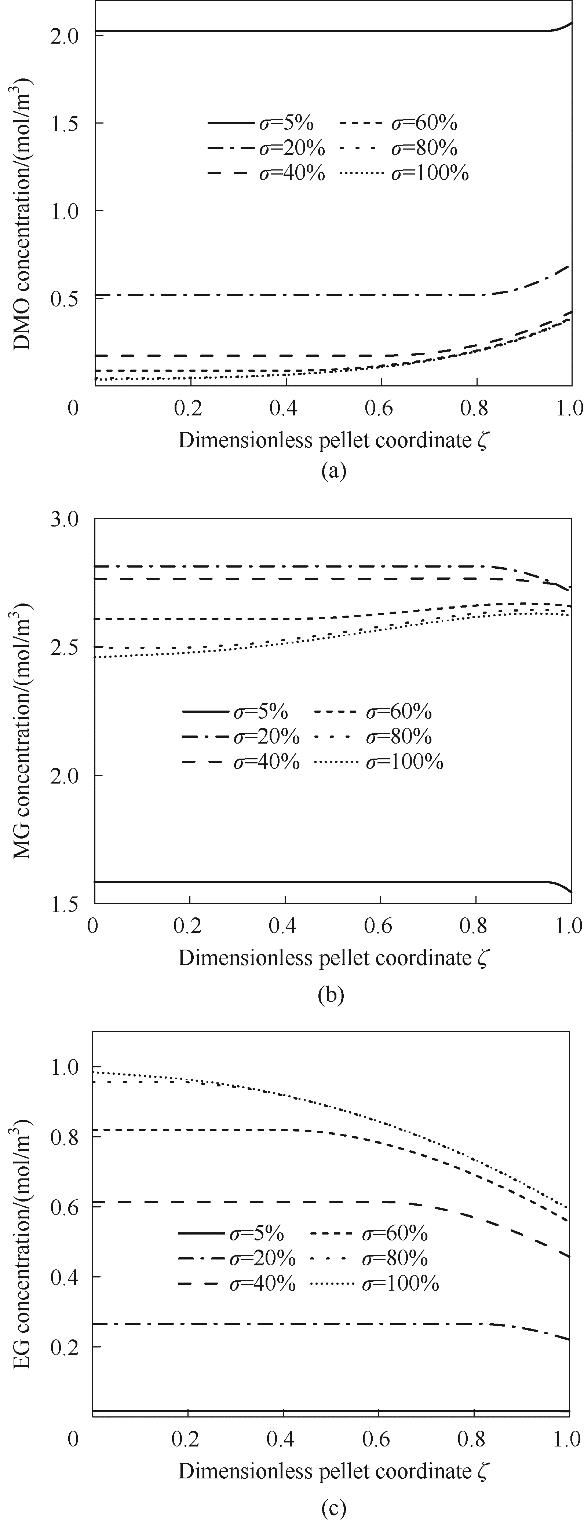
图8 床层z=0.4 m处不同蛋壳厚度的催化剂颗粒内部DMO、MG及EG的浓度分布
Fig.8 The concentration distribution of DMO, MG, and EG inside catalyst pellets with different relative eggshell thicknesses at z=0.4m along the catalyst bed height
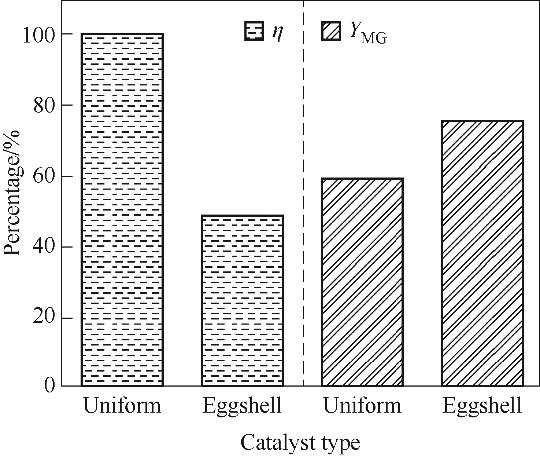
图9 均匀型催化剂与蛋壳型催化剂(σ=20%)的活性组分用量和MG收率比较
Fig.9 The comparison of active component loading and MG yield between the homogeneous and the eggshell pellet catalysts
| 序号 | T/K | WHSV/h-1 | H/D | XDMO/% | SMG/% | SEG/% |
|---|---|---|---|---|---|---|
| 1 | 493.45 | 0.5 | 100 | 99.96 | 58.76 | 41.24 |
| 2 | 493.45 | 0.75 | 100 | 99.85 | 71.55 | 28.45 |
| 3 | 493.25 | 1 | 40 | 98.18 | 76.12 | 23.88 |
| 4 | 493.25 | 1 | 80 | 99.02 | 75.69 | 24.31 |
| 5 | 493.25 | 1 | 100 | 98.99 | 77.29 | 22.71 |
| 6 | 493.25 | 1 | 120 | 99.32 | 75.77 | 24.23 |
| 7 | 493.15 | 1.25 | 100 | 97.86 | 84.07 | 15.93 |
| 8 | 493.15 | 1.5 | 100 | 96.59 | 86.26 | 13.74 |
| 9 | 487.85 | 0.5 | 100 | 99.95 | 61.19 | 38.81 |
| 10 | 488.25 | 0.75 | 100 | 99.30 | 79.11 | 20.89 |
| 11 | 488.45 | 1 | 40 | 96.01 | 86.55 | 13.45 |
| 12 | 488.25 | 1 | 80 | 97.90 | 84.75 | 15.25 |
| 13 | 488.25 | 1 | 100 | 98.20 | 84.53 | 15.47 |
| 14 | 488.25 | 1 | 120 | 98.43 | 84.34 | 15.66 |
| 15 | 488.15 | 1.25 | 100 | 94.76 | 89.78 | 10.22 |
| 16 | 488.25 | 1.5 | 100 | 92.43 | 91.29 | 8.71 |
| 17 | 483.05 | 0.5 | 100 | 99.83 | 73.17 | 26.83 |
| 18 | 483.25 | 0.75 | 100 | 97.22 | 86.76 | 13.24 |
| 19 | 482.55 | 1 | 40 | 84.12 | 92.76 | 7.24 |
| 20 | 482.85 | 1 | 80 | 93.16 | 90.67 | 9.33 |
| 21 | 483.15 | 1 | 100 | 94.90 | 89.97 | 10.03 |
| 22 | 482.95 | 1 | 120 | 95.69 | 89.69 | 10.31 |
| 23 | 483.05 | 1.25 | 100 | 88.97 | 93.30 | 6.70 |
| 24 | 483.15 | 1.5 | 100 | 84.31 | 94.59 | 5.41 |
| 25 | 477.85 | 0.5 | 100 | 99.03 | 81.27 | 18.73 |
| 26 | 478.05 | 0.75 | 100 | 92.74 | 91.20 | 8.80 |
| 27 | 478.15 | 1 | 40 | 69.59 | 95.05 | 4.95 |
| 28 | 478.05 | 1 | 80 | 84.68 | 94.10 | 5.90 |
| 29 | 478.05 | 1 | 100 | 87.53 | 93.67 | 6.33 |
| 30 | 477.95 | 1 | 120 | 90.42 | 93.36 | 6.64 |
| 31 | 477.95 | 1.25 | 100 | 76.65 | 95.92 | 4.08 |
| 32 | 477.95 | 1.5 | 100 | 72.81 | 96.55 | 3.45 |
| 33 | 473.05 | 0.5 | 100 | 95.26 | 88.39 | 11.61 |
| 34 | 473.25 | 0.75 | 100 | 81.91 | 95.03 | 4.97 |
| 35 | 473.15 | 1 | 40 | 59.68 | 96.70 | 3.30 |
| 36 | 473.15 | 1 | 80 | 71.13 | 96.46 | 3.54 |
| 37 | 473.25 | 1 | 100 | 74.83 | 96.31 | 3.69 |
| 38 | 473.15 | 1 | 120 | 79.64 | 96.07 | 3.93 |
| 39 | 473.35 | 1.25 | 100 | 60.44 | 97.68 | 2.32 |
| 40 | 473.35 | 1.5 | 100 | 53.17 | 97.97 | 2.03 |
表A1 动力学实验结果
Table A1 Results of kinetic experiments
| 序号 | T/K | WHSV/h-1 | H/D | XDMO/% | SMG/% | SEG/% |
|---|---|---|---|---|---|---|
| 1 | 493.45 | 0.5 | 100 | 99.96 | 58.76 | 41.24 |
| 2 | 493.45 | 0.75 | 100 | 99.85 | 71.55 | 28.45 |
| 3 | 493.25 | 1 | 40 | 98.18 | 76.12 | 23.88 |
| 4 | 493.25 | 1 | 80 | 99.02 | 75.69 | 24.31 |
| 5 | 493.25 | 1 | 100 | 98.99 | 77.29 | 22.71 |
| 6 | 493.25 | 1 | 120 | 99.32 | 75.77 | 24.23 |
| 7 | 493.15 | 1.25 | 100 | 97.86 | 84.07 | 15.93 |
| 8 | 493.15 | 1.5 | 100 | 96.59 | 86.26 | 13.74 |
| 9 | 487.85 | 0.5 | 100 | 99.95 | 61.19 | 38.81 |
| 10 | 488.25 | 0.75 | 100 | 99.30 | 79.11 | 20.89 |
| 11 | 488.45 | 1 | 40 | 96.01 | 86.55 | 13.45 |
| 12 | 488.25 | 1 | 80 | 97.90 | 84.75 | 15.25 |
| 13 | 488.25 | 1 | 100 | 98.20 | 84.53 | 15.47 |
| 14 | 488.25 | 1 | 120 | 98.43 | 84.34 | 15.66 |
| 15 | 488.15 | 1.25 | 100 | 94.76 | 89.78 | 10.22 |
| 16 | 488.25 | 1.5 | 100 | 92.43 | 91.29 | 8.71 |
| 17 | 483.05 | 0.5 | 100 | 99.83 | 73.17 | 26.83 |
| 18 | 483.25 | 0.75 | 100 | 97.22 | 86.76 | 13.24 |
| 19 | 482.55 | 1 | 40 | 84.12 | 92.76 | 7.24 |
| 20 | 482.85 | 1 | 80 | 93.16 | 90.67 | 9.33 |
| 21 | 483.15 | 1 | 100 | 94.90 | 89.97 | 10.03 |
| 22 | 482.95 | 1 | 120 | 95.69 | 89.69 | 10.31 |
| 23 | 483.05 | 1.25 | 100 | 88.97 | 93.30 | 6.70 |
| 24 | 483.15 | 1.5 | 100 | 84.31 | 94.59 | 5.41 |
| 25 | 477.85 | 0.5 | 100 | 99.03 | 81.27 | 18.73 |
| 26 | 478.05 | 0.75 | 100 | 92.74 | 91.20 | 8.80 |
| 27 | 478.15 | 1 | 40 | 69.59 | 95.05 | 4.95 |
| 28 | 478.05 | 1 | 80 | 84.68 | 94.10 | 5.90 |
| 29 | 478.05 | 1 | 100 | 87.53 | 93.67 | 6.33 |
| 30 | 477.95 | 1 | 120 | 90.42 | 93.36 | 6.64 |
| 31 | 477.95 | 1.25 | 100 | 76.65 | 95.92 | 4.08 |
| 32 | 477.95 | 1.5 | 100 | 72.81 | 96.55 | 3.45 |
| 33 | 473.05 | 0.5 | 100 | 95.26 | 88.39 | 11.61 |
| 34 | 473.25 | 0.75 | 100 | 81.91 | 95.03 | 4.97 |
| 35 | 473.15 | 1 | 40 | 59.68 | 96.70 | 3.30 |
| 36 | 473.15 | 1 | 80 | 71.13 | 96.46 | 3.54 |
| 37 | 473.25 | 1 | 100 | 74.83 | 96.31 | 3.69 |
| 38 | 473.15 | 1 | 120 | 79.64 | 96.07 | 3.93 |
| 39 | 473.35 | 1.25 | 100 | 60.44 | 97.68 | 2.32 |
| 40 | 473.35 | 1.5 | 100 | 53.17 | 97.97 | 2.03 |
| 1 | Zhou R J, Yan W Q, Cao Y Q, et al. Probing the structure sensitivity of dimethyl oxalate partial hydrogenation over Ag nanoparticles: a combined experimental and microkinetic study[J]. Chemical Engineering Science, 2022, 259: 117830. |
| 2 | Yang Q C, Fan Y J, Liu C L, et al. A promising alternative potential solution for sustainable and economical development of coal to ethylene glycol industry: dimethyl oxalate to methyl glycolate process[J]. Energy, 2023, 277: 127668. |
| 3 | Xie T H, Ai S, Huang Y C, et al. Synthesis and purification of glycolic acid from the mixture of methyl levulinate and methyl glycolate via acid-mediated hydrolysis reactions and extraction[J]. Separation and Purification Technology, 2021, 268: 118718. |
| 4 | Sun Y, Wang H, Shen J H, et al. Highly effective synthesis of methyl glycolate with heteropolyacids as catalysts[J]. Catalysis Communications, 2009, 10(5): 678-681. |
| 5 | Xu Y, Dou W J, Zhao Y J, et al. Kinetics study for ion-exchange-resin catalyzed hydrolysis of methyl glycolate[J]. Industrial & Engineering Chemistry Research, 2012, 51(36): 11653-11658. |
| 6 | 袁彩彩. 乙醇酸及聚乙醇酸的合成研究[D]. 郑州: 郑州大学, 2019. |
| Yuan C C. Synthesis of glycolic acid and polyglycolic acid[D]. Zhengzhou: Zhengzhou University, 2019. | |
| 7 | 张军伟, 陈建军, 贾彩敬. 一种多柱连续色谱除盐以纯化羟基乙酸的方法: 111635302B[P]. 2021-06-15. |
| Zhang J W, Chen J J, Jia C J. Method of purifying glycolic acid through multi-column continuous chromatographic desalination: 111635302B[P]. 2021-06-15. | |
| 8 | 石磊, 陈飞, 姚杰. 一种制备乙醇酸甲酯并副产甲氧基乙酸甲酯的方法: 107337602A[P]. 2017-11-10. |
| Shi L, Chen F, Yao J. Method for preparing methyl glycolate and by-producing methyl methoxyacetate: 107337602A[P]. 2017-11-10. | |
| 9 | 李锦春. 乙醇酸甲酯的合成及其开发前景[J]. 四川化工与腐蚀控制, 1998, 1(6): 24-27. |
| Li J C. Synthesis and development prospect of methyl glycolate[J]. Sichuan Chemical Industry and Corrosion Control, 1998, 1(6): 24-27. | |
| 10 | Dong G L, Luo Z W, Cao Y Q, et al. Understanding size-dependent hydrogenation of dimethyl oxalate to methyl glycolate over Ag catalysts[J]. Journal of Catalysis, 2021, 401: 252-261. |
| 11 | Chen H M, Tan J J, Zhu Y L, et al. An effective and stable Ni2P/TiO2 catalyst for the hydrogenation of dimethyl oxalate to methyl glycolate[J]. Catalysis Communications, 2016, 73: 46-49. |
| 12 | Niu J C, Ai P P, Guo Q W, et al. The effect of nitrogen doping on hydrogenation capability and stability of Cu-based catalyst in ester hydrogenation to methyl glycolate[J]. Fuel, 2023, 351: 128866. |
| 13 | Xue J, Wu M M, Song Y W, et al. Study on performance of Ag-modified layered copper silicate catalyst for hydrogenation of dimethyl oxalate to methyl glycolate[J]. Journal of Fuel Chemistry and Technology, 2022, 50(8): 1014-1022. |
| 14 | Dong G L, Cao Y Q, Zheng S N, et al. Catalyst consisting of Ag nanoparticles anchored on amine-derivatized mesoporous silica nanospheres for the selective hydrogenation of dimethyl oxalate to methyl glycolate[J]. Journal of Catalysis, 2020, 391: 155-162. |
| 15 | Hu M L, Yan Y, Duan X P, et al. Effective anchoring of silver nanoparticles onto N-doped carbon with enhanced catalytic performance for the hydrogenation of dimethyl oxalate to methyl glycolate[J]. Catalysis Communications, 2017, 100: 148-152. |
| 16 | Jurtz N, Wehinger G D, Srivastava U, et al. Validation of pressure drop prediction and bed generation of fixed‐beds with complex particle shapes using discrete element method and computational fluid dynamics[J]. AIChE Journal, 2020, 66(6): 16967. |
| 17 | 胡鑫. 草酸二甲酯加氢制乙醇酸甲酯 Ag/SiO2蛋壳型催化剂的制备及性能[D]. 上海: 华东理工大学, 2022. |
| Hu X. Preparation and properties of Ag/SiO2 eggshell catalyst for hydrogenation of dimethyl oxalate to methyl glycolate[D]. Shanghai: East China University of Science and Technology, 2022. | |
| 18 | Froment G F, Bischoff K B, Wilde J D. Chemical Reactor Analysis and Design[M]. 3rd ed. New York: Wiley, 1990: 462-474. |
| 19 | Stitt H, Marigo M, Wilkinson S, et al. How good is your model?[J]. Johnson Matthey Technology Review, 2015, 59(2): 74-89. |
| 20 | Dong Y, Keil F J, Korup O, et al. Effect of the catalyst pore structure on fixed-bed reactor performance of partial oxidation of n-butane: a simulation study[J]. Chemical Engineering Science, 2016, 142: 299-309. |
| 21 | Krammer A, Peham M, Lehner M. 2D heterogeneous model of a polytropic methanation reactor[J]. Journal of CO2 Utilization, 2022, 62: 102059. |
| 22 | Bac S, Avci A K. Ethylene oxide synthesis in a wall-coated microchannel reactor with integrated cooling[J]. Chemical Engineering Journal, 2019, 377: 120104. |
| 23 | Marín P, Díez F V, Ordóñez S. Fixed bed membrane reactors for WGSR-based hydrogen production: optimisation of modelling approaches and reactor performance[J]. International Journal of Hydrogen Energy, 2012, 37(6): 4997-5010. |
| 24 | Liu X L, Qin B, Zhang Q F, et al. Optimizing catalyst supports at single catalyst pellet and packed bed reactor levels: a comparison study[J]. AIChE Journal, 2021, 67(8): 1-13. |
| 25 | Solsvik J, Jakobsen H A. A survey of multicomponent mass diffusion flux closures for porous pellets: mass and molar forms[J]. Transport in Porous Media, 2012, 93(1): 99-126. |
| 26 | Solsvik J, Jakobsen H A. Impacts on the reactor performance of intra-particle multicomponent mass diffusion limitations: Knudsen diffusion[J]. Energy Procedia, 2012, 26: 116-124. |
| 27 | Fuller E N, Schettler P D, Giddings J C. New method for prediction of binary gas-phase diffusion coefficients[J]. Industrial & Engineering Chemistry, 2002, 58(5): 18-27. |
| 28 | Thompson C R, Marín P, Díez F V, et al. Evaluation of the use of ceramic foams as catalyst supports for reverse-flow combustors[J]. Chemical Engineering Journal, 2013, 221: 44-54. |
| 29 | Winterberg M, Tsotsas E, Krischke A, et al. A simple and coherent set of coefficients for modelling of heat and mass transport with and without chemical reaction in tubes filled with spheres[J]. Chemical Engineering Science, 2000, 55(5): 967-979. |
| 30 | Tsotsas E. Wärmeleitung und dispersion in durchströmten schüttungen[M]//Stephan P, Kabelac S, Kind M, et al, eds. Springer Reference Technik. Berlin, Heidelberg: Springer Berlin Heidelberg, 2019: 1753-1772. |
| 31 | 李绍芬.反应工程[M]. 3版. 北京: 化学工业出版社, 2013: 50. |
| Li S F. Reaction Engineering[M]. 3rd ed. Beijing: Chemical Industry Press, 2013: 50. | |
| 32 | Luo Z W, Xu X F, Dong G L, et al. Regulating mesopore structures of support toward enhanced selective hydrogenation of dimethyl oxalate to methyl glycolate on Ag catalysts[J]. Chemical Engineering Journal, 2022, 450: 138397. |
| 33 | 邬时海, 卢立义, 谢在库, 等. 非均匀分布催化剂的性能及制备[J]. 工业催化, 2002, 10(6): 48-52. |
| Wu S H, Lu L Y, Xie Z K, et al. Properties and preparation of non-uniform distributed catalysts[J]. Gongye Cuihua (Industrial Catalysis), 2002, 10(6): 48-52. | |
| 34 | Xu X F, Hu X, Luo Z W, et al. Engineering an egg-shell structure for the Ag/SiO2 pellet catalyst for selective hydrogenation of dimethyl oxalate to methyl glycolate[J]. New Journal of Chemistry, 2023, 47(13): 6045-6049. |
| [1] | 张珂, 任维杰, 王梦娜, 范凯锋, 常丽萍, 李佳斌, 马涛, 田晋平. Bunsen反应产物在微通道中的液-液两相混合特性[J]. 化工学报, 2025, 76(2): 623-636. |
| [2] | 李舒月, 王欢, 周少强, 毛志宏, 张永民, 王军武, 吴秀花. 重质颗粒流态化研究现状与展望[J]. 化工学报, 2025, 76(2): 466-483. |
| [3] | 李奕菲, 苏沿霏, 尹甜, 姜浩强, 许志明, 张霖宙, 史权, 徐春明. 基于GC×GC-TOF MS的煤液化产物油分子组成结构表征[J]. 化工学报, 2025, 76(2): 543-553. |
| [4] | 彭子林, 周蕾, 邓庆航, 叶光华, 周兴贵. 包含偏硅酸影响的3D NAND磷酸湿法刻蚀动力学[J]. 化工学报, 2025, 76(2): 645-653. |
| [5] | 贾艳萍, 马艳菊, 管文昕, 杨彬, 张健, 张兰河. 响应面法优化Fe0/H2O2体系降解染料废水的工艺条件及机理[J]. 化工学报, 2025, 76(1): 348-362. |
| [6] | 韩启沃, 刘永峰, 裴普成, 张璐, 姚圣卓. 工作温度对PEMFC水分布、质子传输及性能影响分析[J]. 化工学报, 2025, 76(1): 374-384. |
| [7] | 邓志诚, 杨欢, 王斯民, 王家瑞. 微混燃烧器中微管结构对氢燃料掺混效果与燃烧性能影响[J]. 化工学报, 2025, 76(1): 335-347. |
| [8] | 杨晨, 毛伟, 董兴宗, 田松, 赵锋伟, 吕剑. 选择性加氢脱氯合成烯烃研究进展[J]. 化工学报, 2025, 76(1): 53-70. |
| [9] | 刘萍, 邱雨生, 李世婧, 孙瑞奇, 申晨. 微通道内纳米流体传热流动特性[J]. 化工学报, 2025, 76(1): 184-197. |
| [10] | 韩志敏, 周相宇, 张宏宇, 徐志明. 不同粗糙元结构下CaCO3污垢局部沉积特性[J]. 化工学报, 2025, 76(1): 151-160. |
| [11] | 郭珊, 田雨, 徐永滨, 王朋, 刘治明. 废旧电池再资源化制备高性能中熵合金催化剂及其性能研究[J]. 化工学报, 2025, 76(1): 231-240. |
| [12] | 王瀚彬, 胡帅, 毕丰雷, 李隽森, 贺来宾. 新型波纹翅片金属氢化物反应器的放氢性能有限元分析[J]. 化工学报, 2025, 76(1): 221-230. |
| [13] | 高羡明, 杨汶轩, 卢少辉, 任晓松, 卢方财. 双槽道结构对超疏水表面液滴合并弹跳的影响[J]. 化工学报, 2025, 76(1): 208-220. |
| [14] | 董新宇, 边龙飞, 杨怡怡, 张宇轩, 刘璐, 王腾. 冷却条件下倾斜上升管S-CO2流动与传热特性研究[J]. 化工学报, 2024, 75(S1): 195-205. |
| [15] | 郭骐瑞, 任丽媛, 陈康, 黄翔宇, 马卫华, 肖乐勤, 周伟良. 用于HTPB推进剂浆料的静态混合管数值模拟[J]. 化工学报, 2024, 75(S1): 206-216. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号