化工学报 ›› 2025, Vol. 76 ›› Issue (12): 6366-6375.DOI: 10.11949/0438-1157.20250519
收稿日期:2025-05-12
修回日期:2025-06-09
出版日期:2025-12-31
发布日期:2026-01-23
通讯作者:
杨菲菲
作者简介:周维(1991—),男,博士,讲师,zhouwei@cumt.edu.cn
基金资助:
Wei ZHOU( ), Zhiqian WU, Yapeng QIAO, Xinyu XIE, Feifei YANG(
), Zhiqian WU, Yapeng QIAO, Xinyu XIE, Feifei YANG( )
)
Received:2025-05-12
Revised:2025-06-09
Online:2025-12-31
Published:2026-01-23
Contact:
Feifei YANG
摘要:
酚类加氢脱氧是木质素转化为可再生液体燃料和芳烃化工原料的关键反应。本研究在惰性SiO2载体上负载非贵金属Ni和VO x 物种,得到系列NiV/SiO2催化剂。采用Raman、XRD、H2-TPR和NH3-TPD对催化剂结构和物化性质进行表征,并以间甲酚为木质素模型化合物,考察不同活性位对酚类加氢脱氧反应的催化性能。研究发现,Ni金属主要催化间甲酚加氢和C—C断键反应,对目标产物甲苯的选择性较低。而VO x 物种和Ni金属之间存在强相互作用,适量VO x 的引入能减小Ni金属的粒径,丰富Ni-VO x 界面位点,进而显著促进间甲酚选择性加氢脱氧为甲苯。结合工艺条件的考察和甲基环己醇探针反应,揭示了Ni-VO x 界面上酚类加氢脱氧主要为直接脱氧机理,为高效酚类脱氧催化剂的研制提供一定理论基础。
中图分类号:
周维, 吴之倩, 乔亚鹏, 谢欣雨, 杨菲菲. Ni-VO x 界面高效催化木质素衍生酚类加氢脱氧[J]. 化工学报, 2025, 76(12): 6366-6375.
Wei ZHOU, Zhiqian WU, Yapeng QIAO, Xinyu XIE, Feifei YANG. Efficient catalytic hydrodeoxygenation of lignin derived phenolics on Ni-VO x interface[J]. CIESC Journal, 2025, 76(12): 6366-6375.
| 催化剂 | 晶相粒径①/nm | 酸密度②/ (μmol·g-1) | |
|---|---|---|---|
| Ni | V2O3 | ||
| Ni/SiO2 | 18.0 | — | 15.3 |
| Ni4.3V/SiO2 | 6.3 | — | 181.8 |
| Ni8.6V/SiO2 | 6.5 | 20.4 | 295.4 |
| 4.3V/SiO2 | — | — | 362.7 |
表1 催化剂的晶相粒径和酸性
Table 1 Crystalline size and acid properties of catalysts
| 催化剂 | 晶相粒径①/nm | 酸密度②/ (μmol·g-1) | |
|---|---|---|---|
| Ni | V2O3 | ||
| Ni/SiO2 | 18.0 | — | 15.3 |
| Ni4.3V/SiO2 | 6.3 | — | 181.8 |
| Ni8.6V/SiO2 | 6.5 | 20.4 | 295.4 |
| 4.3V/SiO2 | — | — | 362.7 |
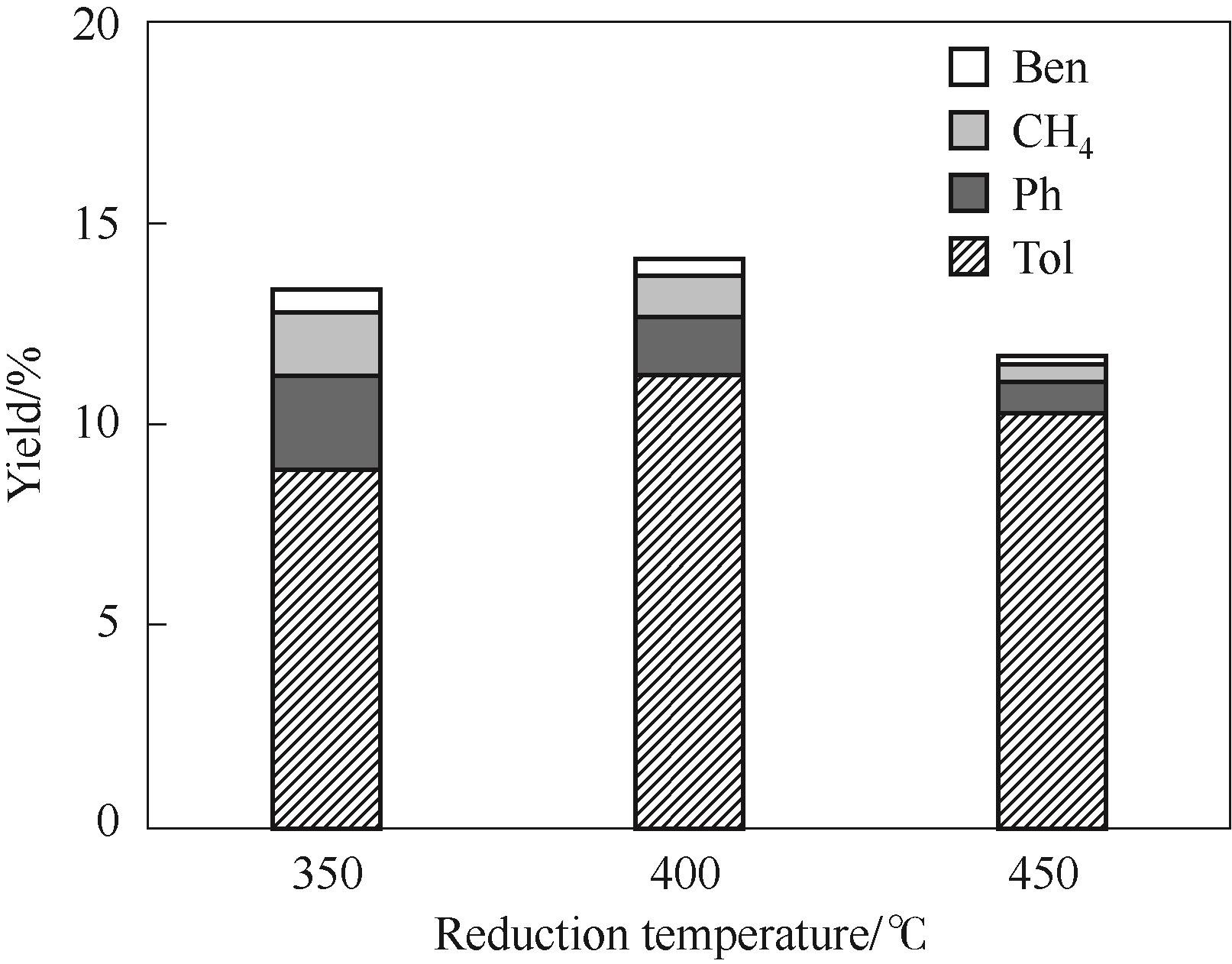
图6 Ni4.3V/SiO2的还原温度对反应性能的影响
Fig.6 Effect of reduction temperature of Ni4.3V/SiO2 on its catalytic performance[Ben: benzene; CH4: methane; Ph: phenol; Tol: toluene. Reaction conditions: 350℃, 1 atm(1 atm=101325 Pa), W/F = 0.05 h, H2/m = 146, TOS = 20 min]
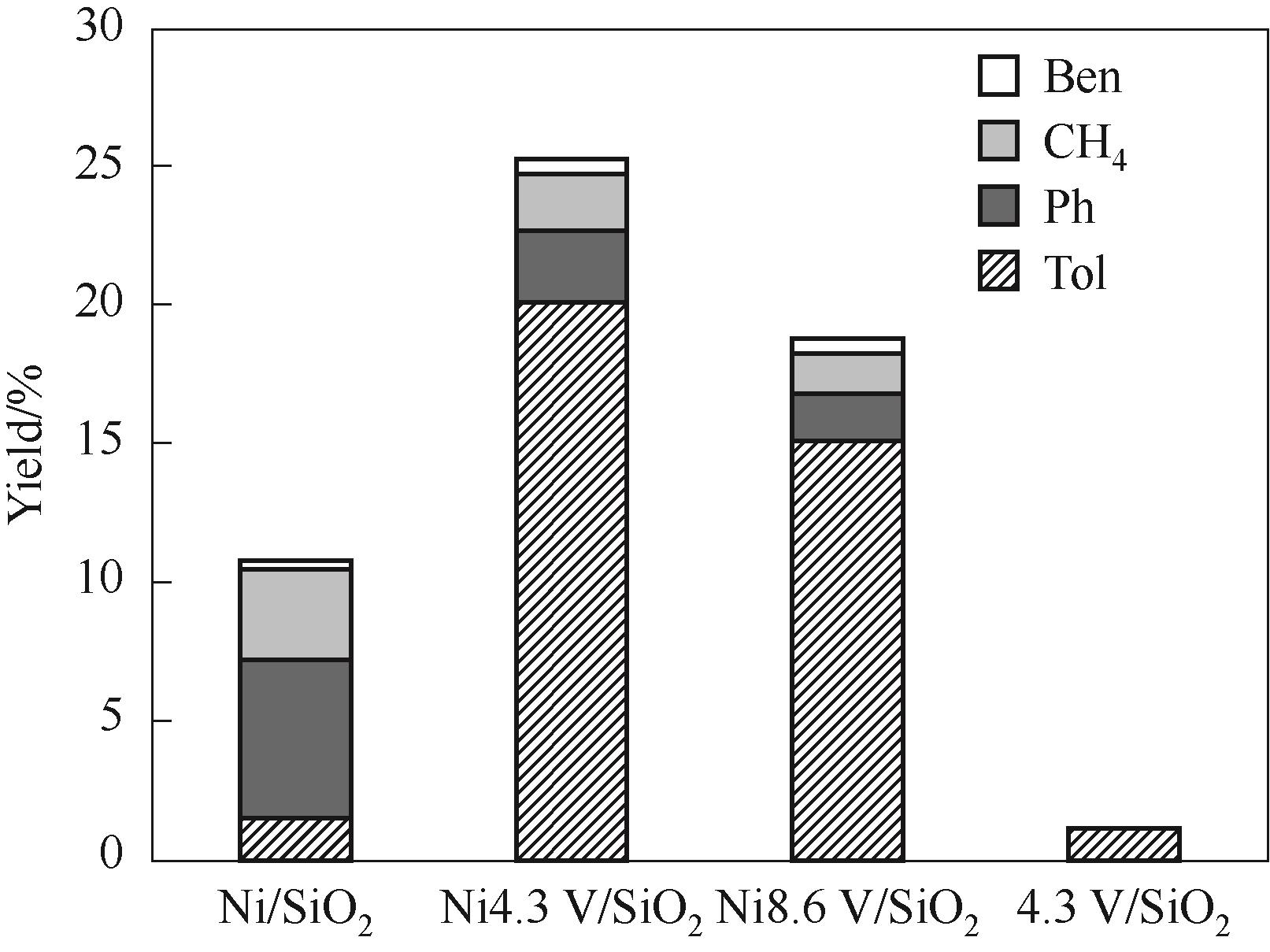
图7 350℃反应温度下不同催化剂在间甲酚加氢脱氧中的催化性能
Fig.7 Catalytic performance of various catalysts in m-cresol hydrodeoxygenation under 350℃(Reaction conditions: 1 atm, W/F = 0.09 h, H2/m = 146, TOS = 20 min)
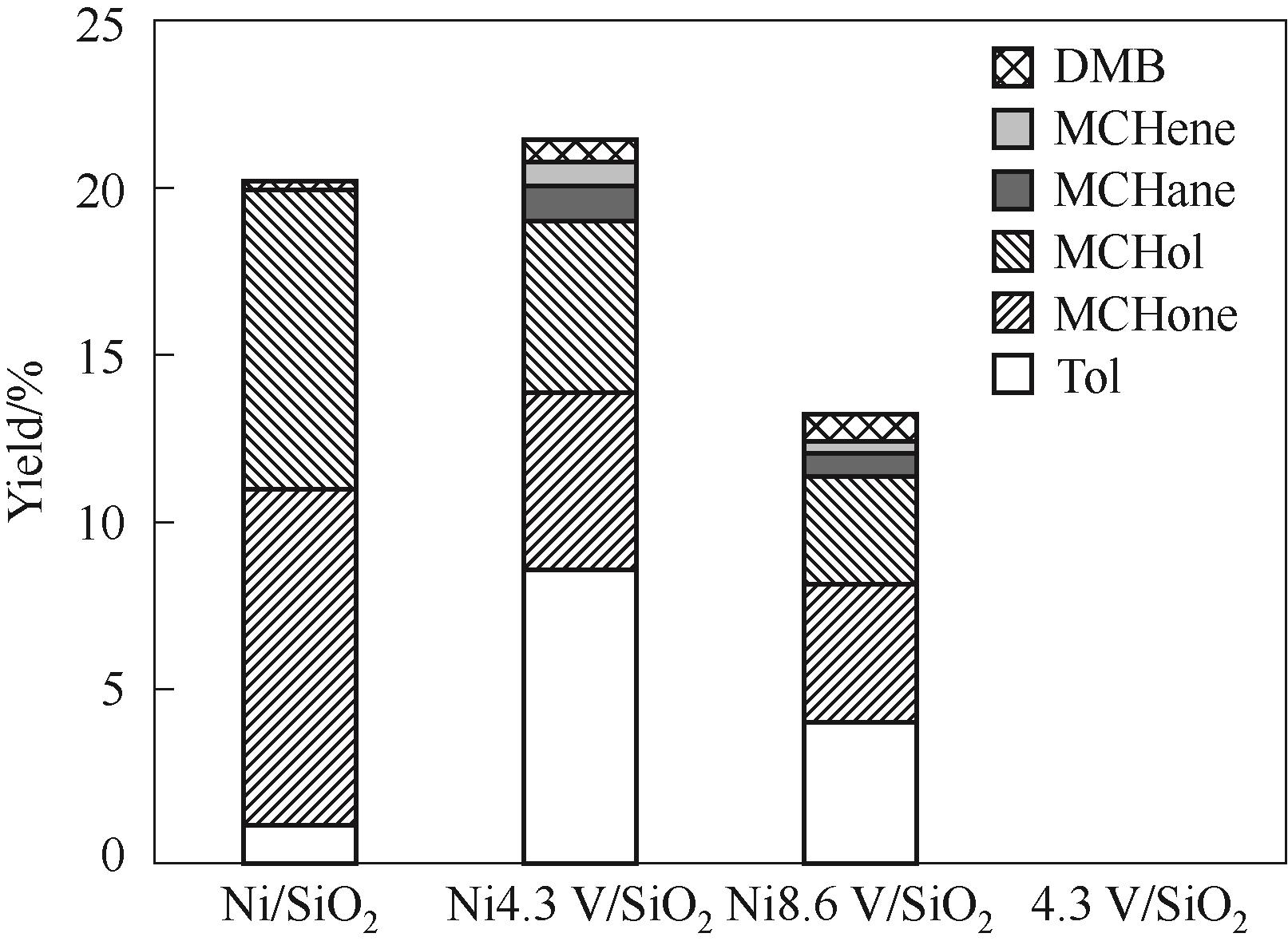
图8 250℃反应温度下不同催化剂在间甲酚加氢脱氧中的催化性能
Fig.8 Catalytic performance of various catalysts in m-cresol hydrodeoxygenation under 250℃(DMB: dimethylbiphenyl compounds; MCHene: methylcyclohexene; MCHane: methylcyclohexane; MCHol: methylcyclohexanol; MCHone: methylcyclohexanone. Reaction conditions: 1 atm, W/F = 0.09 h, H2/m = 146, TOS = 20 min)
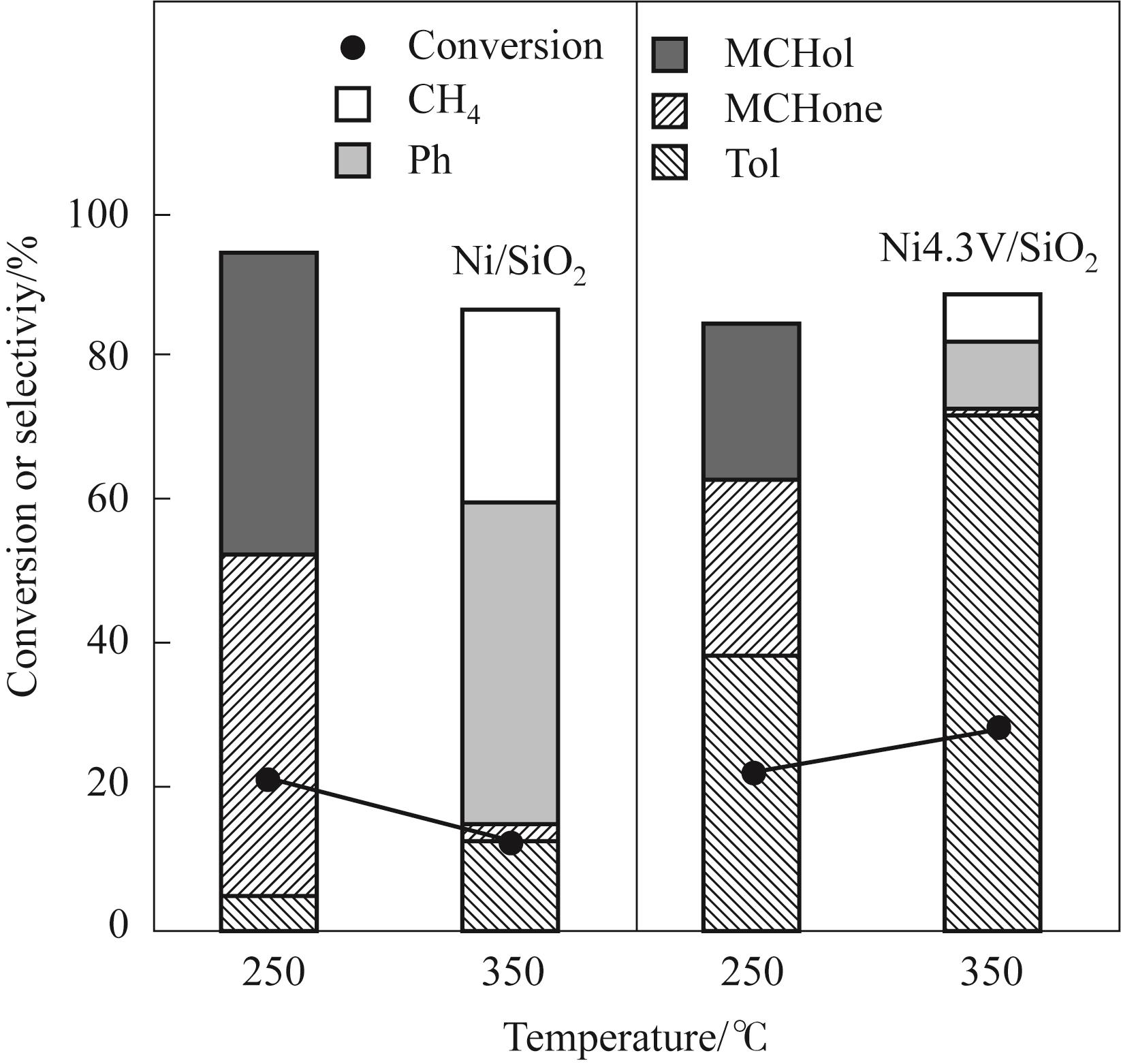
图9 Ni/SiO2和Ni4.3V/SiO2在不同反应温度下的转化率和产物分布差异
Fig.9 Difference of conversion and product distribution between Ni/SiO2 and Ni4.3V/SiO2 under distinct reaction temperature (Reaction conditions: 1 atm, W/F = 0.09 h, H2/m = 146, TOS = 20 min)
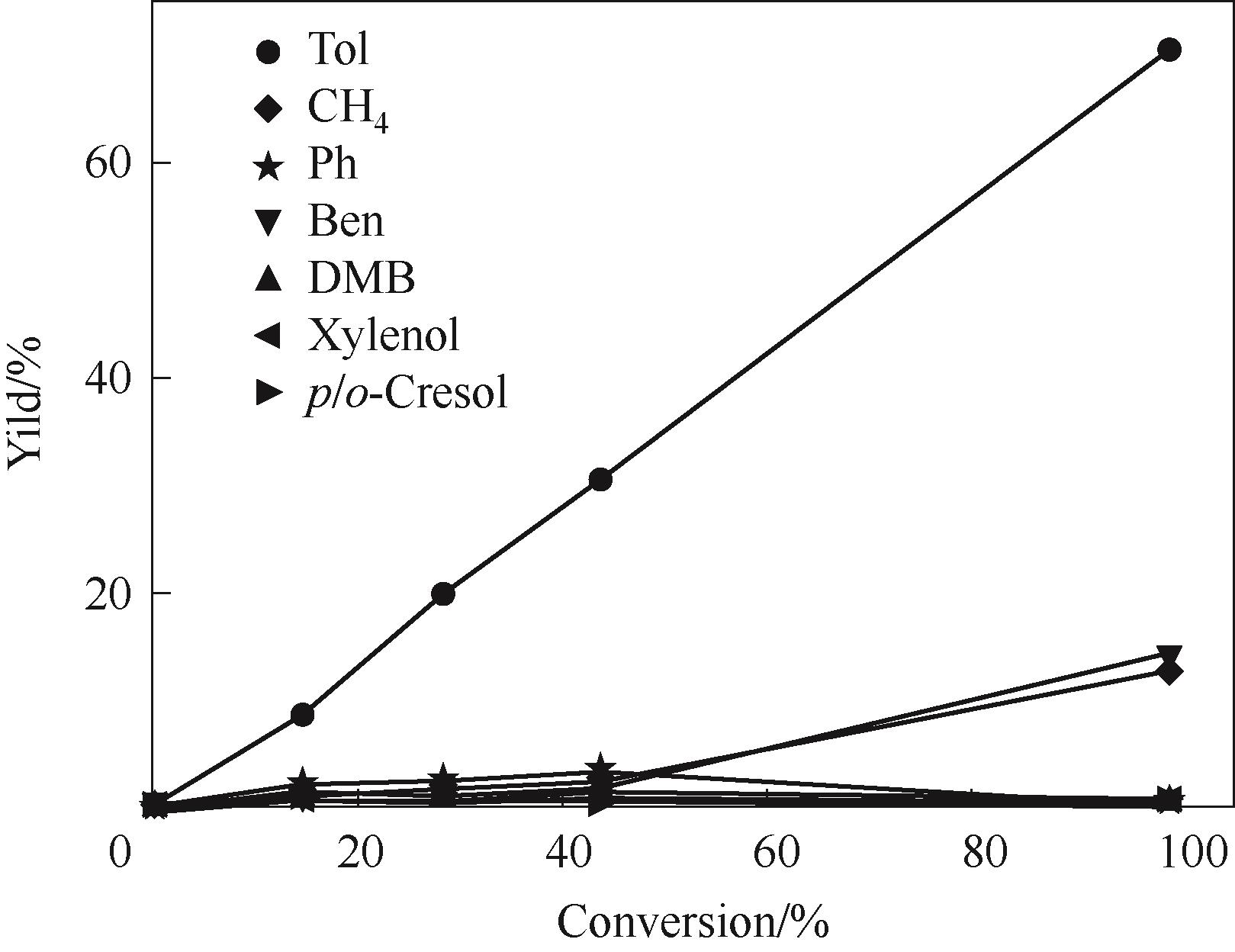
图10 350℃反应温度下Ni4.3V/SiO2催化剂上各个产物收率随间甲酚转化率的变化趋势
Fig.10 Trend of product yield with different m-cresol conversion on Ni4.3V/SiO2 catalyst under 350℃(Reaction conditions: 1 atm, H2/m = 146, TOS = 20 min, W/F is changed to obtain different m-cresol conversion)
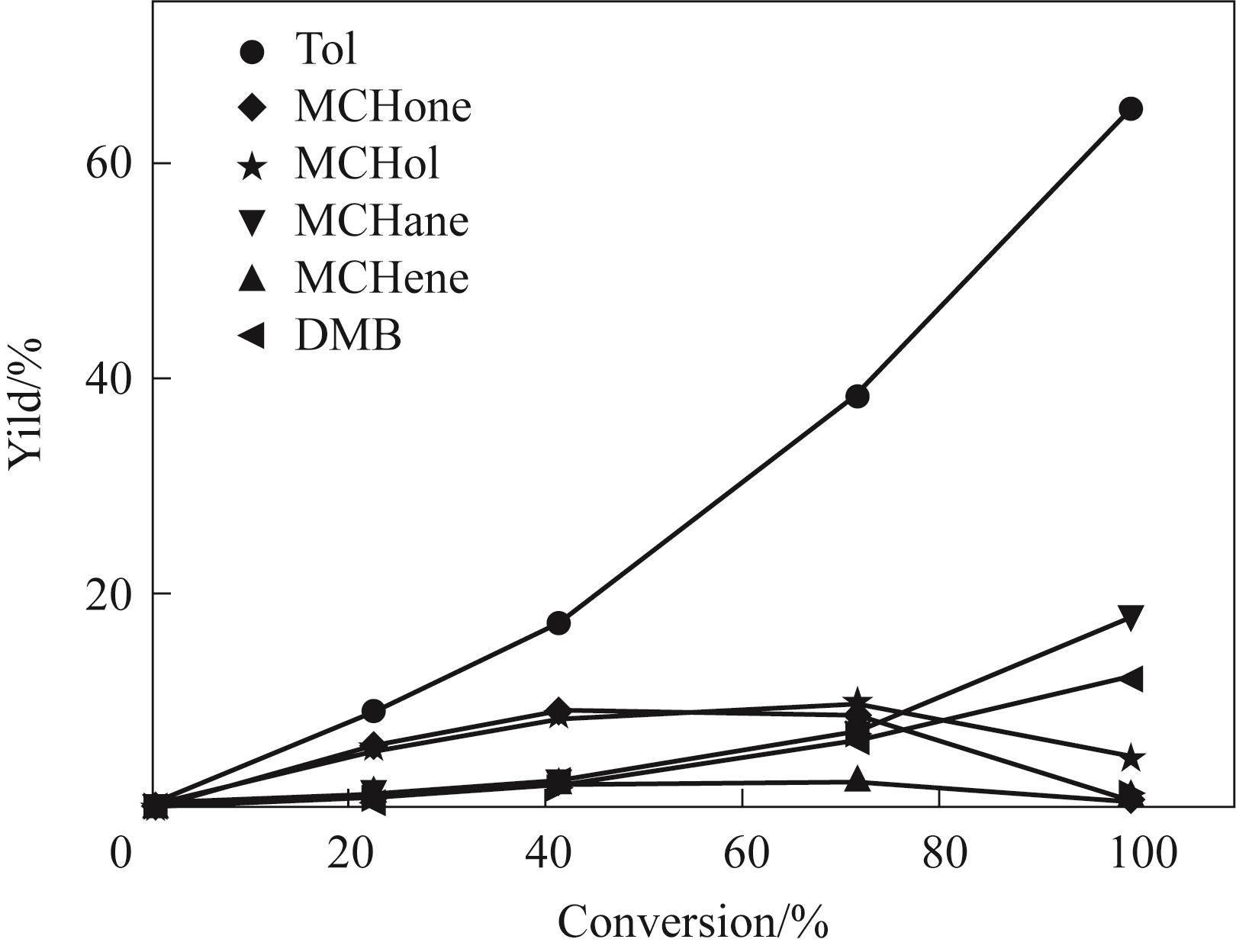
图11 250℃反应温度下Ni4.3V/SiO2催化剂上各个产物收率随间甲酚转化率的变化趋势
Fig.11 Trend of product yield with different m-cresol conversion on Ni4.3V/SiO2 catalyst under 250℃(Reaction conditions: 1 atm, H2/m = 146, TOS = 20 min, W/F is changed to obtain different m-cresol conversion)
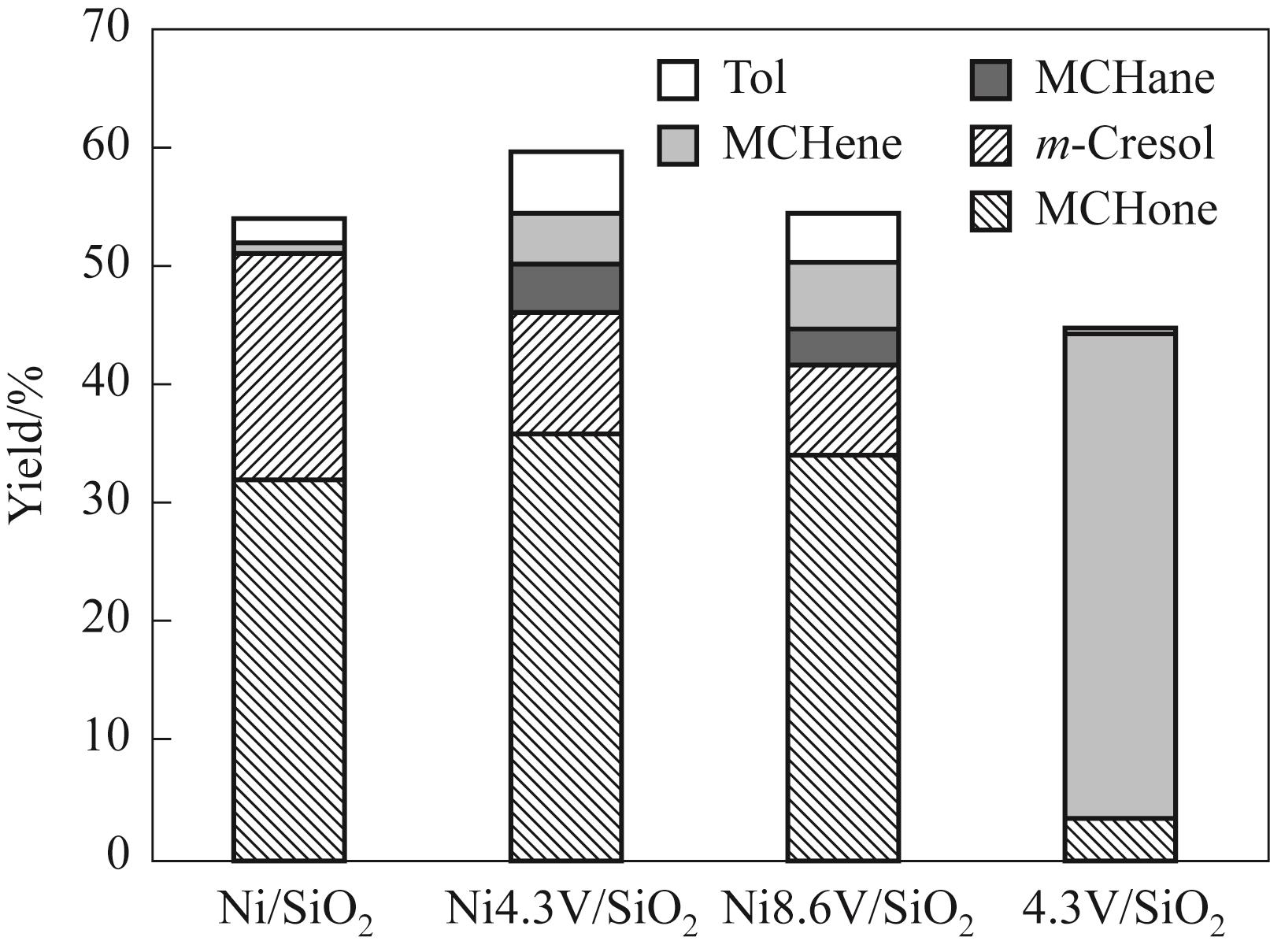
图12 甲基环己醇原料在不同催化剂上反应的产物分布
Fig.12 Product distribution of methylcyclohexane reaction on different catalysts(Reaction conditions: 250℃, 1 atm, H2/m = 146, TOS = 20 min, W/F = 0.065 h)
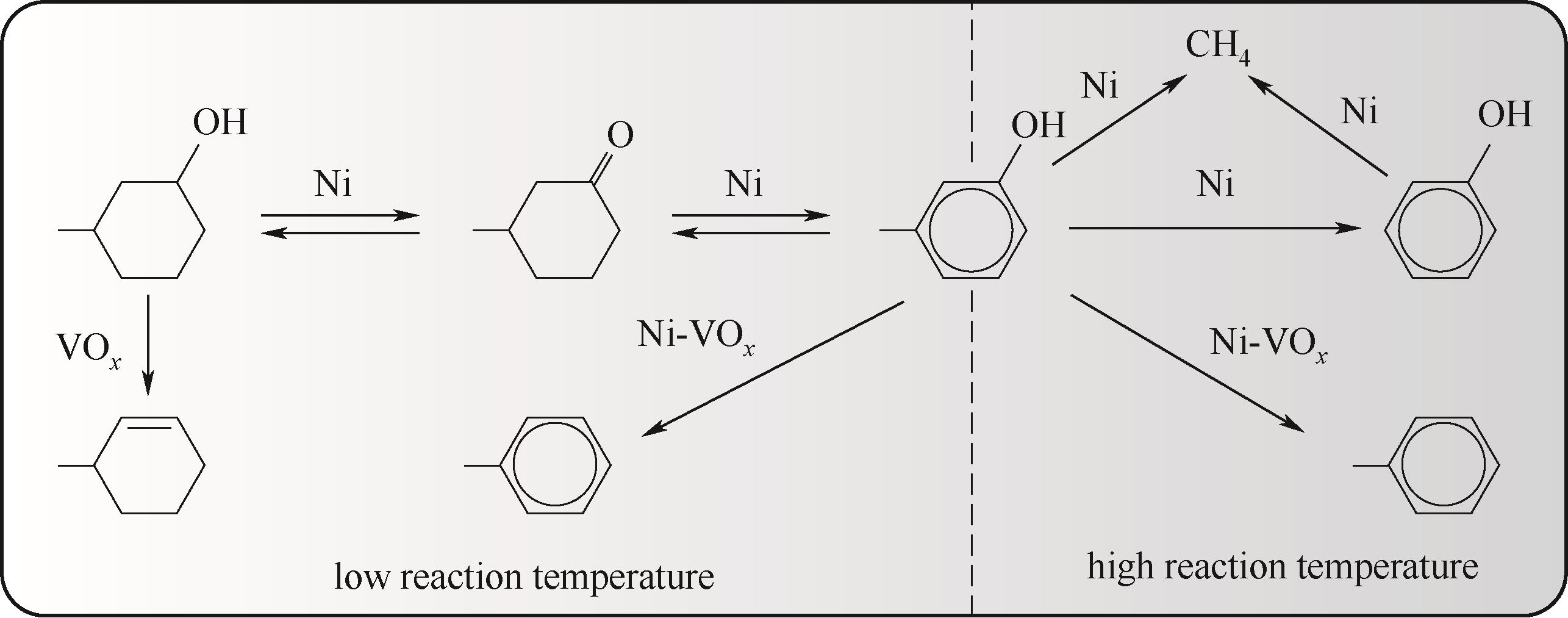
图13 NiV/SiO2催化剂上间甲酚加氢脱氧的主要反应路线和构效关系
Fig.13 Main reaction routes and structure-activity relationships of m-cresol hydrodeoxygenation on NiV/SiO2 catalysts
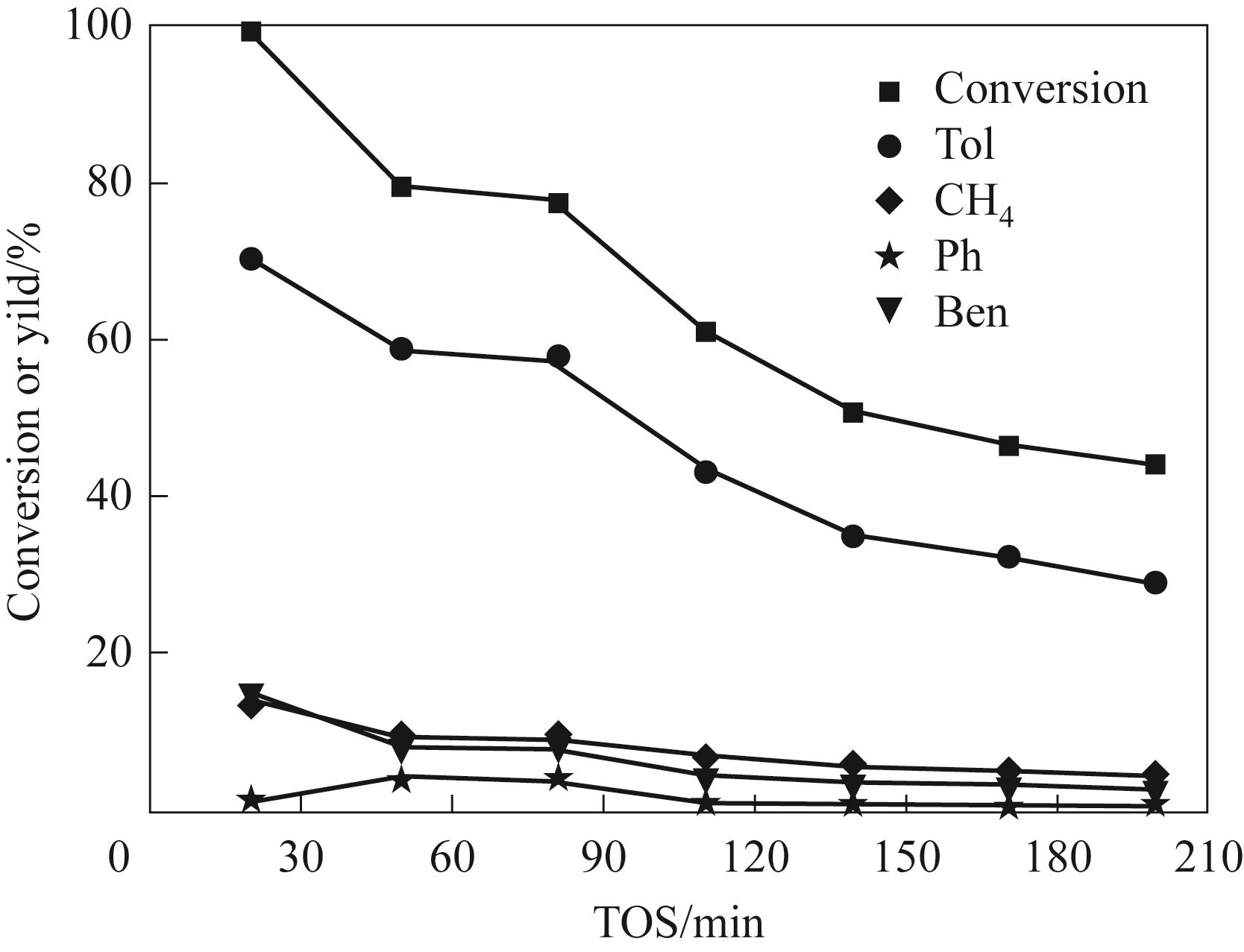
图14 Ni4.3V/SiO2催化剂上间甲酚加氢脱氧的稳定性
Fig.14 The stability of m-cresol hydrodeoxygenation on Ni4.3V/SiO2 catalyst(Reaction conditions: 350℃, 1 atm, W/F = 0.51 h, H2/m = 146)
| [1] | Wang F Q, Ouyang D H, Zhou Z Y, et al. Lignocellulosic biomass as sustainable feedstock and materials for power generation and energy storage[J]. Journal of Energy Chemistry, 2021, 57: 247-280. |
| [2] | Kim J Y, Lee H W, Lee S M, et al. Overview of the recent advances in lignocellulose liquefaction for producing biofuels, bio-based materials and chemicals[J]. Bioresource Technology, 2019, 279: 373-384. |
| [3] | Hoang A T, Ong H C, Rizwanul Fattah I M, et al. Progress on the lignocellulosic biomass pyrolysis for biofuel production toward environmental sustainability[J]. Fuel Processing Technology, 2021, 223: 106997. |
| [4] | Wang C G, Zhang X H, Liu Q, et al. A review of conversion of lignocellulose biomass to liquid transport fuels by integrated refining strategies[J]. Fuel Processing Technology, 2020, 208: 106485. |
| [5] | Liu C J, Wang H M, Karim A M, et al. Catalytic fast pyrolysis of lignocellulosic biomass[J]. Chemical Society Reviews, 2014, 43(22): 7594-7623. |
| [6] | Wang H M, Male J, Wang Y. Recent advances in hydrotreating of pyrolysis bio-oil and its oxygen-containing model compounds[J]. ACS Catalysis, 2013, 3(5): 1047-1070. |
| [7] | Dabros T M H, Stummann M Z, Høj M, et al. Transportation fuels from biomass fast pyrolysis, catalytic hydrodeoxygenation, and catalytic fast hydropyrolysis[J]. Progress in Energy and Combustion Science, 2018, 68: 268-309. |
| [8] | 方辉煌, 吴历洁, 陈伟坤, 等. 生物质基含氧化合物在过渡金属碳化物上加氢脱氧研究进展[J]. 化工学报, 2021, 72(7): 3562-3575. |
| Fang H H, Wu L J, Chen W K, et al. Recent progress on hydrodeoxygenation of biomass-derived oxygenates over transition metal carbides[J]. CIESC Journal, 2021, 72(7): 3562-3575. | |
| [9] | Prasomsri T, Shetty M, Murugappan K, et al. Insights into the catalytic activity and surface modification of MoO3 during the hydrodeoxygenation of lignin-derived model compounds into aromatic hydrocarbons under low hydrogen pressures[J]. Energy & Environmental Science, 2014, 7(8): 2660-2669. |
| [10] | Luo Z C, Zheng Z X, Wang Y C, et al. Hydrothermally stable Ru/HZSM-5-catalyzed selective hydrogenolysis of lignin-derived substituted phenols to bio-arenes in water[J]. Green Chemistry, 2016, 18(21): 5845-5858. |
| [11] | Li C C, Nakagawa Y, Tamura M, et al. Hydrodeoxygenation of guaiacol to phenol over ceria-supported iron catalysts[J]. ACS Catalysis, 2020, 10(24): 14624-14639. |
| [12] | Zhang J H, Sun J M, Wang Y. Recent advances in the selective catalytic hydrodeoxygenation of lignin-derived oxygenates to arenes[J]. Green Chemistry, 2020, 22(4): 1072-1098. |
| [13] | Teles C A, Rabelo-Neto R C, de Lima J R, et al. The effect of metal type on hydrodeoxygenation of phenol over silica supported catalysts[J]. Catalysis Letters, 2016, 146(10): 1848-1857. |
| [14] | Duong N, Tan Q H, Resasco D E. Controlling phenolic hydrodeoxygenation by tailoring metal-O bond strength via specific catalyst metal type and particle size selection[J]. Comptes Rendus Chimie, 2018, 21(3/4): 155-163. |
| [15] | Yang F F, Liu D, Zhao Y T, et al. Size dependence of vapor phase hydrodeoxygenation of m-cresol on Ni/SiO2 catalysts[J]. ACS Catalysis, 2018, 8(3): 1672-1682. |
| [16] | Sun J M, Karim A M, Zhang H, et al. Carbon-supported bimetallic Pd–Fe catalysts for vapor-phase hydrodeoxygenation of guaiacol[J]. Journal of Catalysis, 2013, 306: 47-57. |
| [17] | Hong Y C, Zhang H, Sun J M, et al. Synergistic catalysis between Pd and Fe in gas phase hydrodeoxygenation of m-cresol[J]. ACS Catalysis, 2014, 4(10): 3335-3345. |
| [18] | Hensley A J R, Hong Y C, Zhang R Q, et al. Enhanced Fe2O3 reducibility via surface modification with Pd: characterizing the synergy within Pd/Fe catalysts for hydrodeoxygenation reactions[J]. ACS Catalysis, 2014, 4(10): 3381-3392. |
| [19] | Hong Y C, Zhang S R, Tao F F, et al. Stabilization of iron-based catalysts against oxidation: an in situ ambient-pressure X-ray photoelectron spectroscopy (AP-XPS) study[J]. ACS Catalysis, 2017, 7(5): 3639-3643. |
| [20] | Xiang L, Liu M R, Fan G L, et al. MoO x -decorated ZrO2 nanostructures supporting Ru nanoclusters for selective hydrodeoxygenation of anisole to benzene[J]. ACS Applied Nano Materials, 2021, 4(11): 12588-12599. |
| [21] | Wang C, Mironenko A V, Raizada A, et al. Mechanistic study of the direct hydrodeoxygenation of m-cresol over WO x -decorated Pt/C catalysts[J]. ACS Catalysis, 2018, 8: 7749-7759. |
| [22] | Nie L, de Souza P M, Noronha F B, et al. Selective conversion of m-cresol to toluene over bimetallic Ni–Fe catalysts[J]. Journal of Molecular Catalysis A: Chemical, 2014, 388: 47-55. |
| [23] | Yang F F, Libretto N J, Komarneni M R, et al. Enhancement of m-cresol hydrodeoxygenation selectivity on Ni catalysts by surface decoration of MoO x species[J]. ACS Catalysis, 2019, 9(9): 7791-7800. |
| [24] | Yang F F, Komarneni M R, Libretto N J, et al. Elucidating the structure of bimetallic NiW/SiO2 catalysts and its consequences on selective deoxygenation of m-cresol to toluene[J]. ACS Catalysis, 2021, 11(5): 2935-2948. |
| [25] | Yan F, Wen Z, Wu K, et al. Deoxyalkylation of guaiacol using haggite structured V4O6(OH)4 [J]. Catalysis Science & Technology, 2019, 9(8): 1922-1932. |
| [26] | Cao X C, Long F, Zhang G Y, et al. Selective hydrogenation of methyl palmitate to cetyl alcohol via ternary synergistic catalysis of Ni, oxygen vacancies, and Lewis acid sites under mild reaction conditions[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(29): 9789-9801. |
| [27] | Wu Y J, Sun Y, Liang K L, et al. Enhancing hydrodeoxygenation of bio-oil via bimetallic Ni-V catalysts modified by cross-surface migrated-carbon from biochar[J]. ACS Applied Materials & Interfaces, 2021, 13(18): 21482-21498. |
| [28] | Tian H J, Ross E I, Wachs I E. Quantitative determination of the speciation of surface vanadium oxides and their catalytic activity[J]. The Journal of Physical Chemistry. B, 2006, 110(19): 9593-9600. |
| [29] | Aminzadeh A, Sarikhani-fard H. Raman spectroscopic study of Ni/Al2O3 catalyst[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 1999, 55(7/8): 1421-1425. |
| [30] | Olthof B, Khodakov A, Bell A T, et al. Effects of support composition and pretreatment conditions on the structure of vanadia dispersed on SiO2, Al2O3, TiO2, ZrO2, and HfO2 [J]. The Journal of Physical Chemistry B, 2000, 104(7): 1516-1528. |
| [31] | Ruff P, Schumacher L, Rogg S, et al. Atomic layer deposition-assisted synthesis of embedded vanadia catalysts[J]. ACS Catalysis, 2019, 9(7): 6349-6361. |
| [32] | Abdullah H, Jhuang S J, Shuwanto H, et al. High charge storage of amorphous Ni-doped VO x -modified Ni(OH)2 substrate on a Ni foam cathode in a base solution[J]. ACS Applied Energy Materials, 2023, 6(2): 898-909. |
| [1] | 李相海, 赖德林, 孔纲, 周健. 双仿生表面水下疏油协同机制的分子动力学模拟研究[J]. 化工学报, 2025, 76(9): 4551-4562. |
| [2] | 钱慧慧, 王文婕, 陈文尧, 周兴贵, 张晶, 段学志. 聚丙烯定向转化制芳烃:金属-分子筛协同催化机制[J]. 化工学报, 2025, 76(9): 4838-4849. |
| [3] | 胡国祥, 朱忆魁, 龙华, 刘晓雯, 熊勤钢. 组分配比影响氯化胆碱-乳酸低共熔溶剂碱木质素溶解度的底层机理研究[J]. 化工学报, 2025, 76(9): 4449-4461. |
| [4] | 赵维, 邢文乐, 韩朝旭, 袁兴中, 蒋龙波. g-C3N4基非金属异质结光催化降解水中有机污染物的研究进展[J]. 化工学报, 2025, 76(9): 4752-4769. |
| [5] | 刘卓龙, 甘云华, 屈可扬, 陈宁光, 潘铭晖. 均匀电场对生物柴油小尺度射流扩散燃烧特性影响研究[J]. 化工学报, 2025, 76(9): 4800-4808. |
| [6] | 佟丽丽, 陈英, 艾敏华, 舒玉美, 张香文, 邹吉军, 潘伦. ZnO/WO3异质结光催化环烯烃[2+2]环加成制备高能量密度燃料[J]. 化工学报, 2025, 76(9): 4882-4892. |
| [7] | 李泽权, 蔡天宇, 刘家骏, 陈奇志, 肖沛文, 徐小飞, 赵双良. 木质素基絮凝剂的合成与应用[J]. 化工学报, 2025, 76(9): 4709-4722. |
| [8] | 范夏雨, 孙建辰, 李可莹, 姚馨雅, 商辉. 机器学习驱动液态有机储氢技术的系统优化[J]. 化工学报, 2025, 76(8): 3805-3821. |
| [9] | 杨宁, 李皓男, LIN Xiao, GEORGIADOU Stella, LIN Wen-Feng. 从塑料废弃物到能源催化剂:塑料衍生碳@CoMoO4复合材料在电解水析氢反应中的应用[J]. 化工学报, 2025, 76(8): 4081-4094. |
| [10] | 巢欣旖, 陈文尧, 张晶, 钱刚, 周兴贵, 段学志. 甲醇和乙酸甲酯一步法制丙酸甲酯催化剂的可控制备与性能调控[J]. 化工学报, 2025, 76(8): 4030-4041. |
| [11] | 叶鑫煌, 薛嘉豪, 赵玉来. 可聚型Gemini表面活性剂的制备、表征及其稳定高内相乳液的研究[J]. 化工学报, 2025, 76(8): 4331-4340. |
| [12] | 王御风, 罗小雪, 范鸿亮, 吴白婧, 李存璞, 魏子栋. 耦合电解水制氢的绿色有机电合成——电极界面调控策略综述[J]. 化工学报, 2025, 76(8): 3753-3771. |
| [13] | 郭铮铮, 赵一丹, 王辅强, 裴璐, 靳彦岭, 任芳, 任鹏刚. 异质结构MoS2/RGO/NiFe2O4复合材料的构筑及电磁波吸收性能研究[J]. 化工学报, 2025, 76(7): 3719-3732. |
| [14] | 陆学瑞, 周帼彦, 方琦, 俞孟正, 张秀成, 涂善东. 固体氧化物燃料电池外重整器积炭效应数值模拟研究[J]. 化工学报, 2025, 76(7): 3295-3304. |
| [15] | 陆昕晟, 郭晓镭, 王世丞, 陆海峰, 刘海峰. 秸秆类生物质的粉碎特性研究[J]. 化工学报, 2025, 76(7): 3539-3551. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号