• •
收稿日期:2025-10-23
修回日期:2025-12-10
出版日期:2026-01-21
通讯作者:
夏淑倩
作者简介:孙英华(2001—),女,硕士研究生,1714514289@qq.com
基金资助:
Yinghua SUN( ), Yining WANG, Saiyi SONG, Wuying LI, Shuqian XIA(
), Yining WANG, Saiyi SONG, Wuying LI, Shuqian XIA( )
)
Received:2025-10-23
Revised:2025-12-10
Online:2026-01-21
Contact:
Shuqian XIA
摘要:
溶剂萃取法在抗生素及其他生物活性物质的分离与纯化中发挥着重要作用。本研究利用分子动力学模拟探讨了硫氰酸红霉素在丙酮溶液中的溶解与分散行为。采用GROMACS 2023软件,并基于GAFF力场构建了包含40个硫氰酸红霉素分子的模型,对不同固液比与温度条件下的分散行为进行了分析。结果表明,当固液比为1:3 (g:mL)温度为318.15 K时,体系分散效果最佳,溶解度和分散度均显著提高。通过氢键分析、径向分布函数(RDF)和均方位移(MSD)等指标,定量评估了体系在不同条件下的结构和动力学特征。基于模拟预测结果,设计并开展了相应的溶剂萃取实验,实验结果与模拟趋势一致,进一步证明了模拟预测的可靠性。本研究表明,分子动力学模拟能够在实验前有效预测体系的最优操作条件,并为实验设计提供理论指导。该方法不仅有助于揭示硫氰酸红霉素在丙酮体系中的溶解机理,也为溶剂萃取工艺的优化提供了有价值的参考。
中图分类号:
孙英华, 王一宁, 宋赛依, 李武英, 夏淑倩. 硫氰酸红霉素溶解动力学研究[J]. 化工学报, DOI: 10.11949/0438-1157.20251177.
Yinghua SUN, Yining WANG, Saiyi SONG, Wuying LI, Shuqian XIA. Study on dissolution kinetics of erythromycin thiocyanate[J]. CIESC Journal, DOI: 10.11949/0438-1157.20251177.
| 固液比/(g:mL) | 分子、离子数量 | |||
|---|---|---|---|---|
| 丙酮 | 红霉素 | 硫氰酸根(SCN-) | ||
| 1:2 | 862 | 40 | 40 | |
| 1:2.5 | 1080 | 40 | 40 | |
| 1:3 | 1294 | 40 | 40 | |
| 1:3.5 | 1510 | 40 | 40 | |
| 1:4 | 1726 | 40 | 40 | |
| 1:4.5 | 1940 | 40 | 40 | |
表1 盒子中红霉素、硫氰酸根及丙酮的数量
Table 1 Numbers of erythromycin, thiocyanate ions, and acetone molecules in the simulation box
| 固液比/(g:mL) | 分子、离子数量 | |||
|---|---|---|---|---|
| 丙酮 | 红霉素 | 硫氰酸根(SCN-) | ||
| 1:2 | 862 | 40 | 40 | |
| 1:2.5 | 1080 | 40 | 40 | |
| 1:3 | 1294 | 40 | 40 | |
| 1:3.5 | 1510 | 40 | 40 | |
| 1:4 | 1726 | 40 | 40 | |
| 1:4.5 | 1940 | 40 | 40 | |
| Number | Atom | Charge | Number | Atom | Charge |
|---|---|---|---|---|---|
| 1 | C1 | 0.382 | 60 | H60 | -0.0590 |
| 2 | C2 | 0.232 | 61 | H61 | 0.0866 |
| 3 | C3 | 0.372 | 62 | H62 | -0.0181 |
| 4 | O4 | -0.490 | 63 | H63 | -0.0181 |
| 5 | C5 | 0.791 | 64 | H64 | 0.403 |
| 6 | C6 | -0.438 | 65 | H65 | 0.107 |
| 7 | O7 | -0.553 | 66 | H66 | 0.107 |
| 8 | C8 | 0.207 | 67 | H67 | 0.107 |
| 9 | C9 | -0.0753 | 68 | H68 | 0.412 |
| 10 | C10 | 0.288 | 69 | H69 | 0.120 |
| 11 | C11 | 0.486 | 70 | H70 | 0.120 |
| 12 | C12 | -0.095 | 71 | H71 | 0.120 |
| 13 | C13 | 0.0985 | 72 | H72 | 0.411 |
| 14 | C14 | -0.315 | 73 | H73 | -0.068 |
| 15 | C15 | 0.554 | 74 | H74 | 0.0680 |
| 16 | C16 | -0.0871 | 75 | H75 | 0.0680 |
| 17 | C17 | 0.340 | 76 | H76 | 0.0315 |
| 18 | C18 | 0.162 | 77 | H77 | 0.0822 |
| 19 | C19 | 0.286 | 78 | H78 | 0.447 |
| 20 | O20 | -0.494 | 79 | H79 | 0.0676 |
| 21 | C21 | 0.819 | 80 | H80 | 0.0676 |
| 22 | C22 | 0.0580 | 81 | H81 | 0.0676 |
| 23 | C23 | 0.137 | 82 | H82 | 0.118 |
| 24 | O24 | -0.639 | 83 | H83 | 0.118 |
| 25 | C25 | -0.391 | 84 | H84 | 0.118 |
| 26 | O26 | -0.590 | 85 | H85 | 0.397 |
| 27 | C27 | -0.397 | 86 | H86 | 0.0669 |
| 28 | O28 | -0.703 | 87 | H87 | 0.0669 |
| 29 | O29 | -0.546 | 88 | H88 | 0.0669 |
| 30 | C30 | 0.593 | 89 | H89 | 0.0356 |
| 31 | O31 | -0.479 | 90 | H90 | 0.0356 |
| 32 | C32 | -0.311 | 91 | H91 | 0.0356 |
| 33 | C33 | 0.239 | 92 | C92 | -0.170 |
| 34 | C34 | 0.0497 | 93 | H93 | 0.0626 |
| 35 | O35 | -0.629 | 94 | H94 | 0.0422 |
| 36 | N36 | -0.205 | 95 | H95 | 0.0422 |
| 37 | C37 | -0.151 | 96 | H96 | 0.0422 |
| 38 | C38 | -0.348 | 97 | C97 | -0.494 |
| 39 | O39 | -0.620 | 98 | C98 | -0.481 |
| 40 | C40 | -0.255 | 99 | H99 | 0.122 |
| 41 | O41 | -0.444 | 100 | H100 | 0.122 |
| 42 | C42 | 0.0898 | 101 | H101 | 0.122 |
| 43 | O43 | -0.548 | 102 | H102 | 0.131 |
| 44 | O44 | -0.483 | 103 | H103 | 0.131 |
| 45 | H45 | -0.0509 | 104 | H104 | 0.131 |
| 46 | H46 | -0.0198 | 105 | C105 | -0.369 |
| 47 | H47 | -0.0857 | 106 | H106 | 0.0996 |
| 48 | H48 | 0.114 | 107 | H107 | 0.0996 |
| 49 | H49 | 0.114 | 108 | H108 | 0.0996 |
| 50 | H50 | 0.0118 | 109 | C109 | -0.175 |
| 51 | H51 | -0.1349 | 110 | H110 | 0.0414 |
| 52 | H52 | 0.0352 | 111 | H111 | 0.0414 |
| 53 | H53 | 0.0352 | 112 | H112 | 0.0414 |
| 54 | H54 | 0.0106 | 113 | C113 | 0.437 |
| 55 | H55 | 0.0768 | 114 | H114 | 0.00945 |
| 56 | H56 | 0.0768 | 115 | C115 | -0.403 |
| 57 | H57 | 0.0768 | 116 | H116 | 0.0969 |
| 58 | H58 | 0.0447 | 117 | H117 | 0.0969 |
| 59 | H59 | -0.00492 | 118 | H118 | 0.0969 |
表2 红霉素分子中的原子电荷
Table 2 Atomic charge in erythromycin molecules
| Number | Atom | Charge | Number | Atom | Charge |
|---|---|---|---|---|---|
| 1 | C1 | 0.382 | 60 | H60 | -0.0590 |
| 2 | C2 | 0.232 | 61 | H61 | 0.0866 |
| 3 | C3 | 0.372 | 62 | H62 | -0.0181 |
| 4 | O4 | -0.490 | 63 | H63 | -0.0181 |
| 5 | C5 | 0.791 | 64 | H64 | 0.403 |
| 6 | C6 | -0.438 | 65 | H65 | 0.107 |
| 7 | O7 | -0.553 | 66 | H66 | 0.107 |
| 8 | C8 | 0.207 | 67 | H67 | 0.107 |
| 9 | C9 | -0.0753 | 68 | H68 | 0.412 |
| 10 | C10 | 0.288 | 69 | H69 | 0.120 |
| 11 | C11 | 0.486 | 70 | H70 | 0.120 |
| 12 | C12 | -0.095 | 71 | H71 | 0.120 |
| 13 | C13 | 0.0985 | 72 | H72 | 0.411 |
| 14 | C14 | -0.315 | 73 | H73 | -0.068 |
| 15 | C15 | 0.554 | 74 | H74 | 0.0680 |
| 16 | C16 | -0.0871 | 75 | H75 | 0.0680 |
| 17 | C17 | 0.340 | 76 | H76 | 0.0315 |
| 18 | C18 | 0.162 | 77 | H77 | 0.0822 |
| 19 | C19 | 0.286 | 78 | H78 | 0.447 |
| 20 | O20 | -0.494 | 79 | H79 | 0.0676 |
| 21 | C21 | 0.819 | 80 | H80 | 0.0676 |
| 22 | C22 | 0.0580 | 81 | H81 | 0.0676 |
| 23 | C23 | 0.137 | 82 | H82 | 0.118 |
| 24 | O24 | -0.639 | 83 | H83 | 0.118 |
| 25 | C25 | -0.391 | 84 | H84 | 0.118 |
| 26 | O26 | -0.590 | 85 | H85 | 0.397 |
| 27 | C27 | -0.397 | 86 | H86 | 0.0669 |
| 28 | O28 | -0.703 | 87 | H87 | 0.0669 |
| 29 | O29 | -0.546 | 88 | H88 | 0.0669 |
| 30 | C30 | 0.593 | 89 | H89 | 0.0356 |
| 31 | O31 | -0.479 | 90 | H90 | 0.0356 |
| 32 | C32 | -0.311 | 91 | H91 | 0.0356 |
| 33 | C33 | 0.239 | 92 | C92 | -0.170 |
| 34 | C34 | 0.0497 | 93 | H93 | 0.0626 |
| 35 | O35 | -0.629 | 94 | H94 | 0.0422 |
| 36 | N36 | -0.205 | 95 | H95 | 0.0422 |
| 37 | C37 | -0.151 | 96 | H96 | 0.0422 |
| 38 | C38 | -0.348 | 97 | C97 | -0.494 |
| 39 | O39 | -0.620 | 98 | C98 | -0.481 |
| 40 | C40 | -0.255 | 99 | H99 | 0.122 |
| 41 | O41 | -0.444 | 100 | H100 | 0.122 |
| 42 | C42 | 0.0898 | 101 | H101 | 0.122 |
| 43 | O43 | -0.548 | 102 | H102 | 0.131 |
| 44 | O44 | -0.483 | 103 | H103 | 0.131 |
| 45 | H45 | -0.0509 | 104 | H104 | 0.131 |
| 46 | H46 | -0.0198 | 105 | C105 | -0.369 |
| 47 | H47 | -0.0857 | 106 | H106 | 0.0996 |
| 48 | H48 | 0.114 | 107 | H107 | 0.0996 |
| 49 | H49 | 0.114 | 108 | H108 | 0.0996 |
| 50 | H50 | 0.0118 | 109 | C109 | -0.175 |
| 51 | H51 | -0.1349 | 110 | H110 | 0.0414 |
| 52 | H52 | 0.0352 | 111 | H111 | 0.0414 |
| 53 | H53 | 0.0352 | 112 | H112 | 0.0414 |
| 54 | H54 | 0.0106 | 113 | C113 | 0.437 |
| 55 | H55 | 0.0768 | 114 | H114 | 0.00945 |
| 56 | H56 | 0.0768 | 115 | C115 | -0.403 |
| 57 | H57 | 0.0768 | 116 | H116 | 0.0969 |
| 58 | H58 | 0.0447 | 117 | H117 | 0.0969 |
| 59 | H59 | -0.00492 | 118 | H118 | 0.0969 |
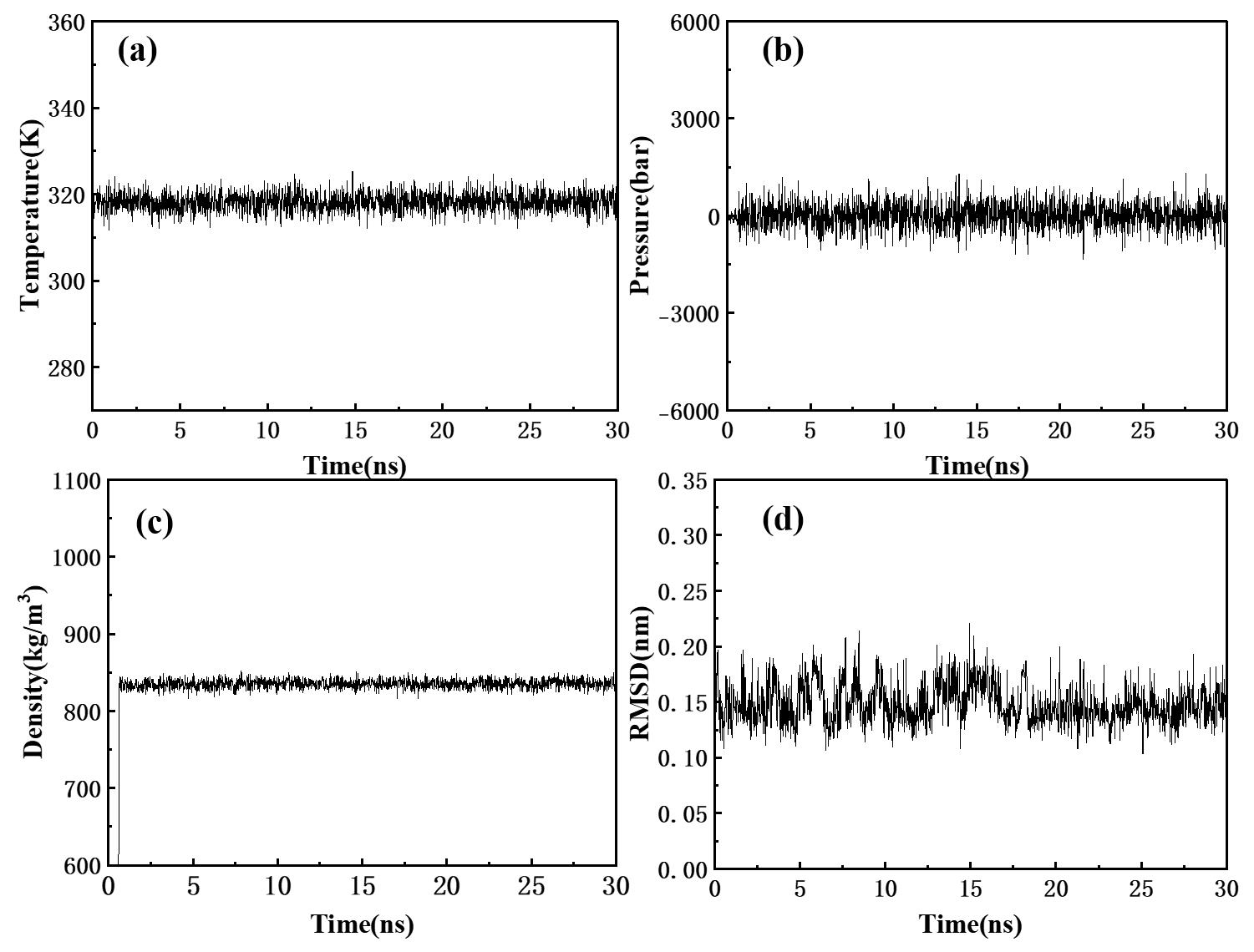
图3 硫氰酸红霉素在丙酮溶液中的体系平衡性质随时间的变化曲线:(a)温度;(b)压力;(c)密度和(d)RMSD
Fig.3 Time evolution of system equilibrium properties of erythromycin thiocyanate in acetone solution: (a) temperature; (b) pressure; (c) density; and (d) RMSD
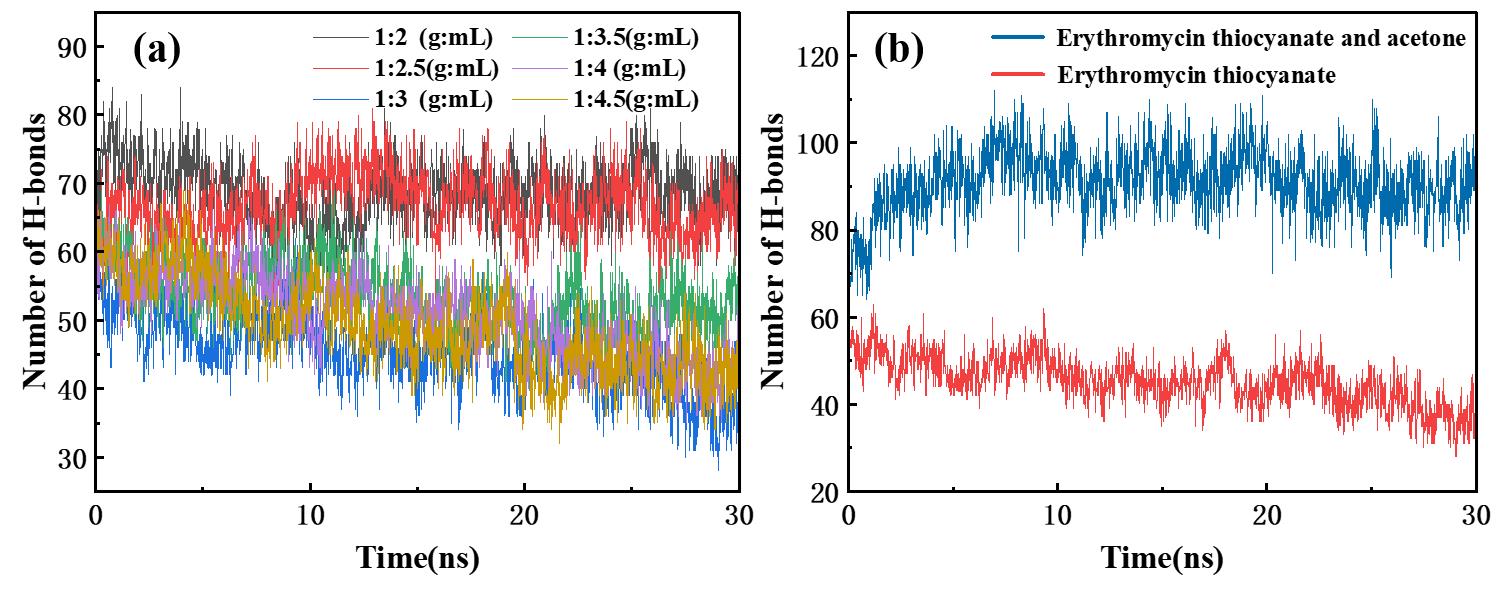
图4 (a)不同固液比条件下硫氰酸红霉素分子间氢键数量随时间变化;(b)1:3 (g:mL)固液比条件下硫氰酸红霉素与丙酮分子间的氢键数目随时间的变化
Fig.4 (a)The number of hydrogen bonds between erythromycin thiocyanate molecules changed with time under different solid-liquid ratios. (b)The number of hydrogen bonds between erythromycin thiocyanate and acetone molecules changed with time under 1:3 (g:mL) solid-liquid ratio
| 不同固液比(g:mL) | ||||||
|---|---|---|---|---|---|---|
| 1:2 | 1:2.5 | 1:3 | 1:3.5 | 1:4 | 1:4.5 | |
| τ(E–E)(ps) | 8.75 | 6.77 | 3.11 | 3.89 | 4.32 | 4.54 |
| k(E–E) | 0.114 | 0.148 | 0.322 | 0.257 | 0.231 | 0.22 |
| τ(E–A) (ps) | 2.57 | 3.78 | 6.45 | 4.35 | 4.79 | 3.98 |
| k(E–A) | 0.389 | 0.265 | 0.155 | 0.23 | 0.209 | 0.251 |
表3 不同固液比条件下氢键的寿命及衰减速率
Table 3 H-bond lifetimes and decay rates at different solid-liquid ratios
| 不同固液比(g:mL) | ||||||
|---|---|---|---|---|---|---|
| 1:2 | 1:2.5 | 1:3 | 1:3.5 | 1:4 | 1:4.5 | |
| τ(E–E)(ps) | 8.75 | 6.77 | 3.11 | 3.89 | 4.32 | 4.54 |
| k(E–E) | 0.114 | 0.148 | 0.322 | 0.257 | 0.231 | 0.22 |
| τ(E–A) (ps) | 2.57 | 3.78 | 6.45 | 4.35 | 4.79 | 3.98 |
| k(E–A) | 0.389 | 0.265 | 0.155 | 0.23 | 0.209 | 0.251 |
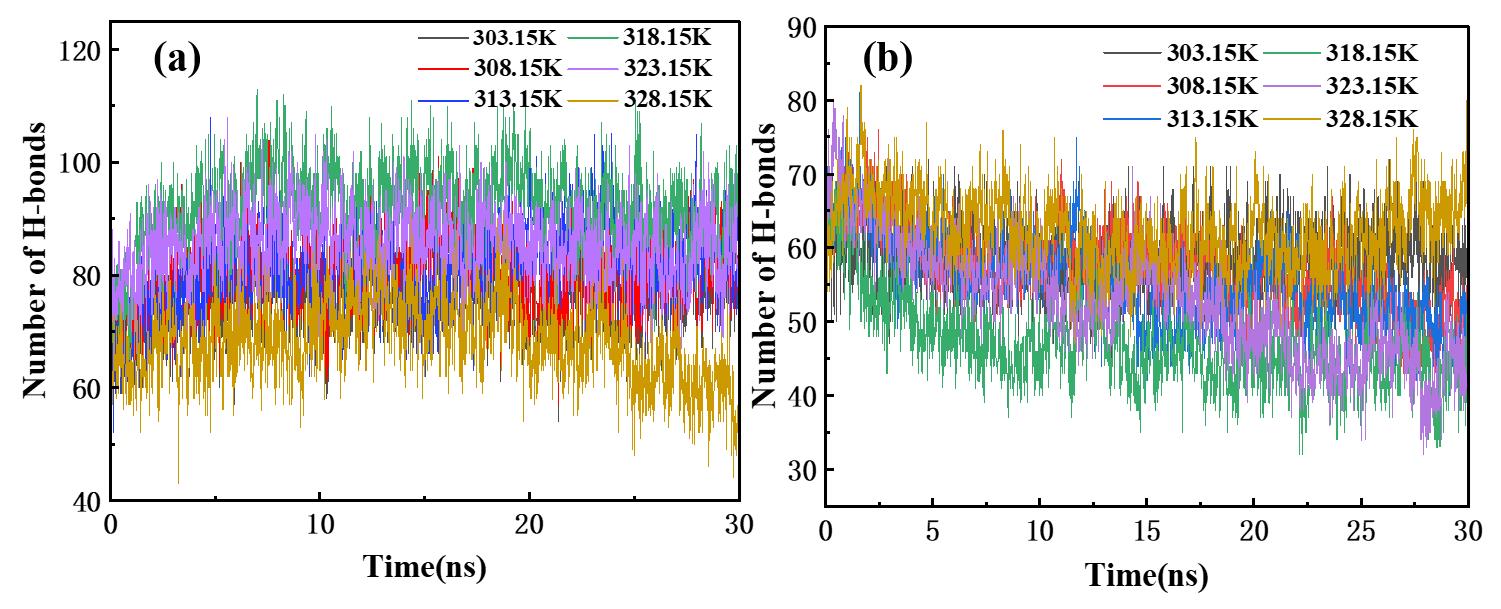
图5 (a)不同温度条件下硫氰酸红霉素分子间氢键数量随时间变化;(b)不同温度条件下硫氰酸红霉素分子与丙酮分子间的氢键数目随时间的变化
Fig.5 (a) Time evolution of the number of intermolecular hydrogen bonds between erythromycin thiocyanate molecules at different temperatures, (b) Time evolution of the number of hydrogen bonds between erythromycin thiocyanate and acetone molecules at different temperatures
| 不同温度(K) | ||||||
|---|---|---|---|---|---|---|
| 303.15 | 308.15 | 313.15 | 318.15 | 323.15 | 328.15 | |
| τ(E–E) (ps) | 7.01 | 6.84 | 5.71 | 3.11 | 2.44 | 1.31 |
| k(E–E) | 0.143 | 0.146 | 0.175 | 0.322 | 0.41 | 0.763 |
| τ(E–A) (ps) | 3.73 | 3.59 | 5.56 | 6.55 | 4.73 | 3.87 |
| k(E–A) | 0.268 | 0.279 | 0.18 | 0.153 | 0.212 | 0.258 |
表4 不同温度条件下氢键的寿命及衰减速率
Table 4 H-bond lifetimes and decay rates at different temperatures
| 不同温度(K) | ||||||
|---|---|---|---|---|---|---|
| 303.15 | 308.15 | 313.15 | 318.15 | 323.15 | 328.15 | |
| τ(E–E) (ps) | 7.01 | 6.84 | 5.71 | 3.11 | 2.44 | 1.31 |
| k(E–E) | 0.143 | 0.146 | 0.175 | 0.322 | 0.41 | 0.763 |
| τ(E–A) (ps) | 3.73 | 3.59 | 5.56 | 6.55 | 4.73 | 3.87 |
| k(E–A) | 0.268 | 0.279 | 0.18 | 0.153 | 0.212 | 0.258 |
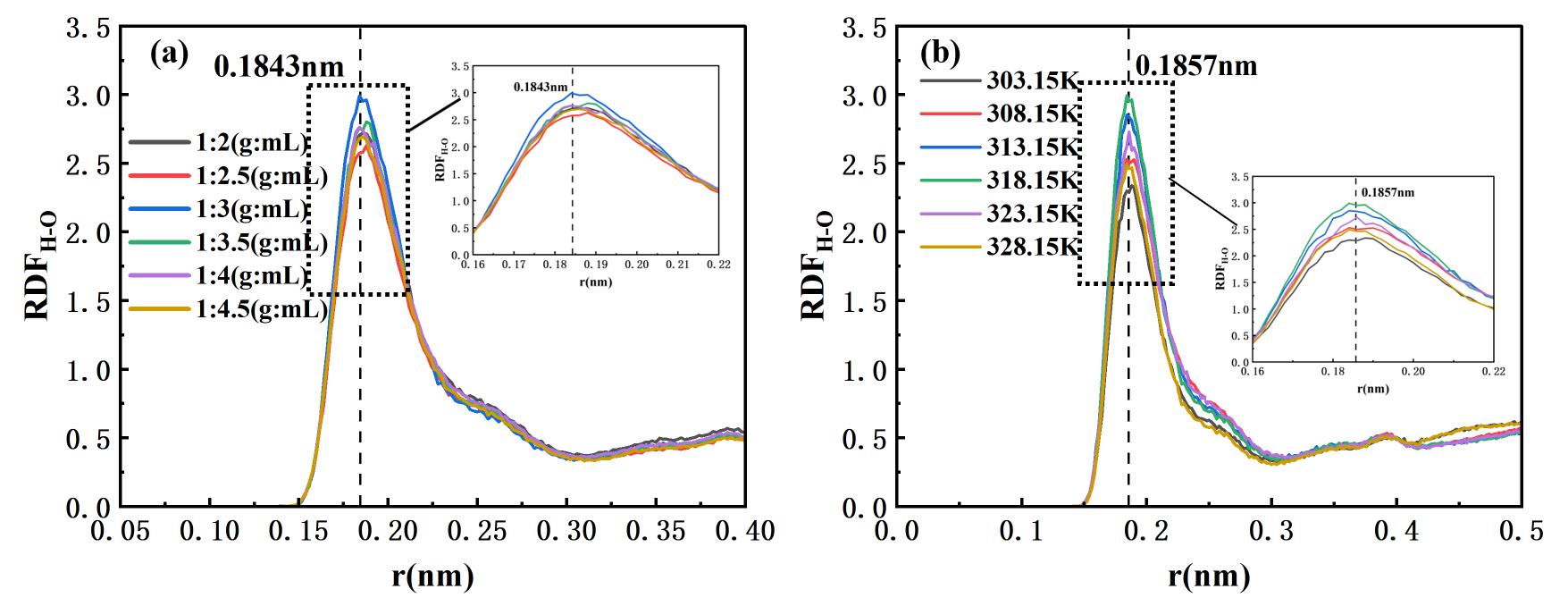
图6 30 ns时丙酮和硫氰酸红霉素体系H-O对的径向分布函数(RDF):(a)固液比对RDF的影响;(b)温度对RDF的影响
Fig.6 Radial distribution functions (RDFs) of H-O pairs between acetone and erythromycin thiocyanate at 30 ns: (a) effect of solid-liquid ratio; (b) effect of temperature
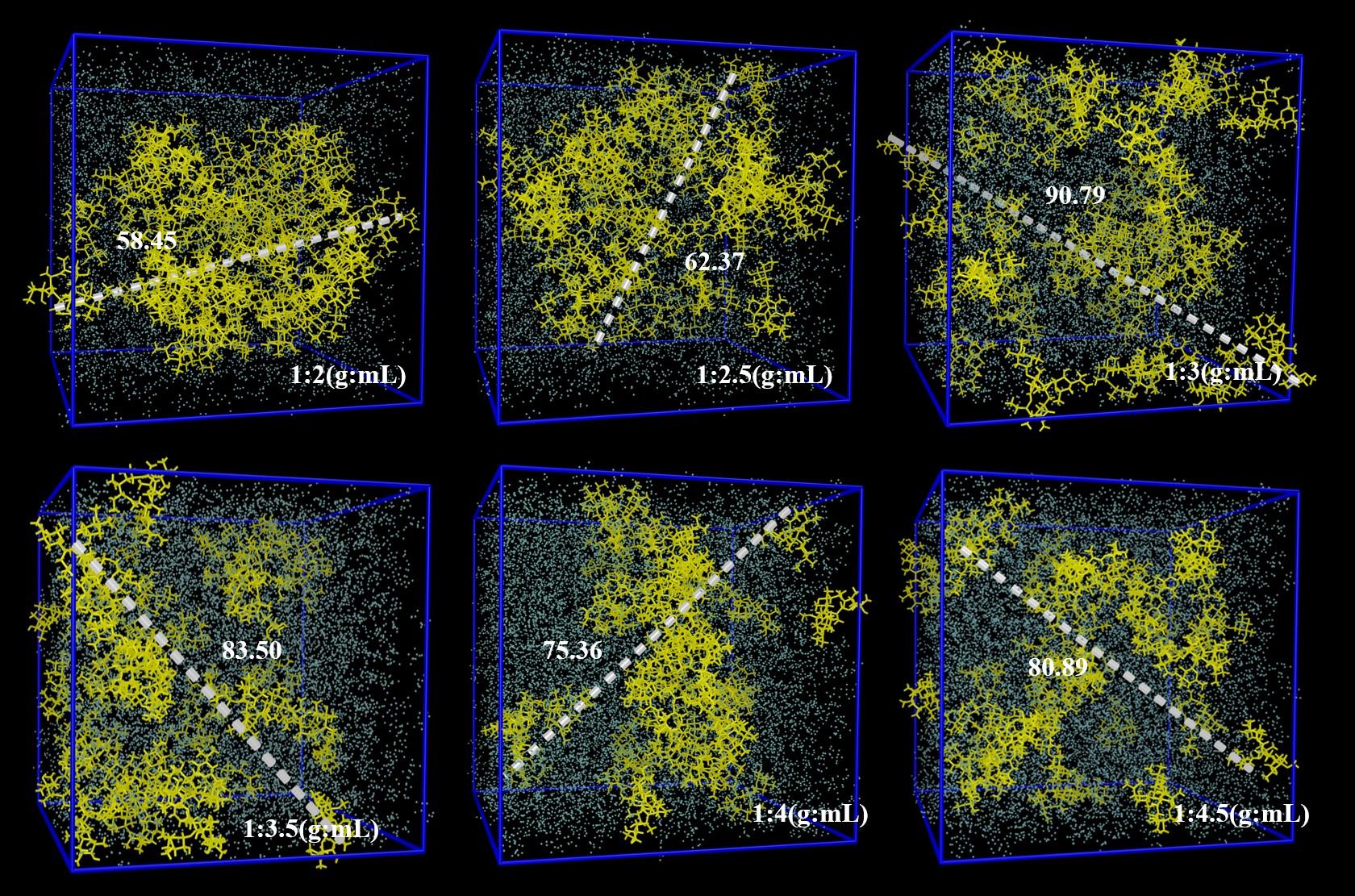
图7 不同固液比(黄色:硫氰酸红霉素分子,蓝色:丙酮分子)团簇在30 ns时的快照注:簇中距离最长的两个原子之间的长度值(单位:Å )已被标出
Fig.7 Snapshots of clusters with different solid-liquid ratios (yellow: erythromycin thiocyanate molecule, blue: acetone molecule)at 30 nsThe length value ( unit : Å ) between the two longest atoms in the cluster is also marked
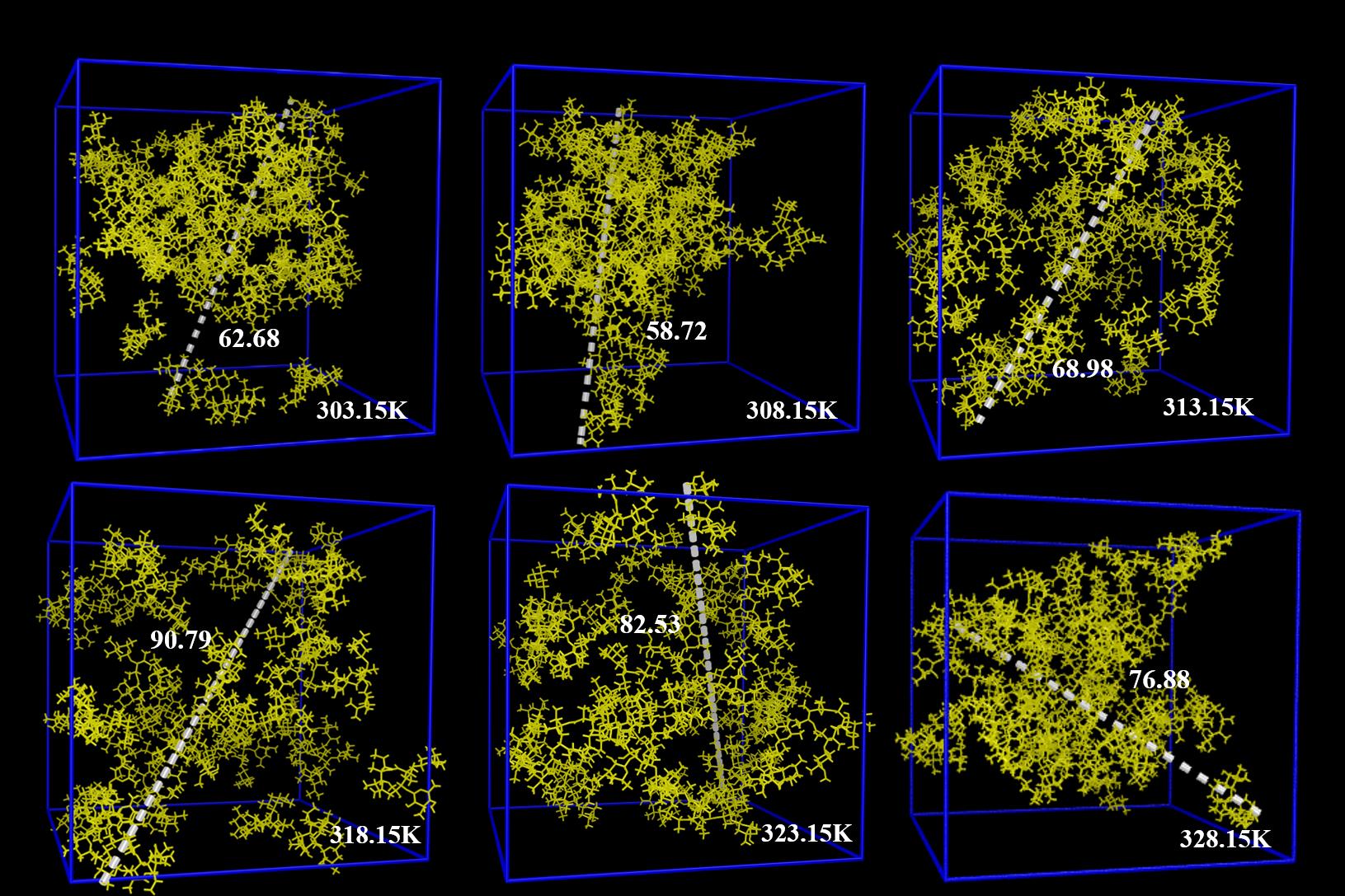
图8 不同温度团簇在30 ns时的快照注:簇中距离最长的两个原子之间的长度值(单位:Å )已被标出
Fig.8 Snapshots of clusters with different temperatures ratios at 30 nsThe length value ( unit : Å ) between the two longest atoms in the cluster is also marked
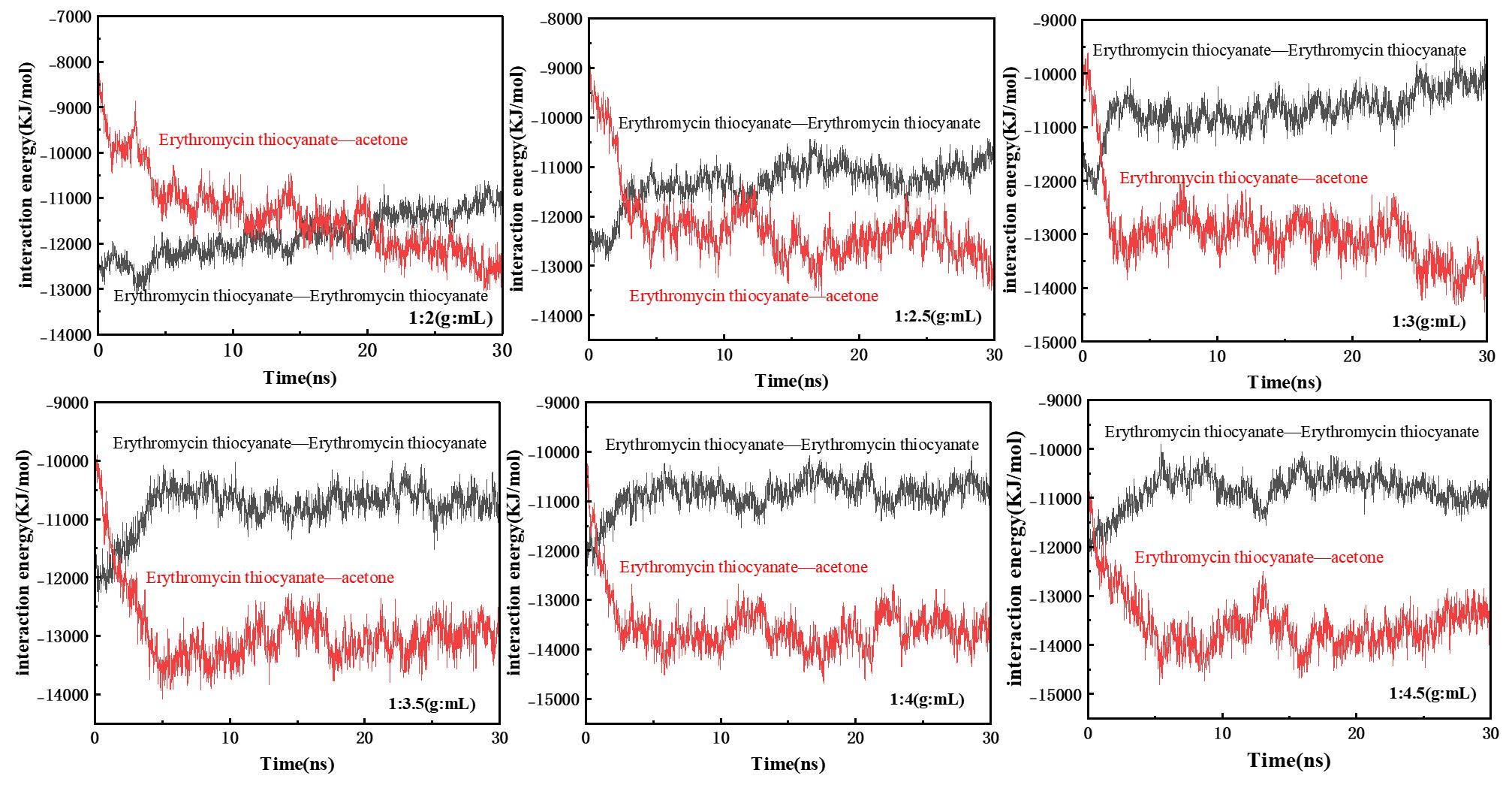
图9 硫氰酸红霉素-硫氰酸红霉素(E-E)、硫氰酸红霉素-丙酮(E-A)在不同固液比条件下的非成键相互作用能随时间的变化
Fig.9 The change of non-bond interaction energy of erythromycin thiocyanate-erythromycin thiocyanate(E-E) and erythromycin thiocyanate-acetone(E-A) with time under different solid-liquid ratio conditions
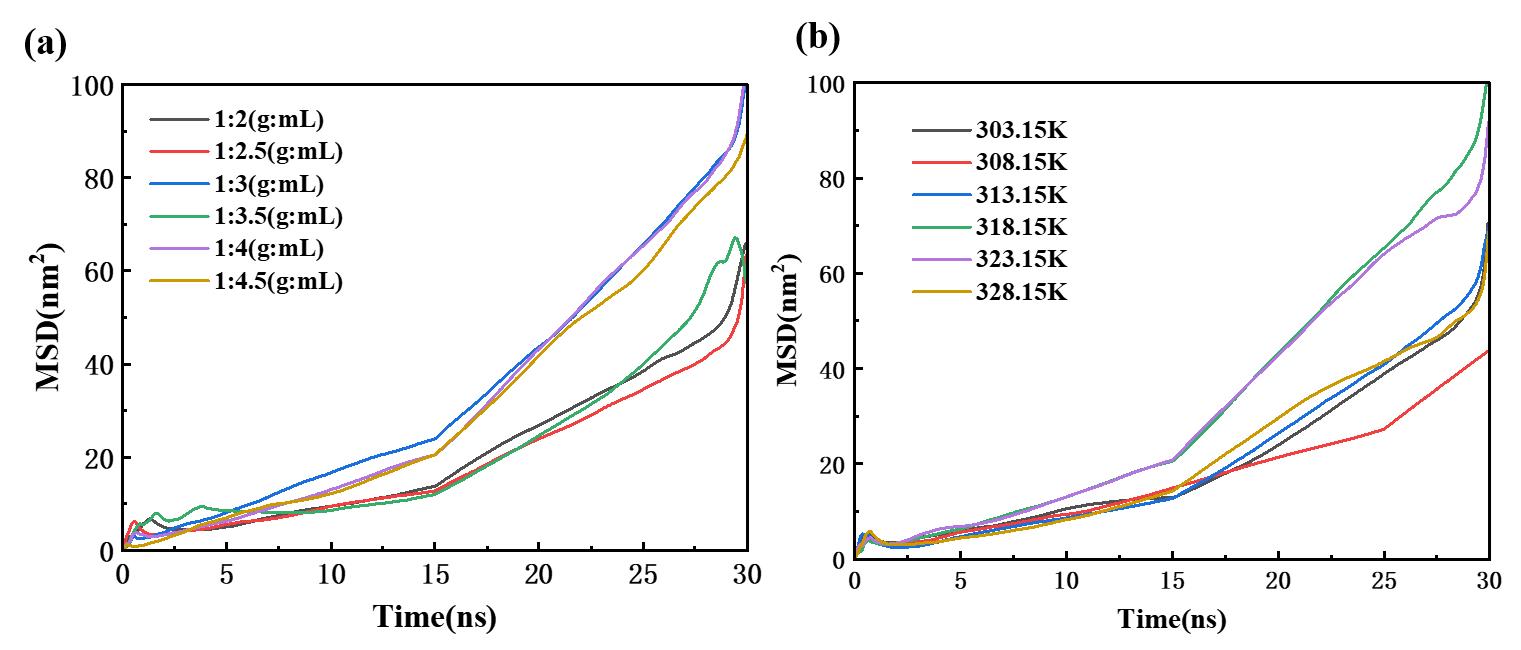
图11 硫氰酸红霉素团簇的均方位移(MSD)随时间的变化:(a)不同固液比条件;(b)不同温度条件
Fig. 11 Mean square displacement (MSD) of erythromycin thiocyanate clusters as a function of time: (a) under different solid-liquid ratios; (b) at different temperatures
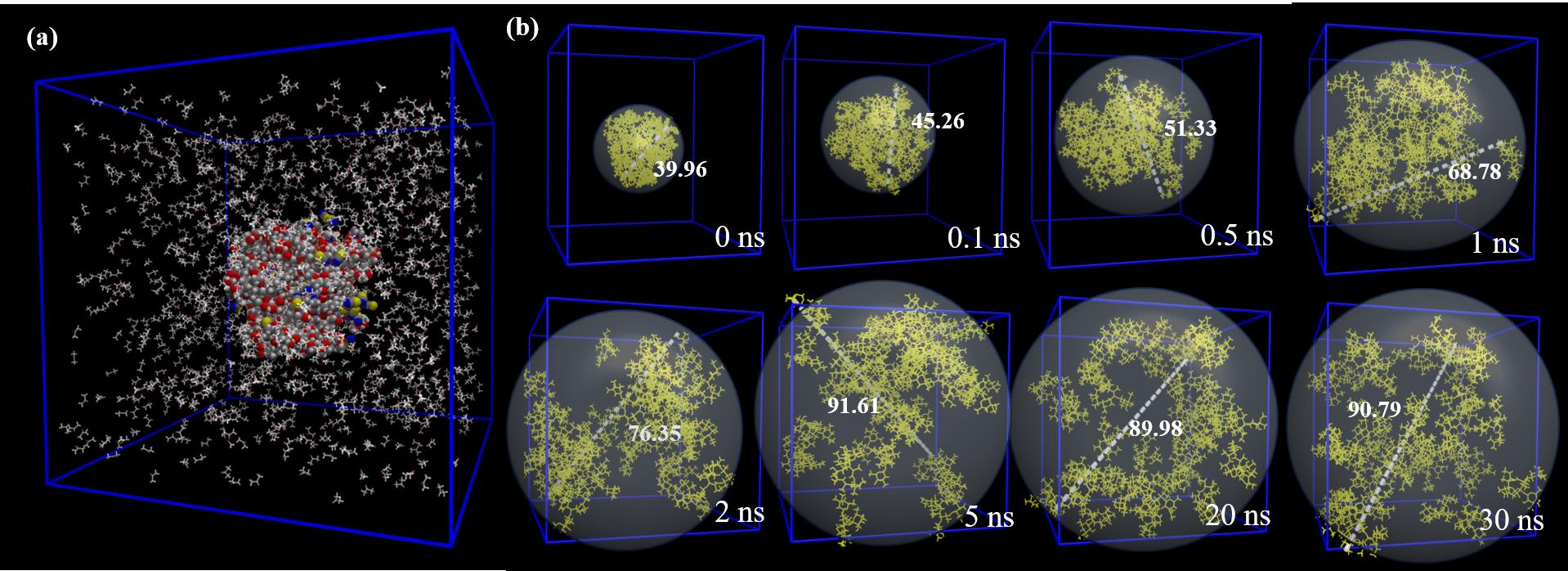
图12 (a)系统模型;(b)在固液比为1:3 (g:mL)丙酮溶液中分散过程中不同时间点的团簇快照
Fig.12 (a) System model; (b) Cluster snapshots at different time points during dispersion in acetone solution with a solid-liquid ratio of 1:3 (g:mL)
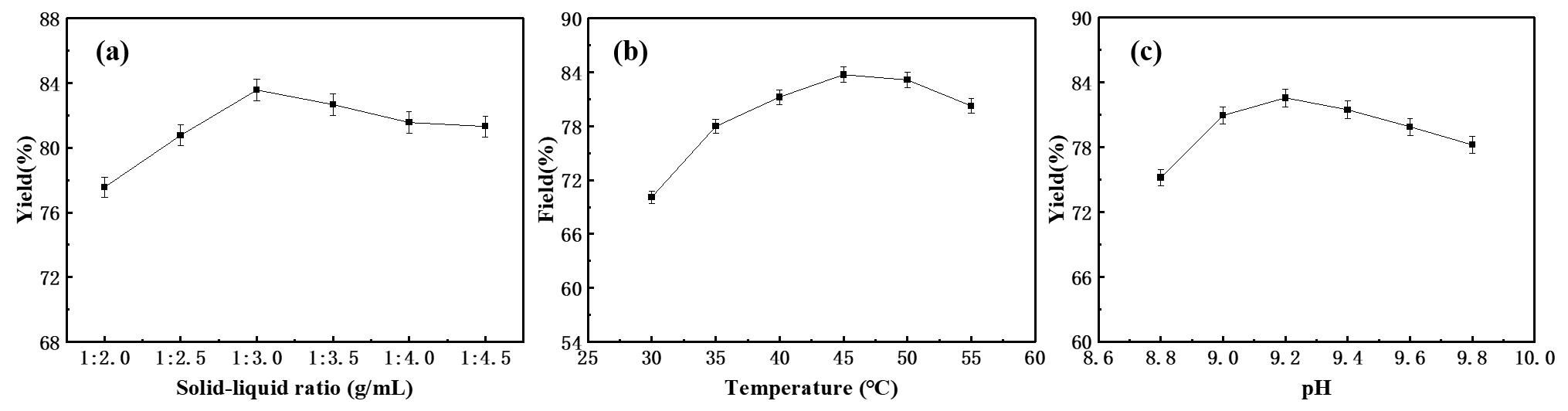
图13 萃取实验条件对硫氰酸红霉素收率的影响:(a)固液比,(b)温度和(c)pH
Fig 13 The effects of extraction conditions on the yield of erythromycin thiocyanate were as follows : (a) Solid-liquid ratio, (b) temperature and (c) pH
| [1] | 毛培学. 反应结晶制备硫氰酸红霉素过程研究[D]. 上海:华东理工大学,2012. |
| Mao P X. The study of preparation of erythromycin thiocyanate by reactive crystallization[D]. Shanghai: East China University of Science and Technology, 2012. | |
| [2] | 姬红明, 王勇平, 张永乐, 等. 水相结晶硫氰酸红霉素及其转红霉素碱工艺研究[J]. 化工与医药工程, 2014, 35(2): 26-29, 39. |
| Ji H M, Wang Y P, Zhang Y L, et al. Research on processes of erythromycin thiocyanate crystallization and production of erythromycin with crude erythromycin thiocyanate[J]. Chemical and Pharmaceutical Engineering, 2014, 35(2): 26-29, 39. | |
| [3] | 徐飞, 李桂水, 楼文君. 膜分离技术在发酵液提取浓缩中的应用[J]. 过滤与分离, 2006, 16(2): 26-29. |
| Xu F, Li G S, Lou W J. Applications of membrane separation technology in extractionand concentration of fermentation liquor[J]. Journal of Filtration & Separation, 2006, 16(2): 26-29. | |
| [4] | Zakrzewska M E, Nunes A V M, Barot A R, et al. Extraction of antibiotics using aqueous two-phase systems based on ethyl lactate and thiosulphate salts[J]. Fluid Phase Equilibria, 2021, 539: 113022. |
| [5] | Anike O, Cuhorka J, Ezeogu N, et al. Separation of Antibiotics Using Two Commercial Nanofiltration Membranes-Experimental Study and Modelling[J]. Membranes, 2024, 14(12): 248. |
| [6] | Li L, Zhao J, Yang T, et al. High-speed countercurrent chromatography as an efficient technique for large separation of plant polyphenols: A review[J]. Food Research International, 2022, 153: 110956. |
| [7] | 刘亚红. 青霉素提纯工艺生产现状和研究进展[J]. 生物技术世界, 2014(11): 168. |
| Liu Y H. Production status and research progress of penicillin purification process[J]. Biotech World, 2014(11): 168. | |
| [8] | Todd D B. Solvent extraction[M]//Vogel H C, Todaro C M. Fermentation and Biochemical Engineering Handbook. Oxford: Elsevier, 2014: 225-238. |
| [9] | Li Z, Qin F, Bao H, et al. Study on new solvent extraction systems for erythromycin[J]. Journal of Chemical Technology & Biotechnology, 2005, 80(7): 772-781. |
| [10] | Oka H, Harada K I, Ito Y, et al. Separation of antibiotics by counter-current chromatography[J]. Journal of Chromatography A, 1998, 812(1/2): 35-52. |
| [11] | Endo S, Pfennigsdorff A, Goss K U. Salting-out effect in aqueous NaCl solutions: trends with size and polarity of solute molecules[J]. Environmental Science & Technology, 2012, 46(3): 1496-1503. |
| [12] | Gezahegn T, Tegegne B, Zewge F, et al. Salting-out assisted liquid–liquid extraction for the determination of ciprofloxacin residues in water samples by high performance liquid chromatography–diode array detector[J]. BMC Chemistry, 2019, 13(1): 28. |
| [13] | Xiao J X, Sivars U, Tjerneld F. Phase behavior and protein partitioning in aqueous two-phase systems of cationic–anionic surfactant mixtures[J]. Journal of Chromatography B: Biomedical Sciences and Applications, 2000, 743(1-2): 327-338. |
| [14] | da Silva L H M, Coimbra J S R, de A Meirelles A J. Equilibrium Phase Behavior of Poly(ethylene glycol) + Potassium Phosphate + Water Two-Phase Systems at Various pH and Temperatures[J]. Journal of Chemical & Engineering Data, 1997, 42(2): 398-401. |
| [15] | Feng T, Li M M, Zhou J J, et al. Application of molecular dynamics simulation in food carbohydrate research—a review[J]. Innovative Food Science & Emerging Technologies, 2015, 31: 1-13. |
| [16] | Raut V P, Agashe M A, Stuart S J, et al. Molecular dynamics simulations of Peptide-Surface interactions[J]. Langmuir, 2005, 21(4): 1629-1639. |
| [17] | Hollingsworth S A, Dror R O. Molecular dynamics simulation for all[J]. Neuron, 2018, 99(6): 1129-1143. |
| [18] | Russina O, Caminiti R, Méndez-Morales T, et al. How does lithium nitrate dissolve in a protic ionic liquid [J]. Journal of Molecular Liquids, 2015, 205: 16-21. |
| [19] | Lanaro G, Patey G N. Molecular dynamics simulation of NaCl dissolution[J]. The Journal of Physical Chemistry B, 2015, 119(11): 4275-4283. |
| [20] | Anand A, Patey G N. Mechanism of urea crystal dissolution in water from molecular dynamics simulation[J]. The Journal of Physical Chemistry B, 2018, 122(3): 1213-1222. |
| [21] | 覃星添, 胡殿赫, 秦利明, 等. 基于分子动力学的纤维素在氯化胆碱/尿素中的溶解行为模拟研究[J]. 中国造纸, 2025, 44(7): 24-32. |
| Qin X T, Hu D H, Qin L M, et al. Simulation study of dissolution behavior of cellulose in choline chloride/urea based on molecular dynamics[J]. China Pulp & Paper, 2025, 44(7): 24-32. | |
| [22] | Platon V M, Dragoi B, Marin L. Erythromycin formulations—a journey to advanced drug delivery[J]. Pharmaceutics, 2022, 14(10): 2180. |
| [23] | Tyteca D, Schanck A, Dufrêne Y F, et al. The macrolide antibiotic azithromycin interacts with lipids and affects membrane organization and fluidity: Studies on Langmuir-blodgett monolayers, liposomes and J774 macrophages[J]. The Journal of Membrane Biology, 2003, 192(3): 203-215. |
| [24] | 郭金梅, 李斌, 肖华平, 等. 3种固体助溶剂对硫氰酸红霉素助溶作用的研究[J]. 现代畜牧科技, 2023(6): 14-17. |
| Guo J M, Li B, Xiao H P, et al. Study on the solubilization effect of three solid cosolvents on erythromycin thiocyanate[J]. Modern Animal Husbandry Science & Technology, 2023(6): 14-17. | |
| [25] | 王华旗. 基于氢键作用的固体分散体结晶性及载体性能的分子模拟与实验研究[D]. 北京: 北京化工大学, 2025. |
| Wang H Q. Molecular simulations and experiments to explore the crystallinity and carrier properties of solid dispersions based on hydrogen bond[D]. Beijing: Beijing University of Chemical Technology, 2025. | |
| [26] | Abraham M J, Murtola T, Schulz R, et al. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers[J]. SoftwareX, 2015, 1: 19-25. |
| [27] | Sprenger K G, Jaeger V W, Pfaendtner J. The general AMBER force field (GAFF) can accurately predict thermodynamic and transport properties of many ionic liquids[J]. The Journal of Physical Chemistry. B, 2015, 119(18): 5882-5895. |
| [28] | Huang J T, Yin Q X, Hao H X, et al. Solid-liquid equilibrium of erythromycin thiocyanate dihydrate in four mono-solvents and three binary solvent mixtures[J]. Journal of Molecular Liquids, 2017, 234: 408-416. |
| [29] | 李顺阳. 储氢气瓶内衬异质界面连接强度和氢透性分子模拟[D]. 太原: 太原科技大学, 2023. |
| Li S Y. Molecular simulation of heterogeneous interfacial connection strength and hydrogen permeability in hydrogen storage cylinder liners[D]. Taiyuan: Taiyuan University of Science and Technology, 2023. | |
| [30] | Grimme S, Ehrlich S, Goerigk L. Effect of the damping function in dispersion corrected density functional theory[J]. Journal of Computational Chemistry, 2011, 32(7): 1456-1465. |
| [31] | Hohenberg P, Kohn W. Inhomogeneous electron gas[J]. Physical Review, 1964, 136(3B): B864-B871. |
| [32] | Martínez L, Andrade R, Birgin E G, et al. PACKMOL: a package for building initial configurations for molecular dynamics simulations[J]. Journal of Computational Chemistry, 2009, 30(13): 2157-2164. |
| [33] | Canzar S, El-Kebir M, Pool R, et al. Charge group partitioning in biomolecular simulation[J]. Journal of Computational Biology, 2013, 20(3): 188-198. |
| [34] | Hestenes M R, Stiefel E. Methods of conjugate gradients for solving linear systems[J]. Journal of Research of the National Bureau of Standards, 1952, 49(6): 409. |
| [35] | Darden T, York D, Pedersen L. Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems[J]. The Journal of Chemical Physics, 1993, 98(12): 10089-10092. |
| [36] | Sun G X, Zhao Y, Liang W Z. Aggregation-induced emission mechanism of dimethoxy-tetraphenylethylene in water solution: molecular dynamics and QM/MM investigations[J]. Journal of Chemical Theory and Computation, 2015, 11(5): 2257-2267. |
| [37] | Bussi G, Donadio D, Parrinello M. Canonical sampling through velocity rescaling[J]. The Journal of Chemical Physics, 2007, 126(1): 014101. |
| [38] | Bernetti M, Bussi G. Pressure control using stochastic cell rescaling[J]. The Journal of Chemical Physics, 2020, 153(11): 114107. |
| [39] | Corti D S. Isothermal-isobaric ensemble for small systems[J]. Physical Review E, 2001, 64(1): 016128. |
| [40] | Lu T, Chen F W. Multiwfn: A multifunctional wavefunction analyzer[J]. Journal of Computational Chemistry, 2012, 33(5): 580-592. |
| [41] | Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 16, Revision C.01[CP/OL]. Wallingford, CT: Gaussian, Inc., 2016. [2026-01-13]. . |
| [42] | 赵东, 汪云燕, 赵健, 等. NaCl对杉木中水分子扩散影响的机理研究[J]. 北京林业大学学报, 2021, 43(7): 140-148. |
| Zhao D, Wang Y Y, Zhao J, et al. Mechanism of NaCl effect on diffusion of water molecules in Cunninghamia lanceolata[J]. Journal of Beijing Forestry University, 2021, 43(7): 140-148. | |
| [43] | Yu X X, Wu Y N, Wang J K, et al. Experimental assessment and modeling of the solubility of malonic acid in different solvents[J]. Chemical Engineering & Technology, 2018, 41(6): 1098-1107. |
| [44] | Pattanayak S K, Prashar N, Chowdhuri S. Effect of temperature and pressure on the structure, dynamics, and hydrogen bond properties of liquid N-methylacetamide: a molecular dynamics study[J]. The Journal of Chemical Physics, 2011, 134(15): 154506. |
| [45] | Luzar A, Chandler D. Hydrogen-bond kinetics in liquid water[J]. Nature, 1996, 379(6560): 55-57. |
| [46] | 郭佳, 黄延智. 硫氰酸红霉素降低杂质B精制工艺研究[J]. 当代化工研究, 2024(6): 153-155. |
| Guo J, Huang Y Z. Study on refining process of erythromycin thiocyanate for reducing impurity B[J]. Modern Chemical Research, 2024(6): 153-155. |
| [1] | 丁昊, 王林, 刘豪. R290/R245fa汽液相平衡混合规则对比研究[J]. 化工学报, 2025, 76(S1): 9-16. |
| [2] | 曾宁, 郭振江, 陈建华, 张子轩, 曾玉娇, 肖炘, 刘松林, 薛绍秀, 周智武, 卢振明, 王利民. 二水湿法磷酸工艺中非水溶磷的分子动力学模拟[J]. 化工学报, 2025, 76(9): 4539-4550. |
| [3] | 刘峰, 韩春硕, 张益, 刘彦成, 郁林军, 申家伟, 高晓泉, 杨凯. 高温高盐环境下单烃链和双烃链表面活性剂对油水界面性质影响的微观机理研究[J]. 化工学报, 2025, 76(6): 2939-2957. |
| [4] | 赵清萍, 张敏, 赵辉, 王刚, 邱永福. 乙烯氢甲酯化合成丙酸甲酯的氢键作用机制及反应动力学研究[J]. 化工学报, 2025, 76(6): 2701-2713. |
| [5] | 朱峰, 赵跃, 马凤翔, 刘伟. 改性UIO-66对SF6/N2混合气体及其分解产物的吸附特性[J]. 化工学报, 2025, 76(4): 1604-1616. |
| [6] | 徐芳, 张锐, 崔达, 王擎. ReaxFF-MD揭示木质素热解反应机制的分子动力学研究[J]. 化工学报, 2025, 76(3): 1253-1263. |
| [7] | 张奇, 张睿, 郑涛, 曹欣, 刘植昌, 刘海燕, 徐春明, 张荣, 孟祥海. 基于分子模拟的新型双阳离子质子型离子液体捕集CO2研究[J]. 化工学报, 2025, 76(2): 797-811. |
| [8] | 任珂, 刘新健, 饶中浩. 潜热型功能流体分散稳定性强化及调控机理研究[J]. 化工学报, 2025, 76(11): 5584-5593. |
| [9] | 董纪广, 谢绍雷, 时东, 李丽娟, 赵晨宇, 黄雨婕, 石成龙, 许淘善, 曹大伟. 邻羟基苯甲酸正辛酯萃取体系提锂:协萃剂结构变化对萃取性能影响[J]. 化工学报, 2025, 76(10): 5190-5202. |
| [10] | 张广宇, 付然飞, 孙冰, 袁俊聪, 冯翔, 杨朝合, 徐伟. CO2-环氧丙烷合成碳酸丙烯酯:氢键供体效应研究[J]. 化工学报, 2024, 75(6): 2243-2251. |
| [11] | 李俊, 赵亮, 高金森, 徐春明. 不同馏分油分级分质加工中萃取技术研究进展[J]. 化工学报, 2024, 75(4): 1065-1080. |
| [12] | 贾海林, 曾锦祥, 潘荣锟, 潘仕利, 周凯旋. 无氟泡沫灭火剂真火实验与分子动力学模拟[J]. 化工学报, 2024, 75(10): 3825-3834. |
| [13] | 徐娜, 李子璇, 刘子璐, 吕耀东, 张释文. 溶液环境对液相纳米颗粒体系分散稳定性的影响[J]. 化工学报, 2024, 75(10): 3815-3824. |
| [14] | 王尤佳, 赵亮, 高金森, 徐春明. 柴油烃类族组成分离技术研究进展[J]. 化工学报, 2024, 75(1): 20-32. |
| [15] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号