化工学报 ›› 2019, Vol. 70 ›› Issue (6): 2092-2101.DOI: 10.11949/0438-1157.20190028
吴金芝1( ),王琳琳1,2(
),王琳琳1,2( ),陈小鹏1,2,童张法1,2,范孝雄1,梁保芳1
),陈小鹏1,2,童张法1,2,范孝雄1,梁保芳1
收稿日期:2019-01-09
修回日期:2019-04-01
出版日期:2019-06-05
发布日期:2019-06-05
通讯作者:
王琳琳
作者简介:<named-content content-type="corresp-name">吴金芝</named-content>(1990—),女,硕士研究生,<email>1491893326@qq.com</email>
基金资助:
Jinzhi WU1( ),Linlin WANG1,2(
),Linlin WANG1,2( ),Xiaopeng CHEN1,2,Zhangfa TONG1,2,Xiaoxiong FAN1,Baofang LIANG1
),Xiaopeng CHEN1,2,Zhangfa TONG1,2,Xiaoxiong FAN1,Baofang LIANG1
Received:2019-01-09
Revised:2019-04-01
Online:2019-06-05
Published:2019-06-05
Contact:
Linlin WANG
摘要:
改进了Ellis平衡釜的保温、搅拌与压力控制系统,建立了一套可视窗口的实验装置。测定了莰烯+(+)-3-蒈烯体系在313.15、373.15和433.15 K下的汽液平衡数据;采用面积法和Van Ness检验法对实验数据进行热力学一致性检验,所有数据均符合Gibbs-Duhenm的热力学一致性;选用Aspen plus软件中NRTL、Wilson和UNIQUAC模型,由最大似然法对目标函数进行优化,实现对实验数据的关联。结果表明,气相组成和平衡压力的估算值与实验值的最大平均相对偏差为0.0128和0.0009,最大均方根偏差为0.0003和0.0012 kPa,相对挥发度的估算值与实验值的绝对平均偏差分别为0.09%、0.03%和0.04%。
中图分类号:
吴金芝, 王琳琳, 陈小鹏, 童张法, 范孝雄, 梁保芳. 莰烯+(+)-3-蒈烯体系汽液平衡数据的测定与关联[J]. 化工学报, 2019, 70(6): 2092-2101.
Jinzhi WU, Linlin WANG, Xiaopeng CHEN, Zhangfa TONG, Xiaoxiong FAN, Baofang LIANG. Determination and correlation of vapor-liquid equilibrium data for camphene+(+)-3-carene system[J]. CIESC Journal, 2019, 70(6): 2092-2101.
| No. | P/kPa | x 1 | y 1 | α 12 |
|---|---|---|---|---|
| 1 | 1.09 | 0.0000 | 0.0000 | — |
| 2 | 1.10 | 0.0499 | 0.0585 | 1.1825 |
| 3 | 1.11 | 0.1248 | 0.1447 | 1.1863 |
| 4 | 1.12 | 0.1748 | 0.2012 | 1.1885 |
| 5 | 1.13 | 0.2247 | 0.2566 | 1.1906 |
| 6 | 1.14 | 0.2747 | 0.3111 | 1.1923 |
| 7 | 1.15 | 0.3247 | 0.3646 | 1.1937 |
| 8 | 1.16 | 0.3746 | 0.4171 | 1.1949 |
| 9 | 1.17 | 0.3996 | 0.4431 | 1.1953 |
| 10 | 1.18 | 0.4496 | 0.4942 | 1.1960 |
| 11 | 1.19 | 0.4994 | 0.5441 | 1.1962 |
| 12 | 1.20 | 0.5494 | 0.5932 | 1.1961 |
| 13 | 1.21 | 0.5998 | 0.6418 | 1.1955 |
| 14 | 1.22 | 0.6495 | 0.6888 | 1.1944 |
| 15 | 1.24 | 0.7246 | 0.7582 | 1.1918 |
| 16 | 1.25 | 0.7747 | 0.8035 | 1.1893 |
| 17 | 1.26 | 0.8248 | 0.8482 | 1.1862 |
| 18 | 1.7 | 0.8749 | 0.8921 | 1.1824 |
| 19 | 1.28 | 0.9249 | 0.9355 | 1.1780 |
| 20 | 1.29 | 0.9750 | 0.9786 | 1.1724 |
| 21 | 1.29 | 1.0000 | 1.0000 | — |
表1 莰烯+(+)-3-蒈烯在313.15 K下的汽液平衡实验数据①及相对挥发度
Table 1 VLE data and relative volatility for camphene+(+)-3-carene at 313.15 K
| No. | P/kPa | x 1 | y 1 | α 12 |
|---|---|---|---|---|
| 1 | 1.09 | 0.0000 | 0.0000 | — |
| 2 | 1.10 | 0.0499 | 0.0585 | 1.1825 |
| 3 | 1.11 | 0.1248 | 0.1447 | 1.1863 |
| 4 | 1.12 | 0.1748 | 0.2012 | 1.1885 |
| 5 | 1.13 | 0.2247 | 0.2566 | 1.1906 |
| 6 | 1.14 | 0.2747 | 0.3111 | 1.1923 |
| 7 | 1.15 | 0.3247 | 0.3646 | 1.1937 |
| 8 | 1.16 | 0.3746 | 0.4171 | 1.1949 |
| 9 | 1.17 | 0.3996 | 0.4431 | 1.1953 |
| 10 | 1.18 | 0.4496 | 0.4942 | 1.1960 |
| 11 | 1.19 | 0.4994 | 0.5441 | 1.1962 |
| 12 | 1.20 | 0.5494 | 0.5932 | 1.1961 |
| 13 | 1.21 | 0.5998 | 0.6418 | 1.1955 |
| 14 | 1.22 | 0.6495 | 0.6888 | 1.1944 |
| 15 | 1.24 | 0.7246 | 0.7582 | 1.1918 |
| 16 | 1.25 | 0.7747 | 0.8035 | 1.1893 |
| 17 | 1.26 | 0.8248 | 0.8482 | 1.1862 |
| 18 | 1.7 | 0.8749 | 0.8921 | 1.1824 |
| 19 | 1.28 | 0.9249 | 0.9355 | 1.1780 |
| 20 | 1.29 | 0.9750 | 0.9786 | 1.1724 |
| 21 | 1.29 | 1.0000 | 1.0000 | — |
| No. | P/kPa | x 1 | y 1 | α 12 |
|---|---|---|---|---|
| 1 | 13.05 | 0.0000 | 0.0000 | — |
| 2 | 13.26 | 0.0502 | 0.0653 | 1.3218 |
| 3 | 13.48 | 0.1003 | 0.1285 | 1.3226 |
| 4 | 13.69 | 0.1499 | 0.1891 | 1.3225 |
| 5 | 13.90 | 0.2001 | 0.2486 | 1.3226 |
| 6 | 14.11 | 0.2496 | 0.3056 | 1.3231 |
| 7 | 14.43 | 0.3253 | 0.3895 | 1.3233 |
| 8 | 14.64 | 0.3747 | 0.4423 | 1.3235 |
| 9 | 14.95 | 0.4502 | 0.5202 | 1.3241 |
| 10 | 15.16 | 0.4998 | 0.5695 | 1.3239 |
| 11 | 15.48 | 0.5752 | 0.6420 | 1.3244 |
| 12 | 15.69 | 0.6256 | 0.6888 | 1.3246 |
| 13 | 15.90 | 0.6747 | 0.7332 | 1.3250 |
| 14 | 16.22 | 0.7500 | 0.7991 | 1.3259 |
| 15 | 16.43 | 0.8004 | 0.8417 | 1.3260 |
| 16 | 16.75 | 0.8749 | 0.9027 | 1.3266 |
| 17 | 16.86 | 0.9003 | 0.9230 | 1.3275 |
| 18 | 16.96 | 0.9251 | 0.9425 | 1.3271 |
| 19 | 17.17 | 0.9752 | 0.9812 | 1.3273 |
| 20 | 17.28 | 1.0000 | 1.0000 | — |
表2 莰烯+(+)-3-蒈烯在373.15 K下的汽液平衡实验数据①及相对挥发度
Table 2 VLE data and relative volatility for camphene+(+)-3-carene at 373.15 K
| No. | P/kPa | x 1 | y 1 | α 12 |
|---|---|---|---|---|
| 1 | 13.05 | 0.0000 | 0.0000 | — |
| 2 | 13.26 | 0.0502 | 0.0653 | 1.3218 |
| 3 | 13.48 | 0.1003 | 0.1285 | 1.3226 |
| 4 | 13.69 | 0.1499 | 0.1891 | 1.3225 |
| 5 | 13.90 | 0.2001 | 0.2486 | 1.3226 |
| 6 | 14.11 | 0.2496 | 0.3056 | 1.3231 |
| 7 | 14.43 | 0.3253 | 0.3895 | 1.3233 |
| 8 | 14.64 | 0.3747 | 0.4423 | 1.3235 |
| 9 | 14.95 | 0.4502 | 0.5202 | 1.3241 |
| 10 | 15.16 | 0.4998 | 0.5695 | 1.3239 |
| 11 | 15.48 | 0.5752 | 0.6420 | 1.3244 |
| 12 | 15.69 | 0.6256 | 0.6888 | 1.3246 |
| 13 | 15.90 | 0.6747 | 0.7332 | 1.3250 |
| 14 | 16.22 | 0.7500 | 0.7991 | 1.3259 |
| 15 | 16.43 | 0.8004 | 0.8417 | 1.3260 |
| 16 | 16.75 | 0.8749 | 0.9027 | 1.3266 |
| 17 | 16.86 | 0.9003 | 0.9230 | 1.3275 |
| 18 | 16.96 | 0.9251 | 0.9425 | 1.3271 |
| 19 | 17.17 | 0.9752 | 0.9812 | 1.3273 |
| 20 | 17.28 | 1.0000 | 1.0000 | — |
| No. | P/kPa | x 1 | y 1 | α 12 |
|---|---|---|---|---|
| 1 | 78.73 | 0.0000 | 0.0000 | — |
| 2 | 79.29 | 0.0252 | 0.0321 | 1.2819 |
| 3 | 80.40 | 0.0753 | 0.0945 | 1.2821 |
| 4 | 81.52 | 0.1249 | 0.1547 | 1.2822 |
| 5 | 82.63 | 0.1751 | 0.2140 | 1.2824 |
| 6 | 84.31 | 0.2502 | 0.2997 | 1.2827 |
| 7 | 85.43 | 0.3006 | 0.3554 | 1.2828 |
| 8 | 87.10 | 0.3748 | 0.4348 | 1.2831 |
| 9 | 88.21 | 0.4252 | 0.4870 | 1.2832 |
| 10 | 89.32 | 0.4749 | 0.5372 | 1.2834 |
| 11 | 90.45 | 0.5254 | 0.5869 | 1.2836 |
| 12 | 92.12 | 0.5995 | 0.6578 | 1.2838 |
| 13 | 93.79 | 0.6755 | 0.7277 | 1.2841 |
| 14 | 94.91 | 0.7246 | 0.7716 | 1.2842 |
| 15 | 96.03 | 0.7753 | 0.8159 | 1.2844 |
| 16 | 98.26 | 0.8748 | 0.8998 | 1.2847 |
| 17 | 99.38 | 0.9254 | 0.9410 | 1.2849 |
| 18 | 100.49 | 0.9747 | 0.9802 | 1.2851 |
| 19 | 101.05 | 1.0000 | 1.0000 | — |
表3 莰烯+(+)-3-蒈烯在433.15 K下的汽液平衡实验数据①及相对挥发度
Table 3 VLE data and relative volatility for camphene+(+)-3-carene at 433.15 K
| No. | P/kPa | x 1 | y 1 | α 12 |
|---|---|---|---|---|
| 1 | 78.73 | 0.0000 | 0.0000 | — |
| 2 | 79.29 | 0.0252 | 0.0321 | 1.2819 |
| 3 | 80.40 | 0.0753 | 0.0945 | 1.2821 |
| 4 | 81.52 | 0.1249 | 0.1547 | 1.2822 |
| 5 | 82.63 | 0.1751 | 0.2140 | 1.2824 |
| 6 | 84.31 | 0.2502 | 0.2997 | 1.2827 |
| 7 | 85.43 | 0.3006 | 0.3554 | 1.2828 |
| 8 | 87.10 | 0.3748 | 0.4348 | 1.2831 |
| 9 | 88.21 | 0.4252 | 0.4870 | 1.2832 |
| 10 | 89.32 | 0.4749 | 0.5372 | 1.2834 |
| 11 | 90.45 | 0.5254 | 0.5869 | 1.2836 |
| 12 | 92.12 | 0.5995 | 0.6578 | 1.2838 |
| 13 | 93.79 | 0.6755 | 0.7277 | 1.2841 |
| 14 | 94.91 | 0.7246 | 0.7716 | 1.2842 |
| 15 | 96.03 | 0.7753 | 0.8159 | 1.2844 |
| 16 | 98.26 | 0.8748 | 0.8998 | 1.2847 |
| 17 | 99.38 | 0.9254 | 0.9410 | 1.2849 |
| 18 | 100.49 | 0.9747 | 0.9802 | 1.2851 |
| 19 | 101.05 | 1.0000 | 1.0000 | — |
| T/K | AREA① | Van Ness② | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NRTL | Wilson | UNIQUAC | Result | ||||||
| Value | Result | Δy | ΔP | Δy | ΔP | Δy | ΔP | ||
| 313.15 | 4.5287 | passed | 0.0012 | 0.0004 | 0.0092 | 0.0006 | 0.0027 | 0.0005 | passed |
| 373.15 | 8.6831 | passed | 0.0030 | 0.0013 | 0.0029 | 0.0020 | 0.0034 | 0.0010 | passed |
| 433.15 | 1.3367 | passed | 0.0039 | 0.0010 | 0.0039 | 0.0010 | 0.0037 | 0.0009 | passed |
表4 汽液平衡实验数据的热力学一致性检验
Table 4 Thermodynamic consistency test of vapor-liquid equilibrium experimental data
| T/K | AREA① | Van Ness② | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NRTL | Wilson | UNIQUAC | Result | ||||||
| Value | Result | Δy | ΔP | Δy | ΔP | Δy | ΔP | ||
| 313.15 | 4.5287 | passed | 0.0012 | 0.0004 | 0.0092 | 0.0006 | 0.0027 | 0.0005 | passed |
| 373.15 | 8.6831 | passed | 0.0030 | 0.0013 | 0.0029 | 0.0020 | 0.0034 | 0.0010 | passed |
| 433.15 | 1.3367 | passed | 0.0039 | 0.0010 | 0.0039 | 0.0010 | 0.0037 | 0.0009 | passed |
| model | Binary interaction parameters | RMSD | AAD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| | | | | | y 1 | P/kPa | y 1 | P/kPa | |||
| NRTL | 0.131792 | -0.160414 | 118.289487 | -87.54019 | 0.3 | 0.0000 | 0.0005 | 0.0000 | 0.0004 | ||
| Wilson | 0.124744 | -0.090117 | 70.204871 | -105.15032 | 0.0000 | 0.0005 | 0.0000 | 0.0004 | |||
| UNIQUAC | 0.033377 | 0.008403 | 36.4785569 | -422.10712 | 0.0001 | 0.0004 | 0.0001 | 0.0003 | |||
表5 莰烯+3-蒈烯体系NRTL,Wilson和UNIQUAC活度系数模型的二元相互作用能量参数,以及莰烯组分的气相组成(y 1)和平衡压力(P)的均方根偏差(RMSD)和平均绝对偏差(AAD)
Table 5 Binary interaction energy parameters of NRTL, Wilson and UNIQUAC activity coefficient models for camphene +3-carene systeme, and root mean square deviation (RMSD) and average absolute deviations (AAD) for vapor phase mole fraction (y 1) and equilibrium pressure (P)
| model | Binary interaction parameters | RMSD | AAD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| | | | | | y 1 | P/kPa | y 1 | P/kPa | |||
| NRTL | 0.131792 | -0.160414 | 118.289487 | -87.54019 | 0.3 | 0.0000 | 0.0005 | 0.0000 | 0.0004 | ||
| Wilson | 0.124744 | -0.090117 | 70.204871 | -105.15032 | 0.0000 | 0.0005 | 0.0000 | 0.0004 | |||
| UNIQUAC | 0.033377 | 0.008403 | 36.4785569 | -422.10712 | 0.0001 | 0.0004 | 0.0001 | 0.0003 | |||
| Component | r | q |
|---|---|---|
| camphene | 6.0560 | 4.7600 |
| (+)-3-carene | 6.0541 | 4.7560 |
表6 UNIQUAC模型分子参数r(van der Waals分子体积)和q(van der Waals分子表面积)
Table 6 van der Waals volume (r) and surface area (q) of components for UNIQUAC equation
| Component | r | q |
|---|---|---|
| camphene | 6.0560 | 4.7600 |
| (+)-3-carene | 6.0541 | 4.7560 |
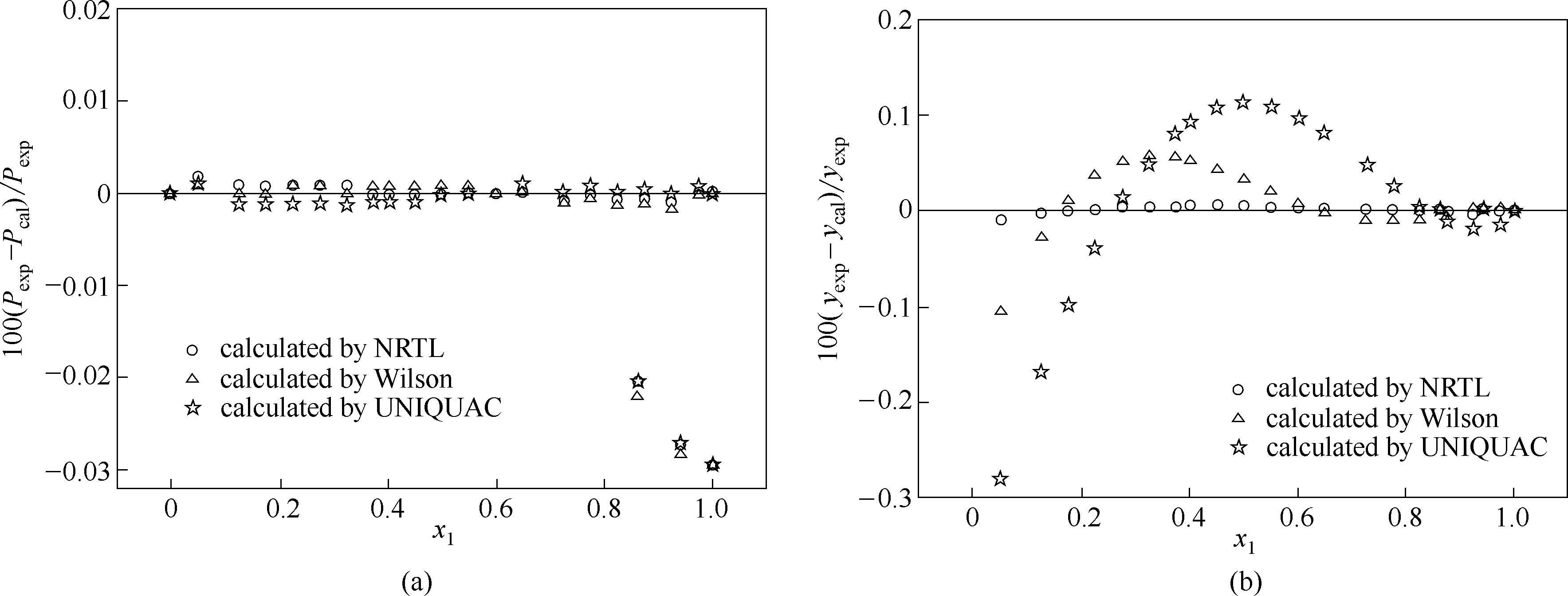
图7 莰烯+(+)-3-蒈烯体系在313.15 K条件下实验值与模型估算值的相对偏差
Fig.7 Relative deviations of experimental data, P exp, and y exp from calculated results of models, P cal and y cal, for camphene+(+)-3-carene system at 313.15 K
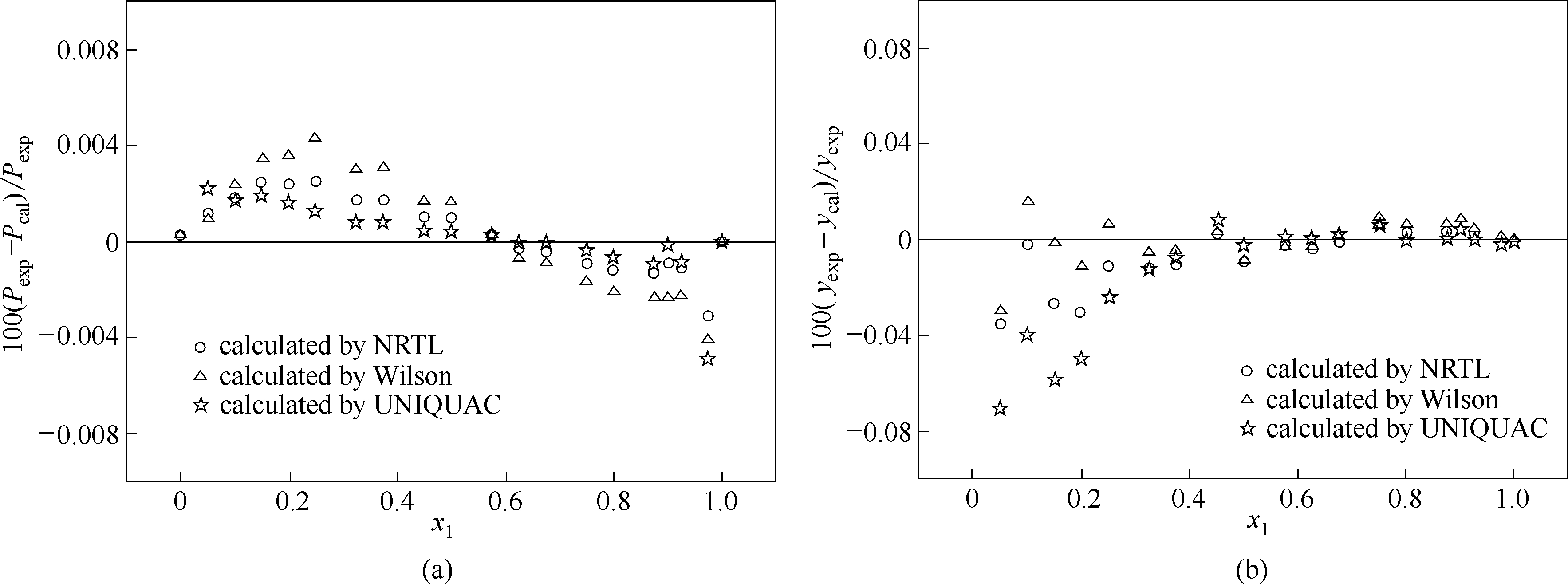
图8 莰烯+(+)-3-蒈烯体系在373.15 K条件下实验值与模型估算值的相对偏差
Fig.8 Relative deviations of experimental data, P exp, and y exp from calculated results of models, P cal and y cal, for camphene+(+)-3-carene system at 373.15 K
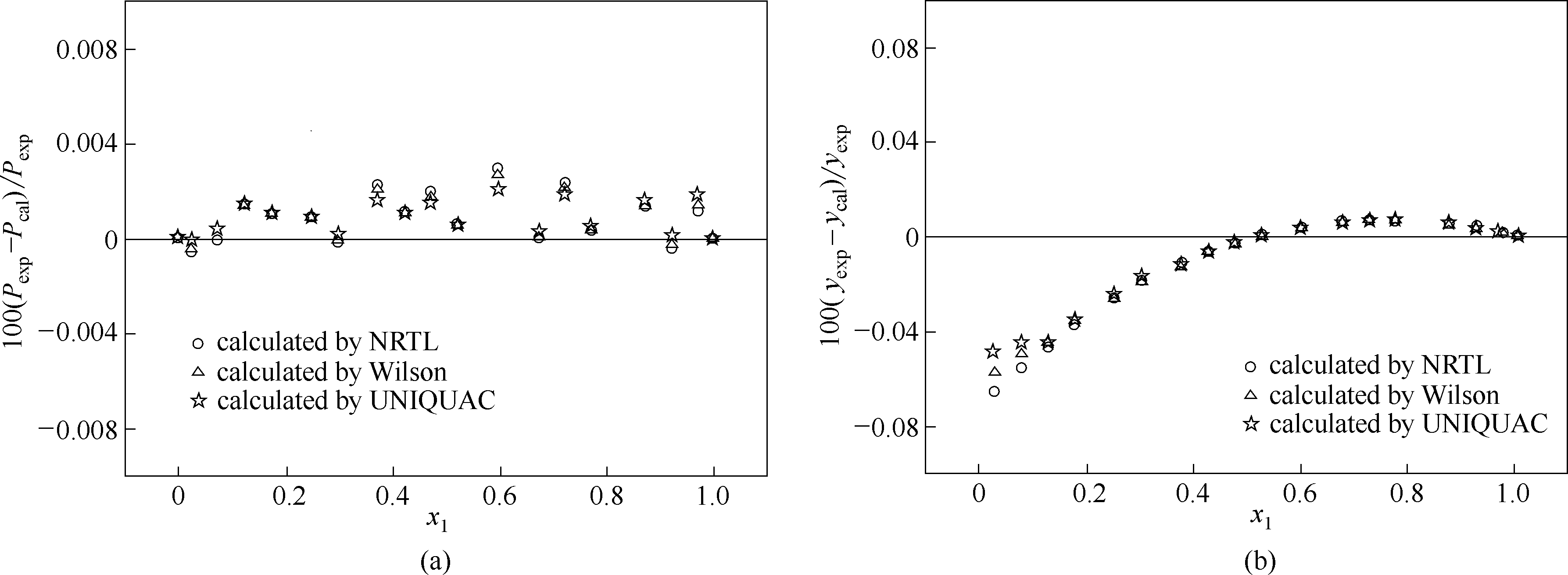
图9 莰烯+(+)-3-蒈烯体系在433.15 K条件下实验值与模型估算值的相对偏差
Fig.9 Relative deviations of experimental data, P exp, and y exp from calculated results of models, P cal and y cal, for camphene+(+)-3-carene system at 433.15 K
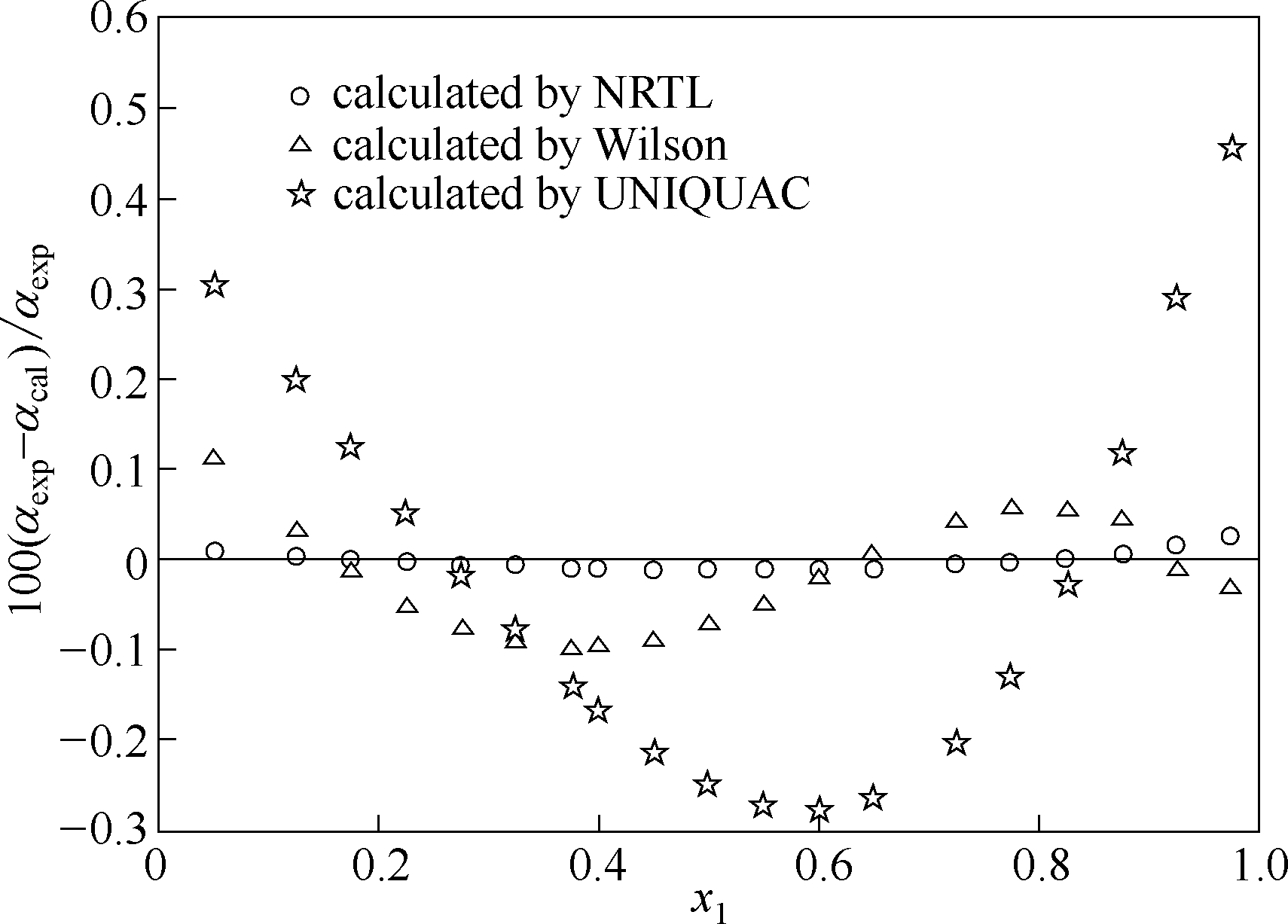
图10 莰烯+(+)-3-蒈烯体系在313.15 K条件下估算值与实验值的相对挥发度的相对偏差
Fig.10 Relative deviation between calculated values and experimental data of relative volatility of camphene+(+)-3-carene system at 313.15 K
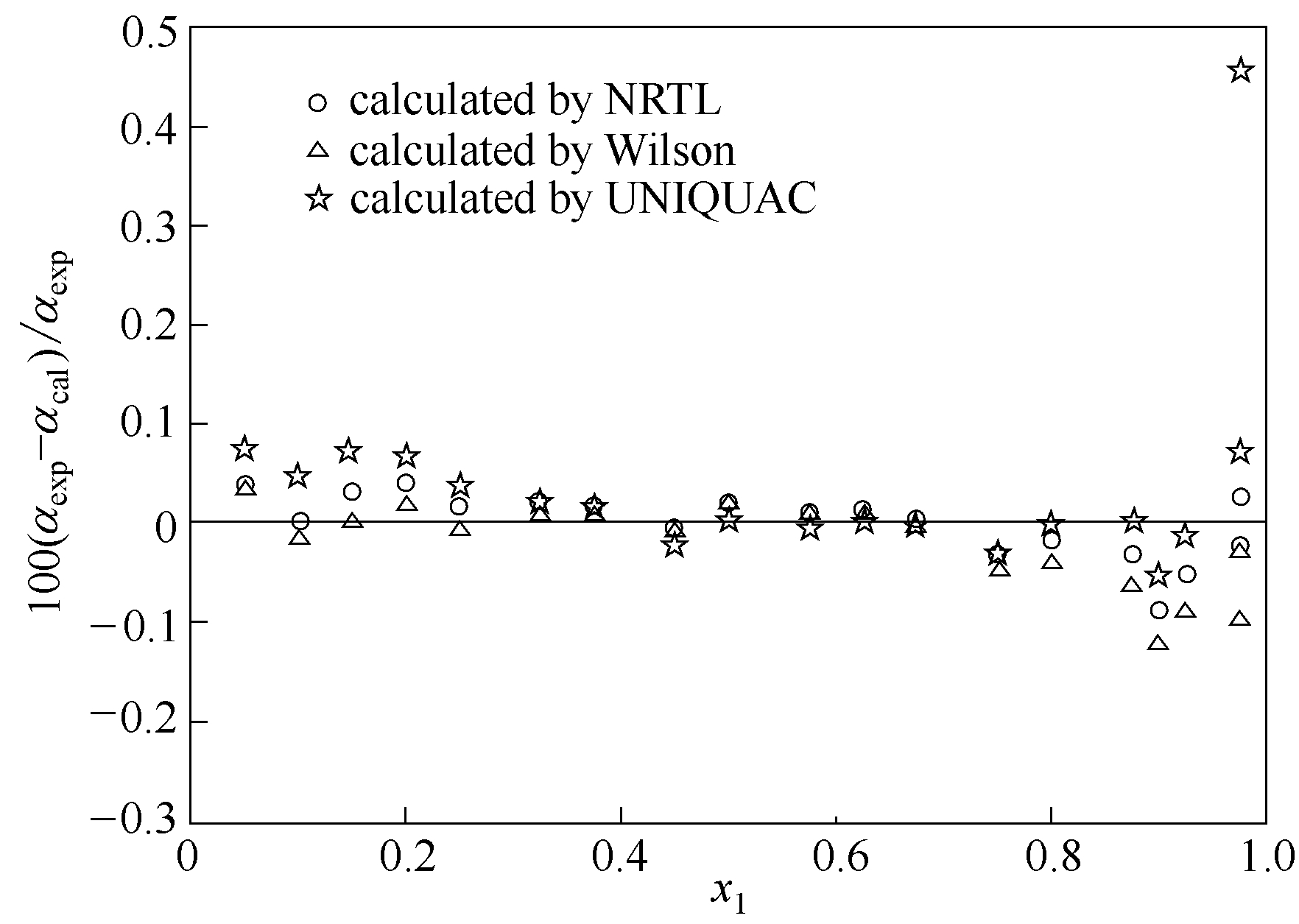
图11 莰烯+(+)-3-蒈烯体系在373.15 K条件下估算值与实验值的相对挥发度的相对偏差
Fig.11 Relative deviation between calculated values and experimental data of relative volatility of camphene+(+)-3-carene system at 373.15 K
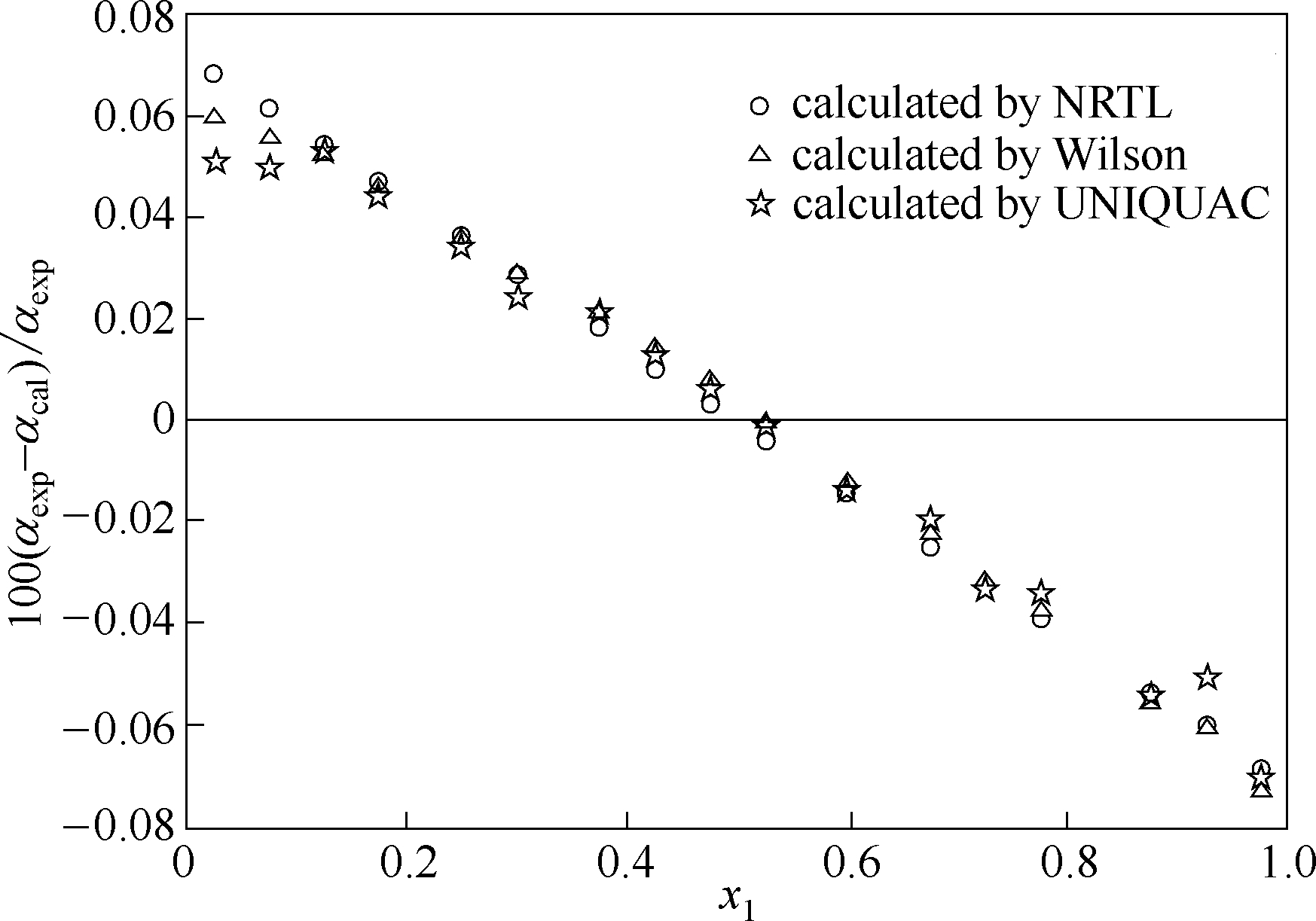
图12 莰烯+(+)-3-蒈烯体系在433.15 K条件下相对挥发度的估算值与实验值的相对偏差
Fig.12 Relative deviation between calculated values and experimental data of relative volatility of camphene+(+)-3-carene system at 433.15 K
| 1 | Rodrigues M F , Bernardo-gil M G . Vapor-liquid equilibrium data of α-pinene + β-pinene + limonene at 80 and 101 kPa[J]. Journal of Chemical Engineering Data, 1996, 41(3): 581-585. |
| 2 | Tong Z F , Kim S H . In Frontiers on Separation Science and Technology[M]. Singapore: World Scientific Publishing Co., 2004. |
| 3 | Okuniewski M , Paduszyński K , Domańska U . Thermodynamic study of molecular interactions in eutectic mixtures containing camphene[J]. The Journal of Physical Chemistry B, 2016, 120(50): 12928-12936. |
| 4 | 李金凤, 梁宁, 孙志, 等 . 水蒸气萃取-气相色谱法同时测定核桃仁挥发油中异喹啉、氧化石竹烯、十六烷[J]. 亚洲传统医学杂志, 2012, 7(6): 246-252. |
| Li J F , Liang N , Sun Z , et al . Simulataneous determination of isoquinoline, caryophyllene oxide, hexadecane in essential oils from juglandis mandshuricae cortex by vapour-vapour extaction combined with gas chromatograph analysis[J]. Asian Journal of Traditional Medicine, 2012, 7(6): 246-252. | |
| 5 | Monteiro J L F , Veloso C O . Catalytic conversion of terpenes into fine chemicals[J]. Topics in Catalysis, 2004, 27(1/2/3/4): 169-180. |
| 6 | Banina O A , Sudarikov D V , Nigmatov A G , et al . Carane amino alcohols as organocatalysts in asymmetric aldol reaction of isatin with acetone[J]. Russian Chemical Bulletin, 2017, 66(2): 293-296. |
| 7 | 王婧 . 3-蒈烯的精制及异构化反应研究[J]. 生物质化学工程, 2014, 48(4): 59-59. |
| Wang J . Study on the purification and isomerization of 3-carene[J]. Biomass Chemical Engineering, 2014, 48(4): 59-59. | |
| 8 | 何丽芝 . 3-蒈烯的制备, 氢化及蒈烷溴化-酯化反应探索研究[J]. 生物质化学工程, 2012, 46(4): 62-63. |
| He L Z . Preparation of 3-carene, hydrogenation and decane bromination-esterification reaction research[J]. Biomass Chemical Engineering, 2012, 46(4): 62-63. | |
| 9 | 孙丽霞, 廖丹葵, 王坤, 等 . α-蒎烯+ β-蒎烯+对伞花烃的减压汽液平衡[J]. 化工学报, 2018, 69(7): 2822-2828. |
| Sun L X , Liao D K , Wang K , et al . Decompression vapor-liquid equilibrium of α-pinene + β-pinene + p-cymene[J]. CIESC Journal, 2018, 69(7): 2822-2828. | |
| 10 | 冯琦, 孙丽霞, 童张法 . UNIFAC模型预测含α-蒎烯体系的汽液平衡数据[J]. 化工学报, 2014, 65(9): 3309-3316. |
| Feng Q , Sun L X , Tong Z F . The UNIFAC model predicts vapor-liquid equilibrium data for α-pinene systems[J]. CIESC Journal, 2014, 65(9): 3309-3316. | |
| 11 | 童张法, 杨征宇, 廖丹葵, 等 . α-蒎烯+柠檬烯和对伞花烃+柠檬烯体系常压汽液平衡的测定与关联[J]. 化工学报, 2009, 60(8): 1877-1882. |
| Tong Z F , Yang Z Y , Liao D K , et al . Determination and correlation of atmospheric vapor-liquid equilibrium of α-pinene + limonene and p-cymene + limonene system[J]. CIESC Journal, 2009, 60(8): 1877-1882. | |
| 12 | Yao G Y , Wang L L , Chen X P , et al . Measurement and correlation of vapor-liquid equilibrium data for binary and ternary systems composed of (-)-β-caryophyllene, p-cymene and 3-carene at 101.33 kPa[J]. The Journal of Chemical Thermodynamics, 2019, 128(8): 215-224. |
| 13 | Kroenlein K G , Diky V , Muzny C D , et al . ThermoLit: NIST Literature report Builder for Thermophysical and Thermochemical Property Measurements[R]. 2012. |
| 14 | Walas S M . Phase Equilibria in Chemical Engineering[M]. London: Butterworth-Heinemann, 1985. |
| 15 | Wisniak J . A new test for the thermodynamic consistency of vapor-liquid equilibrium[J]. Industrial & Engineering Chemistry Research, 1993, 32(7): 1531-1533. |
| 16 | Redlich O , Kiste A T . Algebraic representation of thermodynamic properties and the classification of solutions[J]. Industrial & Engineering Chemistry Research, 1948, 40(2): 345-348. |
| 17 | Van Ness H C , Byer S M , Gibbs R E . Vapour-liquid equilibrium(Ⅰ): An appraisal of data reduction methods[J]. AIChE Journal, 1973, 19(2): 238-244. |
| 18 | Renon H , Prausnitz J M . Local compositions in thermodynamic excess functions for liquid mixtures[J]. AIChE Journal, 1968, 14(1): 135-144. |
| 19 | Wilson G M . Vapour-liquid equilibrium (XI): A new expression for the excess free energy of mixing[J]. Journal of American Chemical Society, 1964, 86(2): 127-130. |
| 20 | Abrams D S , Prausnitz J M . Statistical thermodynamics of liquid mixtures: a new expression for the excess Gibbs energy of partly or completely miscible systems[J]. AIChE Journal, 1975, 21(7): 116-128. |
| 21 | Liu L X , Fu D L , Sun Y H , et al . Isobaric vapor-liquid equilibrium for two binary systems of n-heptane + sec-butyl acetate and methylcyclohexane + sec-butyl acetate under atmosphere[J]. Journal of Chemical & Engineering Data, 2018, 63(5): 1403-1407. |
| 22 | Sun Y H , Fu D L , Ma S T , et al . Isobaric vapor-liquid equilibrium data for two binary systems n-hexane + 1, 2-dimethoxyethane and methylcyclopentane + 1, 2-dimethoxyethane at 101.3 kPa[J]. Journal of Chemical & Engineering Data, 2018, 63(2): 395-401. |
| 23 | 童张法, 王坤, 孙丽霞, 等 . α-蒎烯+对伞花烃和β-蒎烯+对伞花烃体系减压汽液平衡数据的测定与关联[J]. 高校化学工程学报, 2011, 25(5): 734-739. |
| Tong Z F , Wang K , Sun L X , et al . Determination and correlation of vapor-liquid equilibrium data of α-pinene + p-cymene and β-pinene + p-cymene system[J]. Journal of Chemical Engineering of Chinese Universities, 2011, 25(5): 734-739. | |
| 24 | Mathias P M . Guidelines for the analysis of vapour-liquid equilibrium data[J]. Journal of Chemical Engineering Data, 2017, 62(8): 2231-2233. |
| 25 | Mathias P M . Effect of VLE uncertainties on the design of separation sequences by distillation-study of the benzene-chloroform-acetone system[J]. Fluid Phase Equilibria, 2016, 408(9): 265-272. |
| 26 | Prausnitz J M , Lichtenthaler R N , de Azevedo E G . Molecular thermodynamics of fluid-phase equilibria[M]. Singapore: Pearson Education, 1998. |
| 27 | 徐勉, 吴亮衡, 张杰 . 2,4-TDI/共沸剂二元体系的汽液平衡数据的测定及共沸组成分析[J]. 化工学报, 2016, 67(5): 1675-1676. |
| Xu M , Wu L H , Zhang J . Determination of vapor-liquid equilibrium data and azeotropic composition analysis of 2,4-TDI/azeotropic binary system[J]. CIESC Journal, 2016, 67(5): 1675-1676. | |
| 28 | Danner R P , Gess M A . A data base standard for the evaluation of vapor-liquid equilibrium models[J]. Fluid Phase Equilib., 1990, 56(1): 285-301 |
| 29 | Avoseh F , IwarereE S A , Narasigadu C , et al . Vapor-liquid equilibrium for methyl isobutyl ketone (MIBK)+(1-propanol or 2-propanol) binary mixtures[J]. Journal of Chemical & Engineering Data, 2017, 62(7): 2014-2020. |
| 30 | Van Ness H C . Thermodynamics in the treatment of vapor/liquid equilibrium (VLE) data[J]. Pure and Applied Chemistry, 1995, 67(6): 859-872. |
| 31 | 西德尔, 亨利 . 分离过程原理[M]. 朱开宏, 吴俊生, 译. 上海: 华东理工大学出版社, 2007: 78. |
| Seader J D , Henlye E J . Separation Process Principles [M]. Zhu K H, Wu J S, trans. Shanghai: East China University of Science and Technology Press, 2007: 78. | |
| 32 | Yang C , Feng Y , Cheng B , et al . Vapor-liquid equilibria for three binary systems of N-methylethanolamine, N-methyldiethanolamine, and ethylene glycol at P = (40.0, 30.0, and 20.0) kPa[J]. Journal of Chemical Engineering Data, 2013, 58(8): 2272-2279. |
| 33 | Zhang L Z , Xu D M , Gao J . Measurements and correlations of density, viscosity, and vapour-liquid equilibrium for fluoro alcohols[J]. Journal of Chemical Thermodynamics, 2016, 102(7): 155-163. |
| [1] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [2] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [3] | 张家庆, 蒋榕培, 史伟康, 武博翔, 杨超, 刘朝晖. 煤基/石油基火箭煤油高参数黏温特性与组分特性研究[J]. 化工学报, 2023, 74(2): 653-665. |
| [4] | 黄志豪, 李光熙, 唐桂华, 李小龙, 范元鸿. 单侧加热方形通道内超临界水传热研究[J]. 化工学报, 2022, 73(4): 1523-1533. |
| [5] | 汪森林, 李照志, 邵应娟, 钟文琪. 超临界二氧化碳垂直管内传热恶化数值模拟研究[J]. 化工学报, 2022, 73(3): 1072-1082. |
| [6] | 张苗, 杨洪海, 尹勇, 徐悦, 沈俊杰, 卢心诚, 施伟刚, 王军. 氧化石墨烯/水脉动热管的启动及传热特性[J]. 化工学报, 2022, 73(3): 1136-1146. |
| [7] | 韩昌亮, 辛镜青, 于广滨, 刘俊秀, 许麒澳, 姚安卡, 尹鹏. 微通道内超临界氮气三维热流场实验与数值模拟[J]. 化工学报, 2022, 73(2): 653-662. |
| [8] | 李广, 庄大伟, 谢丽懿, 丁国良, 郑立宇, 龙春仙, 江波. 含油R32管内流动沸腾换热特性测试及关联式开发[J]. 化工学报, 2022, 73(2): 612-621. |
| [9] | 宋卓栋, 张作毅, 潘学龙, 王云芳. 聚甲醛二甲醚体系汽液平衡数据的测定与关联[J]. 化工学报, 2020, 71(S1): 1-6. |
| [10] | 李建涛, 姚秀颖, 刘璐, 卢春喜. 气固流化床外取热器内流动和换热特性分析[J]. 化工学报, 2020, 71(7): 3031-3041. |
| [11] | 王志奇, 贺妮, 罗兰, 夏小霞, 左青松. 水平管内R245fa/R141b沸腾换热特性的实验研究[J]. 化工学报, 2020, 71(4): 1588-1596. |
| [12] | 孙中建,杨博,齐楚,李宏光. 面向工业混杂系统故障检测的扩展数据逻辑分析方法[J]. 化工学报, 2020, 71(11): 5237-5245. |
| [13] | 李涛, 沙娇, 赵瑞, 张鹏帅, 刘士琪, 李玉, 任保增. 双季戊四醇在3种混合溶剂中的固-液相平衡[J]. 化工学报, 2020, 71(1): 245-253. |
| [14] | 朱晨阳, 杨峰, 刘向阳, 何茂刚. 高压肉豆蔻酸甲酯比定压热容的实验测量[J]. 化工学报, 2019, 70(S2): 15-19. |
| [15] | 雷兴国, 王庆锋, 李中. 基于合作博弈的管道外腐蚀多层次灰色动态评价[J]. 化工学报, 2019, 70(6): 2386-2396. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号