化工学报 ›› 2019, Vol. 70 ›› Issue (6): 2377-2385.DOI: 10.11949/j.issn.0438-1157.20181536
孙燕1,2( ),蓝际荣1,2,郭莉1,2,孙朋1,2,叶恒朋1,2,杜冬云1,2(
),蓝际荣1,2,郭莉1,2,孙朋1,2,叶恒朋1,2,杜冬云1,2( ),占伟1,2
),占伟1,2
收稿日期:2019-01-02
修回日期:2019-03-07
出版日期:2019-06-05
发布日期:2019-06-05
通讯作者:
杜冬云
作者简介:<named-content content-type="corresp-name">孙燕</named-content>(1994—),女,硕士研究生,<email>YanSun0123@163.com</email>
基金资助:
Yan SUN1,2( ),Jirong LAN1,2,Li GUO1,2,Peng SUN1,2,Hengpeng YE1,2,Dongyun DU1,2(
),Jirong LAN1,2,Li GUO1,2,Peng SUN1,2,Hengpeng YE1,2,Dongyun DU1,2( ),Wei ZHAN1,2
),Wei ZHAN1,2
Received:2019-01-02
Revised:2019-03-07
Online:2019-06-05
Published:2019-06-05
Contact:
Dongyun DU
摘要:
通过对工业废弃物电解锰渣(electrolytic manganese residues, EMRs)进行改性制备As(Ⅲ)吸附材料(改性EMRs),探究了NaOH用量、超声及微波对其表面结构及吸附性能的影响。结果表明:该工业废渣在固液比M(EMRs)∶V(NaOH, aq) = 1∶10(C NaOH,aq = 2.0 mol·L-1)条件下,经超声反应(200 W)2 h脱除大部分Si、S、Ca后,再微波(700 W)反应5 min以使Fe、Mn等活性吸附基团在其表面沉积,最后经105℃烘干制得改性EMRs。SEM结果表明,EMRs改性后表面形成片层纳米结构,对砷具有良好的吸附性能,可将初始As(Ⅲ)浓度为50 mg·L-1废水出水中砷降至0.042 mg·L-1,符合国家地表水环境质量标准Ⅰ类水质量要求(GB 3838—2002);同时,经3% NaOH溶液再生处理后可继续使用。XPS结果表明,改性EMRs吸附砷性能与其表面Fe3O4、FeOOH、MnO2等对As(Ⅲ)具有吸附作用或氧化作用的活性物种的增多密切相关。
中图分类号:
孙燕, 蓝际荣, 郭莉, 孙朋, 叶恒朋, 杜冬云, 占伟. 利用电解锰渣制备As(Ⅲ)吸附材料及其性能研究[J]. 化工学报, 2019, 70(6): 2377-2385.
Yan SUN, Jirong LAN, Li GUO, Peng SUN, Hengpeng YE, Dongyun DU, Wei ZHAN. Preparation of As(Ⅲ) adsorbent material by electrolytic manganese slag and its properties[J]. CIESC Journal, 2019, 70(6): 2377-2385.
| Si | Al | Ca | Mg | Fe | S | Mn |
|---|---|---|---|---|---|---|
| 23.99 | 2.1 | 10.53 | 1.95 | 7.24 | 25.92 | 4.82 |
表1 电解锰废渣主要组成成分
Table 1 Main components of EMR/%
| Si | Al | Ca | Mg | Fe | S | Mn |
|---|---|---|---|---|---|---|
| 23.99 | 2.1 | 10.53 | 1.95 | 7.24 | 25.92 | 4.82 |
| Element | EMRs | EMRs+ NaOH | EMRs+ NaOH+ ultrasound | EMRs + NaOH+ ultrasound+ microwave |
|---|---|---|---|---|
| Ca | 8.53 | 6.12 | 3.89 | 3.91 |
| S | 15.02 | 12.56 | 0.24 | 0.12 |
| Mn | 4.82 | 3.78 | 3.95 | 5.57 |
| Fe | 7.24 | 4.24 | 3.85 | 8.17 |
| Si | 23.99 | 13.22 | 7.13 | 7.51 |
| O | 40.23 | 42.25 | 43.22 | 41.25 |
表2 不同改性方法获得的各改性EMRs样品的主要含量变化
Table 2 Main content changes of EMRs samples with different modification methods/%(mass)
| Element | EMRs | EMRs+ NaOH | EMRs+ NaOH+ ultrasound | EMRs + NaOH+ ultrasound+ microwave |
|---|---|---|---|---|
| Ca | 8.53 | 6.12 | 3.89 | 3.91 |
| S | 15.02 | 12.56 | 0.24 | 0.12 |
| Mn | 4.82 | 3.78 | 3.95 | 5.57 |
| Fe | 7.24 | 4.24 | 3.85 | 8.17 |
| Si | 23.99 | 13.22 | 7.13 | 7.51 |
| O | 40.23 | 42.25 | 43.22 | 41.25 |
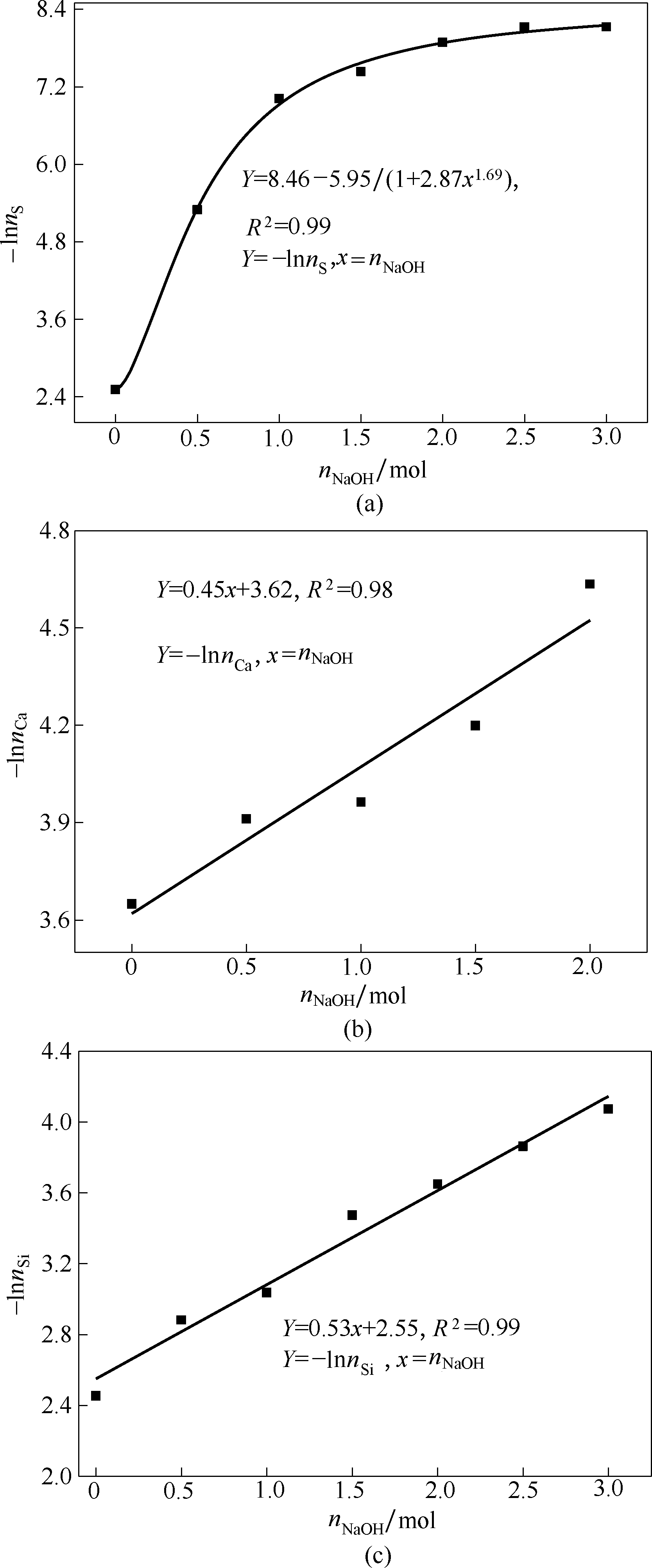
图4 NaOH物质的量与硫(a)、钙(b)和硅(c)物质减少量的关系
Fig.4 Relationship between amount of NaOH substances and reduction of sulfur (a), calcium (b) and silicon (c) substances
| Item | Fe/ (mg·L-1) | Mn/ (mg·L-1) | Si/ (mg·L-1) | Ca/ (mg·L-1) | Na/ (mg·L-1) | SO4 2?/ (mg·L-1) | As/ (mg·L-1) | pH | Chromaticity (dilution factor) |
|---|---|---|---|---|---|---|---|---|---|
| after material treatment | 0.068 | 0.005 | 0.056 | 0.21 | 0.008 | 0.25 | 0. 042 | 7.3 | 5 |
| GB 3838—2002 | 0.3 | 0.1 | — | — | — | 250 | 0.1—0.05 | 6—9 | 50—180 |
表3 模拟废水在改性EMRs吸附后出水性质
Table 3 Characteristics of synthesis wastewater after treated with material as compared with that shown in GB 3838—2002
| Item | Fe/ (mg·L-1) | Mn/ (mg·L-1) | Si/ (mg·L-1) | Ca/ (mg·L-1) | Na/ (mg·L-1) | SO4 2?/ (mg·L-1) | As/ (mg·L-1) | pH | Chromaticity (dilution factor) |
|---|---|---|---|---|---|---|---|---|---|
| after material treatment | 0.068 | 0.005 | 0.056 | 0.21 | 0.008 | 0.25 | 0. 042 | 7.3 | 5 |
| GB 3838—2002 | 0.3 | 0.1 | — | — | — | 250 | 0.1—0.05 | 6—9 | 50—180 |
| 1 | 姚瑛瑛, 郭莉, 胡中求, 等 .超声辅助碱浸铜冶炼烟灰中铜砷分离[J]. 化工学报, 2018, 69(9): 3983-3992. |
| Yao Y Y , Guo L , Hu Z Q , et al . Separation of copper and arsenic in copper smelting dust by Na2S-NaOH leaching assisted with ultrasound method[J]. CIESC Journal, 2018, 69(9): 3983-3992. | |
| 2 | 彭昌军, 姜秀丽, 计红芳, 等 . 铁锰复合氧化物对As(Ⅲ)、As(Ⅴ)的吸附研究及其在沼液中的应用[J]. 化工学报, 2014, 65(5): 1848-1855. |
| Peng C J , Jiang X L , Ji H F , et al . Adsorption behavior of Fe-Mn binary oxide towards As(Ⅲ) and As(V) and its application in biogas slurry[J]. CIESC Journal, 2014, 65(5): 1849-1855. | |
| 3 | Sun Z , Yi Y U , Pang S , et al . Manganese-modified activated carbon fiber (Mn-ACF): novel efficient adsorbent for arsenic[J]. Applied Surface Science, 2013, 284(11): 100-106. |
| 4 | Chen H Y , Lv K L , Du Y , et al . Microwave-assisted rapid synthesis of Fe2O3/ACF hybrid for high efficient As(Ⅴ) removal[J]. J. Alloy. Compd., 2016, 674(5): 399-405. |
| 5 | Zhang Y , Yang M , Gao Y . Preparation and adsorption mechanism of rare earth-doped adsorbent for arsenic(Ⅴ) removal from groundwater[J]. Sci. China Ser. B, 2003, 46(3): 252-258. |
| 6 | Halter W E , Pfeifer H . Arsenic(Ⅴ) adsorption onto α-Al2O3 between 25 and 70℃ [J]. Applied Geochemistry, 2001, 16(7): 793-802. |
| 7 | Stauffer R E , Thompson J M . Arsenic and antimony in geothermal waters of Yellow Stone National Park, Wyoming, USA[J]. Geochimica et Cosmochimica Acta, 1984, 48(12): 2547-2561. |
| 8 | Pascua C S , Minato M , Yokoyama S . Uptake of dissolved arsenic during the retrieval of silica from spent geothermal brine[J]. Geothermics, 2007, 36(3): 230-242. |
| 9 | Romero L , Alonso H , Campamo P . Arsenic enrichment in waters and sediments of the Rio Loa (Second Region, Chile) [J]. Applied Geochemistry, 2003, 18(9): 1399-1416. |
| 10 | Berg M , Tran H C , Nguyen T C , et al . Arsenic contamination of groundwater and drinking water in Vietnam: a human health threat[J]. Environmental Science & Technology, 2001, 35(13): 2621 -2626. |
| 11 | Zhang Y X , Ye Y J , Liu Z L , et al . Monodispersed hierarchical aluminum/iron oxides composites micro/nanoflowers for efficient removal of As(V) and Cr(VI) ions from water[J]. Journal of Alloys and Compounds, 2016, 662(25): 421-430. |
| 12 | Zhou J M , Chen S , Liu J , et al . Adsorption kinetic and species variation of arsenic for As(V) removal by biologically mackinawite (FeS) [J]. Chemical Engineering Journal, 2018, 354(8): 237-244. |
| 13 | 牛莎莎, 王志兴, 郭华军, 等 .电解锰阳极渣还原浸出锰[J]. 中国有色金属学报, 2012, 22(9): 2662-2666. |
| Niu S S , Wang Z X , Guo H J , et al . Reductive leaching of manganese from manganese anode slag[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(9): 2662-2666. | |
| 14 | Shu J , Liu R , Liu Z . Solidification/stabilization of electrolytic manganese residue using phosphate resource and low-grade MgO/CaO[J]. Journal of Hazardous Materials, 2016, 317(5): 267-274. |
| 15 | Xin B , Chen B , Duan N . Extraction of manganese from electrolytic manganese residue by bioleaching[J]. Bioresource Technology, 2011, 102(2): 1683-1687. |
| 16 | Li C , Zhong H , Wang S , et al . Removal of basic dye (methylene blue) from aqueous solution using zeolite synthesized from electrolytic manganese residue[J]. Journal of Industrial & Engineering Chemistry, 2015, 23: 344-352. |
| 17 | Chen H , Liu R , Liu Z . Immobilization of Mn and NH4 +-N from electrolytic manganese residue waste[J]. Environmental Science & Pollution Research, 2016, 23(12): 12352-12361. |
| 18 | Shu J , Wu H , Liu R , et al . Simultaneous stabilization /solidification of Mn2+ and NH4 +-N from electrolytic manganese residue using MgO and different phosphate resource[J]. Ecotoxicology & Environmental Safety, 2018, 148(4): 220-224. |
| 19 | Li J , Du D , Peng Q , et al . Activation of silicon in the electrolytic manganese residue by mechanical grinding-roasting[J]. Journal of Cleaner Production, 2018, 192(10): 347-353 |
| 20 | 李明强 . 电解锰渣中锰元素的浸取研究[D]. 重庆: 重庆大学, 2015. |
| Li M Q . The leaching of manganese from electrolytic manganese residues[D]. Chongqing: Chongqing University, 2015. | |
| 21 | Shu J , Liu R , Liu Z . Simultaneous removal of ammonia and manganese from electrolytic metal manganese residue leachate using phosphate salt[J]. Journal of Cleaner Production, 2016, 135 (1): 468-475. |
| 22 | 盘俊, 谢能银, 明宪权, 等 . 锰矿浸渣中可溶锰离子的稳定化处理研究[J]. 广西大学学报(自然科学版), 2015, 40(3): 551-557. |
| Pan J , Xie N Y , Ming X Q , et al . The stabilizing treatment for the soluble manganese in manganese leaching slag[J]. Journal of Guangxi University( Nat. Sci. Ed.), 2015, 40(3): 551-557. | |
| 23 | Lu J , Dreisinger D , Gluck T . Electrolytic manganese metal production from manganese carbonate precipitate[J]. Hydrometallurgy, 2016, 161(5): 45-53. |
| 24 | Li J , Peng Q J Du D Y , et al . Activation of silicon in the electrolytic manganese residue by mechanical grinding-roasting[J]. Journal of Cleaner Production, 2018, 192(10): 347-353. |
| 25 | 陈振兴, 李佳, 杜冬云, 等 . 硅酸盐细菌对电解锰渣中有效硅的活化研究[J]. 硅酸盐通报, 2018, 37(11): 3581-3586. |
| Chen Z X , Li J , Du D Y , et al . Study on activation of effective silicon in electrolytic manganese slag by silicate bacteria[J]. Bulletin of the Chinese Ceramic Society, 2018, 37(11): 3581-3586. | |
| 26 | 蓝际荣, 李佳, 杜冬云, 等 . 锰渣堆肥过程中理化性质及基于Tessier法的重金属行为分析[J]. 环境工程学报, 2017, 11(10): 5637-5643. |
| Lan J R , Li J , Du D Y , et al . Physicochemical properties and heavy metals behavior analysis based on Tessier method in composting process of electrolytic manganese residue[J]. Chinese Journal of Environmental Engineering, 2017, 11(10): 5637-5643. | |
| 27 | 彭秋菊, 李佳, 杜冬云, 等 . 响应面法优化电解锰渣的微波活化有效硅工艺条件[J]. 硅酸盐通报, 2018, 37(8): 2548-2554. |
| Peng Q J , Li J , Du D Y , et al . Optimization of microwave activated effective silicon process conditions for electrolytic manganese residue by response surface methodology[J]. Bulletin of the Chinese Ceramic Society, 2018, 37(8): 2548-2554. | |
| 28 | Eslami H , Ehrampoush M H , Esmaeili A , et al . Enhanced coagulation process by Fe-Mn bimetal nano-oxides in combination with inorganic polymer coagulants for improving As(Ⅴ) removal from contaminated water[J]. Journal of Cleaner Production, 2018, 208(10): 384-392. |
| 29 | Lan L J , Zheng B J , Zhang Y , et al . Rapid and effective removal of As ( Ⅲ ) and As(V) using spore@Ti4+ microspheres[J]. Chemosphere, 2018, 206(9): 742-749. |
| 30 | Lee C G , Pedro J , Alvarez J , et al . Arsenic(Ⅴ) removal using an amine-doped acrylic ion exchange fiber: kinetic, equilibrium, and regeneration studies[J]. Journal of Hazardous Materials, 2017, 325 (5): 223-229. |
| 31 | Chen H , Lv K , Du Y , et al . Microwave-assisted rapid synthesis of Fe2O3/ACF hybrid for high efficient As(Ⅴ) removal[J]. Journal of Alloys and Compounds, 2016, 674(5): 399-405. |
| 32 | Wang Y X , Bing Q Y , Fei F J , et al . Removal of As(Ⅴ) from aqueous solution by using cement-porous hematite composite granules as adsorbent[J]. Results in Physics, 2018, 11(12): 23-29. |
| 33 | Paulina M , Jakub M , Tiina L , et al . Toward highly effective and easily separable halloysite-containing adsorbents: the effect of iron oxide particles impregnation and new insight into As(Ⅴ) removal mechanisms[J]. Separation and Purification Technology, 2019, 210(8): 390-401. |
| 34 | Oh C , Rhee S , Oh M , et al . Removal characteristics of As(Ⅲ) and As(V) from acidic aqueous solution by steel making slag[J]. Journal of Hazardous Materials, 2012, 30(4): 147-155. |
| [1] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [2] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [5] | 胡兴枝, 张皓焱, 庄境坤, 范雨晴, 张开银, 向军. 嵌有超小CeO2纳米粒子的碳纳米纤维的制备及其吸波性能[J]. 化工学报, 2023, 74(8): 3584-3596. |
| [6] | 文兆伦, 李沛睿, 张忠林, 杜晓, 侯起旺, 刘叶刚, 郝晓刚, 官国清. 基于自热再生的隔壁塔深冷空分工艺设计及优化[J]. 化工学报, 2023, 74(7): 2988-2998. |
| [7] | 王光宇, 张锴, 张凯华, 张东柯. 微波加热干燥煤泥热质传递及其能耗特性分析[J]. 化工学报, 2023, 74(6): 2382-2390. |
| [8] | 卫雪岩, 钱勇. 微米级铁粉燃料中低温氧化反应特性及其动力学研究[J]. 化工学报, 2023, 74(6): 2624-2638. |
| [9] | 肖川宝, 李林洋, 刘武锋, 钟年丙, 解泉华, 钟登杰, 常海星. 光催化与离子交换吸附耦合有效去除2,4,6-三氯苯酚[J]. 化工学报, 2023, 74(4): 1587-1597. |
| [10] | 陈俊先, 姬忠礼, 赵瑜, 张倩, 周岩, 刘猛, 刘震. 基于微波技术的天然气管道内颗粒物在线检测方法研究[J]. 化工学报, 2023, 74(3): 1042-1053. |
| [11] | 潘煜, 王子航, 王佳韵, 王如竹, 张华. 基于可得然-氯化锂复合吸附剂的除湿换热器热湿性能研究[J]. 化工学报, 2023, 74(3): 1352-1359. |
| [12] | 陈瑞哲, 程磊磊, 顾菁, 袁浩然, 陈勇. 纤维增强树脂复合材料化学回收技术研究进展[J]. 化工学报, 2023, 74(3): 981-994. |
| [13] | 李敏, 阎雪茹, 刘新磊. 苯并咪唑连接聚合物吸附剂和膜研究进展[J]. 化工学报, 2023, 74(2): 599-616. |
| [14] | 鲁文静, 李先锋. 液流电池多孔离子传导膜研究进展[J]. 化工学报, 2023, 74(1): 192-204. |
| [15] | 黄宽, 马永德, 蔡镇平, 曹彦宁, 江莉龙. 油脂催化加氢转化制备第二代生物柴油研究进展[J]. 化工学报, 2023, 74(1): 380-396. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号