化工学报 ›› 2023, Vol. 74 ›› Issue (9): 3742-3755.DOI: 10.11949/0438-1157.20230604
杨学金1( ), 杨金涛1, 宁平2, 王访1, 宋晓双1, 贾丽娟1(
), 杨金涛1, 宁平2, 王访1, 宋晓双1, 贾丽娟1( ), 冯嘉予1(
), 冯嘉予1( )
)
收稿日期:2023-06-21
修回日期:2023-09-01
出版日期:2023-09-25
发布日期:2023-11-20
通讯作者:
贾丽娟,冯嘉予
作者简介:杨学金(1998—),男,硕士研究生,320731920@qq.com
基金资助:
Xuejin YANG1( ), Jintao YANG1, Ping NING2, Fang WANG1, Xiaoshuang SONG1, Lijuan JIA1(
), Jintao YANG1, Ping NING2, Fang WANG1, Xiaoshuang SONG1, Lijuan JIA1( ), Jiayu FENG1(
), Jiayu FENG1( )
)
Received:2023-06-21
Revised:2023-09-01
Online:2023-09-25
Published:2023-11-20
Contact:
Lijuan JIA, Jiayu FENG
摘要:
磷化氢(PH3)是一种来源广泛的剧毒气体,未经处理排放到大气中会对人体和环境造成严重危害。近年来,国家对PH3的排放进行了严格的规定,因此,尾气中PH3的深度净化受到了广泛的关注。干法是主流的PH3净化技术,主要包括吸附法和催化法。相较于湿法,干法具有性能好、稳定性强、耗水量小以及不产生二次污染等优点。本文首先从吸附法入手,分别探讨了复合金属氧化物吸附剂、活性炭基吸附剂及其他材料(分子筛、二氧化硅等)的研究现状,深入分析了各类脱磷吸附剂的结构性质及优缺点。其次对催化分解PH3的机理进行了总结归纳,重点讨论了各类催化剂的构-效关系。最后,介绍了其他干法(燃烧法、等离子体降解法、生物法)在PH3净化领域的应用。在此基础上,讨论了干法脱磷技术的主要优势以及面临的挑战,并对干法脱除PH3技术的发展方向进行了展望。本工作可以为脱磷吸附剂/催化剂的构建和设计提供参考和指导。
中图分类号:
杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755.
Xuejin YANG, Jintao YANG, Ping NING, Fang WANG, Xiaoshuang SONG, Lijuan JIA, Jiayu FENG. Research progress in dry purification technology of highly toxic gas PH3[J]. CIESC Journal, 2023, 74(9): 3742-3755.
| 材料种类 | 活性组分 | 吸附剂 比表面积/(m2/g) | 吸附剂总孔容/(cm3/g) | 吸附剂平均 孔径/nm | 气体组成 | 反应 温度/℃ | 气体总流速/(ml/min) | 质量空速/(ml/(h·g)) | 穿透标准 | 磷容/(mg/g) | 文献 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ce-Cu-Al | CuO | 45.8 | 0.10 | 4.5 | N2 + H2S + PH3 + 1% O2 | 70 | 500 | 10000 | — | 201.9 | [ |
| Cu-Fe-Ce | CuO | 110.27 | 0.16 | 5.64 | N2 + 456 mg/m3 H2S + 911 mg/m3 PH3 + 1% O2 | 70 | — | 30000 | — | 151.7 | [ |
| 30Cu@TiO2 | CuO | 63.77 | 0.31 | — | N2 + 1518 mg/m3 PH3 | 120 | 100 | 30000 | 90% | 135.73 | [ |
| Cu x /TiO2 | CuO | 110.3 | 0.31 | 5.7 | N2 + 1518 mg/m3 PH3+ 1% O2 | 90 | 100 | 60000 | 97% | 136.2 | [ |
| Cu/TiO2 | CuO | — | — | — | N2 + 759 mg/m3 PH3 | 25 | 100 | — | 99% | 108.48 | [ |
| Cu/γ-Al2O3 | CuO | 232 | 0.43 | — | N2 + 76 mg/m3 PH3 | 20 | 60 | — | 90% | 32.09 | [ |
Flower-shaped CuO/AC | CuO | — | — | — | N2 + PH3 + 1.6% O2 | 110 | — | 750 | — | 96.08 | [ |
| Cu x /ACF | CuO | 1768.9 | 0.57 | 0.96 | N2 + H2S + PH3 + 0.5% O2 | 90 | — | 36000 | — | 132.1 | [ |
| Modified walnut-shell ACs | CuO | 1419 | 0.70 | — | N2 + 1518 mg/m3 PH3+ 1% O2 | 120 | 250 | — | 90% | 284.12 | [ |
| Cu/HZSM-5-[S1] | CuO | 192.2 | 0.11 | 2.1 | N2 + 684 mg/m3 H2S + 911 mg/m3 PH3 + 1 % O2 | 90 | 100 | 20000 | 60% | 150.90 | [ |
| Ce1Cu30O x /HZSM-5 | CuO | 282 | 0.16 | 2.26 | N2 + 1214 mg/m3 PH3 + 1 % O2 | 90 | 100 | 15000 | 60% | 114.36 | [ |
| Cu/SBA-15 | CuO | 177.6 | 0.252 | 5.31 | N2 + 304 mg/m3 H2S + 1214 mg/m3 PH3 + 0.5 % O2 | 80 | 100 | 10000 | 60% | 104. 84 | [ |
| Cu-Fe / SBA-15 | CuO | 206.3 | — | 5.49 | N2 + 304 mg/m3 H2S + 1214 mg/m3 PH3 + 0.5 % O2 | 80 | 100 | 10000 | 60% | 120. 05 | [ |
| Cu-Fe /硅藻土 | CuO | 45.33 | 0.415 | 9.74 | N2 + 304 mg/m3 H2S + 1214 mg/m3 PH3 + 0.5 % O2 | 80 | 100 | 10000 | 60% | 29.61 | [ |
表1 铜基材料PH3净化条件对比
Table 1 Comparison of PH3 purification conditions for copper-based materials
| 材料种类 | 活性组分 | 吸附剂 比表面积/(m2/g) | 吸附剂总孔容/(cm3/g) | 吸附剂平均 孔径/nm | 气体组成 | 反应 温度/℃ | 气体总流速/(ml/min) | 质量空速/(ml/(h·g)) | 穿透标准 | 磷容/(mg/g) | 文献 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ce-Cu-Al | CuO | 45.8 | 0.10 | 4.5 | N2 + H2S + PH3 + 1% O2 | 70 | 500 | 10000 | — | 201.9 | [ |
| Cu-Fe-Ce | CuO | 110.27 | 0.16 | 5.64 | N2 + 456 mg/m3 H2S + 911 mg/m3 PH3 + 1% O2 | 70 | — | 30000 | — | 151.7 | [ |
| 30Cu@TiO2 | CuO | 63.77 | 0.31 | — | N2 + 1518 mg/m3 PH3 | 120 | 100 | 30000 | 90% | 135.73 | [ |
| Cu x /TiO2 | CuO | 110.3 | 0.31 | 5.7 | N2 + 1518 mg/m3 PH3+ 1% O2 | 90 | 100 | 60000 | 97% | 136.2 | [ |
| Cu/TiO2 | CuO | — | — | — | N2 + 759 mg/m3 PH3 | 25 | 100 | — | 99% | 108.48 | [ |
| Cu/γ-Al2O3 | CuO | 232 | 0.43 | — | N2 + 76 mg/m3 PH3 | 20 | 60 | — | 90% | 32.09 | [ |
Flower-shaped CuO/AC | CuO | — | — | — | N2 + PH3 + 1.6% O2 | 110 | — | 750 | — | 96.08 | [ |
| Cu x /ACF | CuO | 1768.9 | 0.57 | 0.96 | N2 + H2S + PH3 + 0.5% O2 | 90 | — | 36000 | — | 132.1 | [ |
| Modified walnut-shell ACs | CuO | 1419 | 0.70 | — | N2 + 1518 mg/m3 PH3+ 1% O2 | 120 | 250 | — | 90% | 284.12 | [ |
| Cu/HZSM-5-[S1] | CuO | 192.2 | 0.11 | 2.1 | N2 + 684 mg/m3 H2S + 911 mg/m3 PH3 + 1 % O2 | 90 | 100 | 20000 | 60% | 150.90 | [ |
| Ce1Cu30O x /HZSM-5 | CuO | 282 | 0.16 | 2.26 | N2 + 1214 mg/m3 PH3 + 1 % O2 | 90 | 100 | 15000 | 60% | 114.36 | [ |
| Cu/SBA-15 | CuO | 177.6 | 0.252 | 5.31 | N2 + 304 mg/m3 H2S + 1214 mg/m3 PH3 + 0.5 % O2 | 80 | 100 | 10000 | 60% | 104. 84 | [ |
| Cu-Fe / SBA-15 | CuO | 206.3 | — | 5.49 | N2 + 304 mg/m3 H2S + 1214 mg/m3 PH3 + 0.5 % O2 | 80 | 100 | 10000 | 60% | 120. 05 | [ |
| Cu-Fe /硅藻土 | CuO | 45.33 | 0.415 | 9.74 | N2 + 304 mg/m3 H2S + 1214 mg/m3 PH3 + 0.5 % O2 | 80 | 100 | 10000 | 60% | 29.61 | [ |

图2 (a) 不同酸的使用对Ce-Cu-Al吸附剂性能的影响[42];(b) 三种Ce-Cu-Al吸附剂的H2-TPR谱图[42];(c) 30Cu@TiO2和TiO2的CO2-TPD谱图[32];(d) 不同负载量时XCu@TiO2的BET分析[32];(e) Cu x /TiO2净化PH3机理图[30];(f) XCu@TiO2脱除PH3反应机理[32];(g) Cu x /TiO2的H2-TPR谱图[30];(h) Cu x /TiO2的CO2-TPD谱图[30];(i) Cu/TiO2吸附剂在不同气氛下的PH3失活曲线[27];(j) CuCl2改性γ-Al2O3吸附剂四次再生循环后的磷容[28]
Fig.2 (a) Effect of different acids on the performance of Ce-Cu-Al adsorbents[42]; (b) H2-TPR profiles of three kinds of Ce-Cu-Al adsorbents[42]; (c) CO2-TPD profiles of 30Cu@TiO2 and TiO2[32]; (d) BET analysis of XCu@TiO2 at different loading levels[32]; (e) Cu x /TiO2 purification mechanism of PH3[30]; (f) XCu@TiO2 reaction mechanism for PH3 removal[32]; (g) H2-TPR profiles of Cu x /TiO2[30]; (h) CO2-TPD profiles of Cu x /TiO2[30]; (i) PH3 deactivation curves of Cu/TiO2 adsorbents under different atmospheres[27]; (j) Phosphorus capacity of CuCl2-modified γ-Al2O3 adsorbent after four regeneration cycles[28]
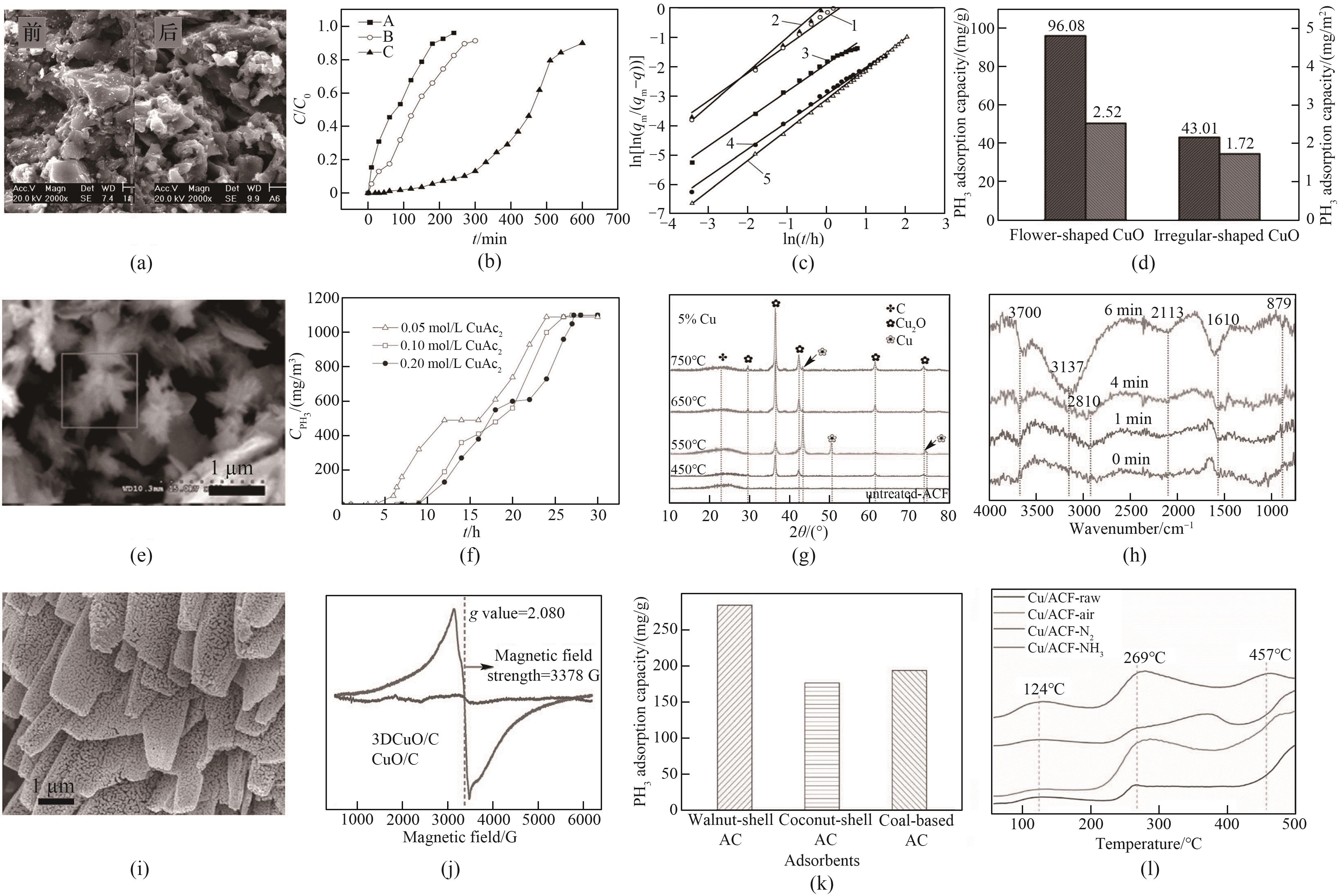
图3 (a) 反应前后吸附剂的SEM图像[52];(b) 不同活性炭吸附剂的PH3穿透曲线[52];(c) PH3在浸渍活性炭上吸附的班厄姆吸附动力学曲线[53];(d) 花型和不规则型CuO/AC吸附剂磷容[44];(e) 花型CuO/AC吸附剂的SEM图像[44];(f) 不同浓度CuAc2浸渍AC的PH3穿透曲线[55];(g) 不同焙烧温度下Cu x /ACF吸附剂的XRD谱图[45];(h) Cu0.15/ACF的原位红外光谱图[45];(i) 3DCuO/C吸附剂的SEM图像[31];(j) 3DCuO/C吸附剂的EPR谱图[31];(k) 三种改性吸附剂的磷容对比[46];(l) Cu/ACF-X的CO2-TPD谱图[60] (1 G=10-4 T)
Fig.3 (a) SEM images of the adsorbent before and after the reaction[52]; (b) PH3 breakthrough curves of different activated carbon adsorbents[52]; (c) Banham adsorption kinetic curve of PH3 adsorption on impregnated activated carbon[53]; (d) Phosphorus capacity of flower-shaped and irregular CuO/AC adsorbents[44]; (e) SEM image of flower type CuO/AC adsorbent[44]; (f) PH3 breakthrough curves of AC impregnated with different concentrations of CuAc2[55]; (g) XRD patterns of Cu x /ACF adsorbents at different calcination temperatures[45]; (h) In situ infrared spectrogram of Cu0.15/ACF[45]; (i) SEM images of 3DCuO/C adsorbent[31]; (j) EPR spectrum of 3DCuO/C adsorbent[31]; (k) Comparison of phosphorus capacity of three modified adsorbents[46]; (l) CO2-TPD profiles of Cu/ACF-X[60]

图4 (a) 不同吸附剂的PH3突破曲线[47];(b) Cu/HZSM-5-[S0]和Cu/HZSM-5-[S1]的CO2-TPD谱图[47];(c) 不同Ce掺杂时吸附剂的突破曲线[29];(d) 不同硝酸浓度改性Cu/SBA-15时吸附剂的PH3吸附/氧化性能[48];(e) Cu/SBA-15的原位IR光谱[48];(f) 不同样品的NH3-TPD曲线[49];(g) 不同含量的Fe掺杂时Cu-Fe/SBA-15吸附剂的BET分析[50];(h) 不同含量的Fe掺杂时Cu-Fe/硅藻土吸附剂的穿透曲线[50];(i)、(j) 不同含量的Ce掺杂时吸附剂的EDS映射(Cu和Ce)图像[29];(k) NaCl溶液改性5A分子筛前后的SEM图像[61];(l) Cu-Fe/硅藻土吸附剂的SEM图像[50]
Fig.4 (a) PH3 breakthrough curves for different adsorbents[47]; (b) CO2-TPD profiles of Cu/HZSM-5-[S0] and Cu/HZSM-5-[S1][47]; (c) Breakthrough curves of adsorbents at different Ce doping[29]; (d) PH3 adsorption/oxidation performance of adsorbents at different nitric acid concentrations modified with Cu/SBA-15[48]; (e) In situ IR spectra of Cu/SBA-15[48]; (f) NH3-TPD curves of different samples[49]; (g) BET analysis of Cu-Fe/SBA-15 adsorbent at different levels of Fe doping[50]; (h) Breakthrough curves of Cu-Fe/diatomite adsorbents at different levels of Fe doping[50]; (i), (j) EDS mapping (Cu and Ce) images of the adsorbent at different levels of Ce doping[29]; (k) SEM images before and after modification of 5A molecular sieve by NaCl solution[61]; (l) SEM images of Cu-Fe/diatomite adsorbent[50]
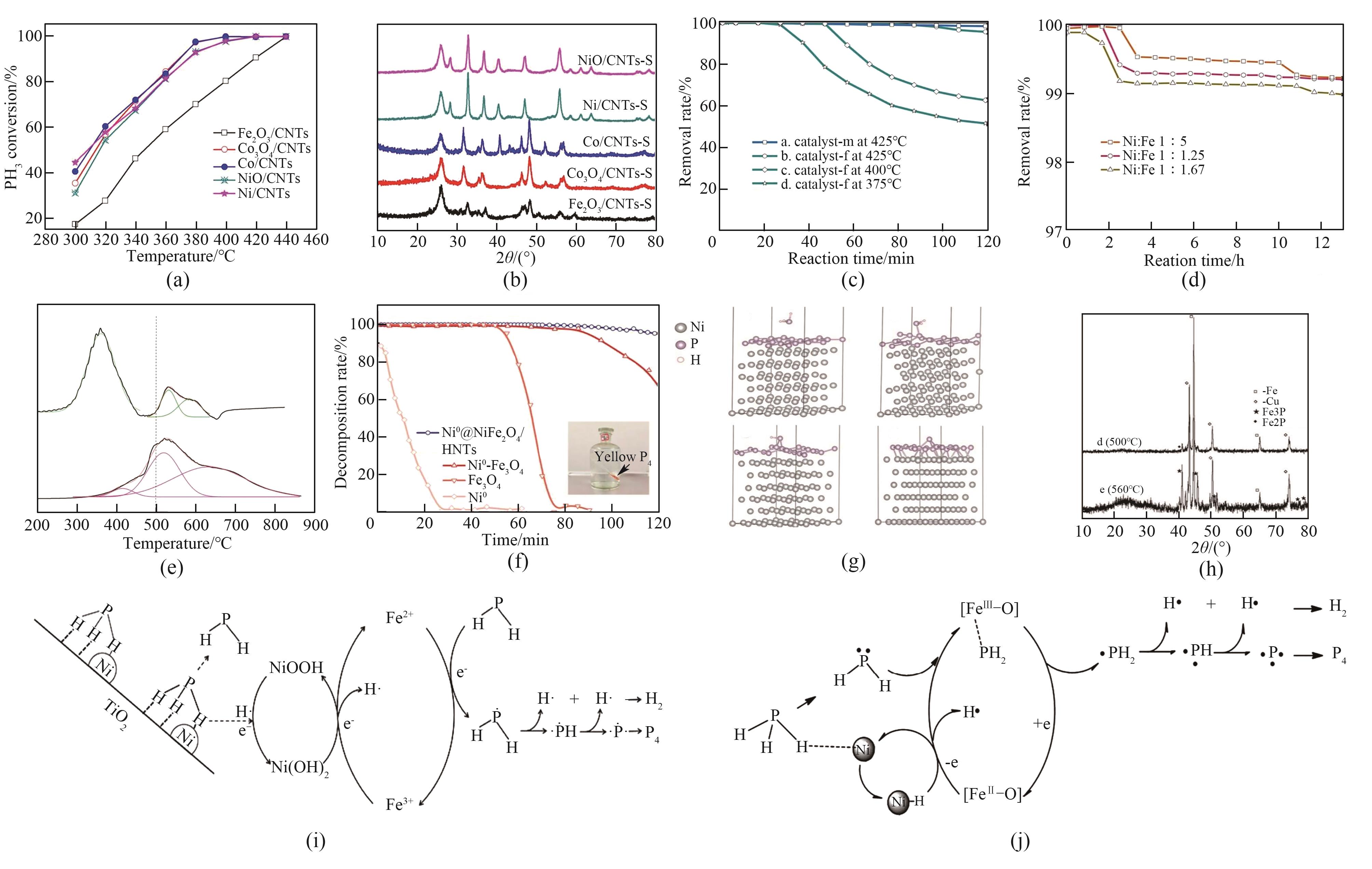
图5 (a) 不同温度下各催化剂的PH3分解性能[67];(b) 催化剂反应后的XRD谱图[67];(c) 额外煅烧还原后和正常煅烧还原后的催化剂在425℃时的PH3分解率和催化剂f在不同温度时的PH3分解率[35];(d) 不同Ni∶Fe摩尔比合成的催化剂m在425℃下的PH3分解率[35];(e) FeNiO/HNTs和BFeNiO/HNTs的H2-TPR谱图[9];(f) 不同纳米材料催化剂的PH3等温分解实验[36];(g) P层表面PH3的不同状态示意图[36];(h) CuFeP催化剂PH3分解产物XRD谱图[34];(i) Ni/Fe3O4/TiO2催化剂催化分解PH3机理[35];(j) FeNi/HNTs催化剂催化分解PH3机理[9]
Fig.5 (a) PH3 decomposition performance of each catalyst at different temperatures[67]; (b) XRD pattern of the catalyst after reaction[67]; (c) PH3 decomposition rates of catalysts after additional calcination reduction and normal calcination reduction at 425℃ and PH3 decomposition rates of catalyst f at different temperatures[35]; (d) PH3 decomposition rates at 425℃ for catalysts m synthesized with different Ni∶Fe molar ratios[35]; (e) H2-TPR profiles of FeNiO/HNTs and BFeNiO/HNTs[9]; (f) PH3 isothermal decomposition test of different nanomaterial catalysts[36]; (g) Schematic diagram of the different states of PH3 on the surface of P-layer[36]; (h) XRD pattern of CuFeP catalyst PH3 decomposition products[34]; (i) Mechanism of catalytic decomposition of PH3 by Ni/Fe3O4/TiO2 catalyst[35]; (j) Mechanism of catalytic decomposition of PH3 by FeNi/HNTs catalysts[9]
| 纳米材料种类 | 活性组分 | 气体组成 | 气体总流速/(ml/min) | 催化剂用量/g | 质量空速/(ml/(h·g)) | 催化分解 温度/℃ | 分解 效率/% | 分解产物 | 文献 |
|---|---|---|---|---|---|---|---|---|---|
| Co/CNTs、Ni/CNTs、 Fe2O3/CNTs | Co3O4、NiO、Fe2O3 | N2+5% PH3 | 60 | 0.3 | 2520 | 400 | 100 | P、H2、CoP、NiP2、FeP | [ |
| Ni/Fe3O4/TiO2 | Fe3O4、Ni | N2+1% PH3 | — | 0.2 | 3000 | 425 | 100 | P4 | [ |
| FeNi/HNTs | Fe3O4、Ni | N2+5% PH3 | — | — | 2520 | 420 | 100 | P+H2 | [ |
| Ni0@Fe3O4/HNTs | Fe3O4、Ni0 | N2+1% PH3 | — | 0.1 | 1200 | 300~450 | 100 | P+H2 | [ |
| CuFeP | Fe、Cu | N2+PH3 | 88 | — | — | 400~500或>800 | 100 | Fe2P+Fe3P | [ |
| Co-P非晶合金 | CoP | N2+PH3 | 70 | — | — | 470 | 99.8 | 高纯P | [ |
表2 几种不同纳米材料催化剂催化分解PH3条件对比
Table 2 Comparison of conditions for catalytic decomposition of PH3 by several different nanomaterial catalysts
| 纳米材料种类 | 活性组分 | 气体组成 | 气体总流速/(ml/min) | 催化剂用量/g | 质量空速/(ml/(h·g)) | 催化分解 温度/℃ | 分解 效率/% | 分解产物 | 文献 |
|---|---|---|---|---|---|---|---|---|---|
| Co/CNTs、Ni/CNTs、 Fe2O3/CNTs | Co3O4、NiO、Fe2O3 | N2+5% PH3 | 60 | 0.3 | 2520 | 400 | 100 | P、H2、CoP、NiP2、FeP | [ |
| Ni/Fe3O4/TiO2 | Fe3O4、Ni | N2+1% PH3 | — | 0.2 | 3000 | 425 | 100 | P4 | [ |
| FeNi/HNTs | Fe3O4、Ni | N2+5% PH3 | — | — | 2520 | 420 | 100 | P+H2 | [ |
| Ni0@Fe3O4/HNTs | Fe3O4、Ni0 | N2+1% PH3 | — | 0.1 | 1200 | 300~450 | 100 | P+H2 | [ |
| CuFeP | Fe、Cu | N2+PH3 | 88 | — | — | 400~500或>800 | 100 | Fe2P+Fe3P | [ |
| Co-P非晶合金 | CoP | N2+PH3 | 70 | — | — | 470 | 99.8 | 高纯P | [ |
| 1 | Widera A, Thöny D, Aebli M, et al. Solid-state investigation, storage, and separation of pyrophoric PH3 and P2H4 with α-Mg formate[J]. Angewandte Chemie International Edition, 2023, 62(13): e202217534. |
| 2 | Devai I, Delaune R D. Evidence for phosphine production and emission from Louisiana and Florida marsh soils[J]. Organic Geochemistry, 1995, 23(3): 277-279. |
| 3 | Geng J J, Niu X J, Jin X C, et al. Simultaneous monitoring of phosphine and of phosphorus species in Taihu Lake sediments and phosphine emission from lake sediments[J]. Biogeochemistry, 2005, 76(2): 283-298. |
| 4 | Hong Y N, Geng J J, Qiao S, et al. Phosphorus fractions and matrix-bound phosphine in coastal surface sediments of the Southwest Yellow Sea[J]. Journal of Hazardous Materials, 2010, 181(1/2/3): 556-564. |
| 5 | Han C, Geng J J, Hong Y N, et al. Free atmospheric phosphine concentrations and fluxes in different wetland ecosystems, China[J]. Environmental Pollution, 2011, 159(2): 630-635. |
| 6 | Chen W Y, Niu X J, An S R, et al. Emission and distribution of phosphine in paddy fields and its relationship with greenhouse gases[J]. Science of the Total Environment, 2017, 599/600: 952-959. |
| 7 | Wang S, Hu Z, Zhang J, et al. Formation of phosphine and its effect on phosphorus retention in constructed wetlands: characteristic and mechanism[J]. Environmental Technology & Innovation, 2022, 28: 102653. |
| 8 | Fu W Y, Zhang X H. Global phosphorus dynamics in terms of phosphine[J]. NPJ Climate and Atmospheric Science, 2020, 3: 51. |
| 9 | Tang X J, Li L L, Shen B X, et al. Halloysite-nanotubes supported FeNi alloy nanoparticles for catalytic decomposition of toxic phosphine gas into yellow phosphorus and hydrogen[J]. Chemosphere, 2013, 91(9): 1368-1373. |
| 10 | Wang Z H, Jiang M, Ning P, et al. Thermodynamic modeling and gaseous pollution prediction of the yellow phosphorus production[J]. Industrial & Engineering Chemistry Research, 2011, 50(21): 12194-12202. |
| 11 | Pidakala P P B, Esfandi K, Afsar S, et al. Effects of phosphine (ECO2FUME®) on ‘Hass’ avocado fruit quality and target pest mortality[J]. New Zealand Journal of Crop and Horticultural Science, 2022: 1-11. |
| 12 | Chiluwal K, Lee B H, Kwon T H, et al. Post-fumigation sub-lethal activities of phosphine and ethyl formate on survivorship, fertility and female sex pheromone production of Callosobruchus chinensis (L.)[J]. Scientific Reports, 2023, 13: 4333. |
| 13 | Bains W, Petkowski J J, Sousa-Silva C, et al. New environmental model for thermodynamic ecology of biological phosphine production[J]. Science of the Total Environment, 2019, 658: 521-536. |
| 14 | Roels J, Verstraete W. Occurrence and origin of phosphine in landfill gas[J]. Science of the Total Environment, 2004, 327(1/2/3): 185-196. |
| 15 | Ma J L, Chen W Y, Niu X, et al. The relationship between phosphine, methane, and ozone over paddy field in Guangzhou, China[J]. Global Ecology and Conservation, 2019, 17: e00581. |
| 16 | 刘树根, 李婷, 宁平, 等. 环境中磷化氢的产生、分布及转化研究进展[J]. 化工进展, 2019, 38(2): 1085-1096. |
| Liu S G, Li T, Ning P, et al. Research progress of the release, distribution and transformation of phosphine in environment[J]. Chemical Industry and Engineering Progress, 2019, 38(2): 1085-1096. | |
| 17 | Tuet W Y, Pierce S A, Racine M C, et al. Cardiopulmonary effects of phosphine poisoning: a preliminary evaluation of milrinone[J]. Toxicology and Applied Pharmacology, 2021, 427: 115652. |
| 18 | Yang L P, Yi H H, Tang X L, et al. Effect of rare earth addition on Cu-Fe/AC adsorbents for phosphine adsorption from yellow phosphorous tail gas[J]. Journal of Rare Earths, 2010, 28: 322-325. |
| 19 | 黄顺祥. 大气污染与防治的过去、现在及未来[J]. 科学通报, 2018, 63(10): 895-919. |
| Huang S X. Air pollution and control: past, present and future[J]. Chinese Science Bulletin, 2018, 63(10): 895-919. | |
| 20 | 王文兴, 柴发合, 任阵海, 等. 新中国成立70年来我国大气污染防治历程、成就与经验[J]. 环境科学研究, 2019, 32(10): 1621-1635. |
| Wang W X, Chai F H, Ren Z H, et al. Process, achievements and experience of air pollution control in China since the founding of the People's Republic of China 70 years ago[J]. Research of Environmental Sciences, 2019, 32(10): 1621-1635. | |
| 21 | 吴满昌, 宁平, 任丙南, 等. 黄磷尾气中总磷及磷化氢的测定[J]. 环境污染与防治, 2004, 26(4): 317-318, 326. |
| Wu M C, Ning P, Ren B N, et al. Determination of total phosphorus and hydrogen phosphide in yellow phosphorus tail gas[J]. Environmental Pollution & Control, 2004, 26(4): 317-318, 326. | |
| 22 | 任占冬, 陈樑. JC系列催化剂上氧化脱除黄磷尾气中PH3、H2S[J]. 天然气化工, 2004, 29(6): 19-23. |
| Ren Z D, Chen L. Oxidative removal of PH3 and H2S from yellow phosphorus tail gas by JC series catalysts[J]. Natural Gas Chemical Industry (C1 Chemistry and Technology), 2004, 29(6): 19-23. | |
| 23 | 任占冬, 陈樑, 宁平, 等. 黄磷尾气净化脱除磷化氢、硫化氢中试试验[J]. 现代化工, 2005, 25(12): 48-50, 52. |
| Ren Z D, Chen L, Ning P, et al. Pilot test of removal of PH3 and H2S from yellow phosphorus tail gas[J]. Modern Chemical Industry, 2005, 25(12): 48-50, 52. | |
| 24 | 张永, 宁平, 徐浩东, 等. 改性活性炭吸附净化黄磷尾气中的PH3 [J]. 环境工程学报, 2007, 1(5): 74-78. |
| Zhang Y, Ning P, Xu H D, et al. Adsorptive removal of PH3 in off-gas of yellow phosphorus by modified activated carbon[J]. Chinese Journal of Environmental Engineering, 2007, 1(5): 74-78. | |
| 25 | Li W C, Bai H, Hsu J N, et al. Metal loaded zeolite adsorbents for phosphine removal[J]. Industrial & Engineering Chemistry Research, 2008, 47(5): 1501-1505. |
| 26 | Hsu J N, Bai H, Li S N, et al. Copper loaded on sol-gel-derived alumina adsorbents for phosphine removal[J]. Journal of the Air & Waste Management Association, 2010, 60(5): 629-635. |
| 27 | Chang S M, Hsu Y Y, Chan T S. Chemical capture of phosphine by a sol-gel-derived Cu/TiO2 adsorbent-interaction mechanisms[J]. The Journal of Physical Chemistry C, 2011, 115(5): 2005-2013. |
| 28 | Zhou Y M, He T F, Liu S F, et al. Adsorption of trace phosphine in circular hydrogen of a polysilicon chemical vapor deposition stove by Cu/γ-Al2O3 [J]. Industrial & Engineering Chemistry Research, 2018, 57(44): 15122-15131. |
| 29 | Feng J Y, Wang F, Wang C, et al. Ce-doping CuO/HZSM-5 as a regenerable sorbent for adsorption-oxidation removal of PH3 at low temperature[J]. Separation and Purification Technology, 2021, 277: 119420. |
| 30 | Feng J Y, Li K, Wang X Q, et al. Two birds with one stone: copper-based adsorbents used for photocatalytic oxidation of Hg0 (gas) after removal of PH3 [J]. Environmental Science & Technology, 2023, 57(11): 4632-4642. |
| 31 | Feng J Y, Ma L X, Wang C, et al. Catalytic decomposition mechanism of PH3 on 3DCuO/C and high value utilization of deactivated catalysts[J]. Small, 2023, 19(28): 2370206. |
| 32 | Jia L J, Yang X J, Hu K Q, et al. Preparation of XCu@TiO2 adsorbent for high-efficient PH3 removal in anaerobic environment and evaluation of desulfurization activity of deactivated adsorbent[J]. Chemical Engineering Journal, 2023, 457: 141277. |
| 33 | 梁培玉, 韩长秀, 林徐明, 等. CoP非晶合金催化分解磷化氢制高纯磷的研究[J]. 南开大学学报(自然科学版), 2006, 39(4): 20-23. |
| Liang P Y, Han C X, Lin X M, et al. Research on decomposition of phosphine to high pure phosphorus using Co-P amorphous alloy as catalyst[J]. Acta Scientiarum Naturalium Universitatis Nankaiensis, 2006, 39(4): 20-23. | |
| 34 | Han C X, Ren J L, Tang X J, et al. Preparation of nanometer FeCuP alloy and its application in decomposition of PH3 [J]. Chinese Chemical Letters, 2007, 18(10): 1285-1288. |
| 35 | Tang X J, Xing C, Ma S H, et al. Highly active Ni/Fe3O4/TiO2 nanocatalysts with tunable interfacial interactions for PH3 decomposition[J]. Environmental Technology, 2021, 42(28): 4426-4433. |
| 36 | Tang X J, Xue J J, Xing C. Catalytic decomposition of toxic phosphine gas on the developed nickel ferrite nanocrystals supported by halloysite nanotubes[J]. Applied Surface Science, 2020, 530: 147264. |
| 37 | 余琼粉, 易红宏, 唐晓龙, 等. 磷化氢在改性活性炭纤维上的吸附等温过程[J]. 中南大学学报(自然科学版), 2010, 41(1): 381-386. |
| Yu Q F, Yi H H, Tang X L, et al. Adsorption isotherm of phosphine onto CoCl2-modified activated carbon fiber[J]. Journal of Central South University (Science and Technology), 2010, 41(1): 381-386. | |
| 38 | Xu X, Miao X, Liao N, et al. Breakthrough analysis for adsorption of phosphine on 5A molecular sieve[J]. Chemical Engineering & Technology, 2011, 34(1): 140-145. |
| 39 | Xu X W, Huang G Q. Effect of 13X zeolite modified with CuCl2 and ZnCl2 for removing phosphine from circular hydrogen of a polysilicon chemical vapor deposition stove[J]. Industrial & Engineering Chemistry Research, 2016, 55(5): 1380-1386. |
| 40 | Xu X W, Huang G Q, Qi S. Properties of AC and 13X zeolite modified with CuCl2 and Cu(NO3)2 in phosphine removal and the adsorptive mechanisms[J]. Chemical Engineering Journal, 2017, 316: 563-572. |
| 41 | Wang Y W, Lin Q, Ning P, et al. Preparation of Ce0.6-Cu60/Al40-[O] catalyst and role of CeO2/CuO in simultaneous removal of H2S and PH3 [J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 87: 44-53. |
| 42 | Wang Y W, Lin Q A, Ning P, et al. Effect of the acid used in the evaporation-induced self-assembly method on Ce-Cu-Al trimetallic composite catalyst for its simultaneous removal of H2S and PH3 [J]. New Journal of Chemistry, 2021, 45(13): 5822-5828. |
| 43 | Wei S, Ning P, Wang C, et al. Research into the simultaneous removal process of H2S and PH3 by Cu-Fe-Ce composite metal oxide adsorbent[J]. Research on Chemical Intermediates, 2020, 46(9): 4017-4032. |
| 44 | Ren Z D, Quan S S, Zhu Y C, et al. Purification of yellow phosphorus tail gas for the removal of PH3 on the spot with flower-shaped CuO/AC[J]. RSC Advances, 2015, 5(38): 29734-29740. |
| 45 | Wang Y W, Ning P, Zhao R H, et al. A Cu-modified active carbon fiber significantly promoted H2S and PH3 simultaneous removal at a low reaction temperature[J]. Frontiers of Environmental Science & Engineering, 2021, 15(6): 1-10. |
| 46 | Yu Q F, Li M, Ning P, et al. Preparation and phosphine adsorption of activated carbon prepared from walnut shells by KOH chemical activation[J]. Separation Science and Technology, 2014, 49(15): 2366-2375. |
| 47 | Feng J Y, Wang F, Wang C, et al. Cu/HZSM-5 sorbent treated by NH3 plasma for low-temperature simultaneous adsorption-oxidation of H2S and PH3 [J]. ACS Applied Materials & Interfaces, 2021, 13(21): 24670-24681. |
| 48 | Li S, Li K, Hao J M, et al. Acid modified mesoporous Cu/SBA-15 for simultaneous adsorption/oxidation of hydrogen sulfide and phosphine[J]. Chemical Engineering Journal, 2016, 302: 69-76. |
| 49 | Song X, Li S, Li K, et al. Preparation of Cu-Fe composite metal oxide loaded SBA-15 and its capacity for simultaneous catalytic oxidation of hydrogen sulfide and phosphine[J]. Microporous and Mesoporous Materials, 2018, 259: 89-98. |
| 50 | Li S, Hao J M, Ning P, et al. Preparation of Cu-Fe nanocomposites loaded diatomite and their excellent performance in simultaneous adsorption/oxidation of hydrogen sulfide and phosphine at low temperature[J]. Separation and Purification Technology, 2017, 180: 23-35. |
| 51 | Rupp E C, Granite E J, Stanko D C. Laboratory scale studies of Pd/γ-Al2O3 sorbents for the removal of trace contaminants from coal-derived fuel gas at elevated temperatures[J]. Fuel, 2013, 108: 131-136. |
| 52 | 蒋明, 宁平, 王重华, 等. 改性活性炭吸附净化低浓度磷化氢[J]. 高校化学工程学报, 2012, 26(4): 685-691. |
| Jiang M, Ning P, Wang Z H, et al. Adsorption purification of low-concentration PH3 by modified activated carbon[J]. Journal of Chemical Engineering of Chinese Universities, 2012, 26(4): 685-691. | |
| 53 | 王学谦, 宁平. 浸渍活性炭吸附低浓度PH3动力学[J]. 中南大学学报(自然科学版), 2011, 42(7): 2162-2166. |
| Wang X Q, Ning P. Kinetics of low concentration PH3 adsorption on impregnatedly activated carbon[J]. Journal of Central South University (Science and Technology), 2011, 42(7): 2162-2166. | |
| 54 | 徐浩东, 宁平, 蒋明, 等. 净化PH3和H2S气体改性活性炭的制备与表征[J]. 环境科学学报, 2008, 28(7): 1365-1369. |
| Xu H D, Ning P, Jiang M, et al. Preparation and characterization of modified activated carbon for purification of PH3 and H2S[J]. Acta Scientiae Circumstantiae, 2008, 28(7): 1365-1369. | |
| 55 | Ning P, Wang X Y, Bart H J, et al. Removal of phosphorus and sulfur from yellow phosphorus off-gas by metal-modified activated carbon[J]. Journal of Cleaner Production, 2011, 19(13): 1547-1552. |
| 56 | Ning P, Yi H H, Yu Q F, et al. Effect of zinc and cerium addition on property of copper-based adsorbents for phosphine adsorption[J]. Journal of Rare Earths, 2010, 28(4): 581-586. |
| 57 | Yi H H, Yu Q F, Tang X L, et al. Phosphine adsorption removal from yellow phosphorus tail gas over CuO-ZnO-La2O3/activated carbon[J]. Industrial & Engineering Chemistry Research, 2011, 50(7): 3960-3965. |
| 58 | 杨丽萍, 易红宏, 唐晓龙, 等. 铜基改性活性炭吸附净化黄磷尾气中的PH3 [J]. 中南大学学报(自然科学版), 2011, 42(5): 1489-1494. |
| Yang L P, Yi H H, Tang X L, et al. Adsorptive purification of PH3 in yellow phosphorus off-gas by Cu-based modified activated carbon[J]. Journal of Central South University (Science and Technology), 2011, 42(5): 1489-1494. | |
| 59 | 李彬, 宁平, 王学谦. 负载酸活性炭净化黄磷尾气中磷化氢[J]. 四川化工, 2004, 7(5): 41-43. |
| Li B, Ning P, Wang X Q. Purification of phosphine from yellow phosphorus tail gas by acid-loaded activated carbon[J]. Sichuan Chemical Industry, 2004, 7(5): 41-43. | |
| 60 | Yang X Y, Li K, Wang C, et al. Cu/ACF adsorbent modified by non-thermal plasma for simultaneous adsorption-oxidation of H2S and PH3 [J]. Journal of Environmental Sciences, 2023, 127: 641-651. |
| 61 | 黄小凤, 谭娟, 宁平. 改性5A分子筛吸附净化PH3的实验研究[J]. 西安建筑科技大学学报(自然科学版), 2011, 43(2): 220-223. |
| Huang X F, Tan J, Ning P. Adsorbing purification of PH3 by modified 5A molecular sieves[J]. Journal of Xi’an University of Architecture & Technology (Natural Science Edition), 2011, 43(2): 220-223. | |
| 62 | Feng J Y, Li K, Li S, et al. Regeneration of the exhausted mesoporous Cu/SBA-15-[N] for simultaneous adsorption-oxidation of hydrogen sulfide and phosphine[J]. Research on Chemical Intermediates, 2020, 46(1): 329-346. |
| 63 | Einicke W D, Enke D, Dvoyashkin M, et al. The mechanism of pseudomorphic transformation of spherical silica gel into MCM-41 studied by PFG NMR diffusometry[J]. Materials, 2013, 6(9): 3688-3709. |
| 64 | Umemoto H. Dependence of the catalytic decomposition of PH3 on wire material[J]. Thin Solid Films, 2015, 575: 9-11. |
| 65 | 李俐俐, 侯小歌, 胡春红, 等. 碳纳米管负载的镍及其氧化物对高毒气体PH3的催化分解性能[J]. 环境化学, 2013, 32(8): 1518-1523. |
| Li L L, Hou X G, Hu C H, et al. Catalytic decomposition of highly toxic PH3 gas over metal Ni and NiO supported on carbon nanotubes[J]. Environmental Chemistry, 2013, 32(8): 1518-1523. | |
| 66 | 李俐俐, 盛东峰, 王进, 等. 碳纳米管负载的非晶态CoP合金和金属Co对高毒气体PH3的催化分解[J]. 环境科学学报, 2013, 33(8): 2158-2165. |
| Li L L, Sheng D F, Wang J, et al. Catalytic decomposition of highly toxic PH3 gas over amorphous CoP alloy and metal Co supported on carbon nanotubes[J]. Acta Scientiae Circumstantiae, 2013, 33(8): 2158-2165. | |
| 67 | Li L L, Chen C, Chen L, et al. Catalytic decomposition of toxic chemicals over iron group metals supported on carbon nanotubes[J]. Environmental Science & Technology, 2014, 48(6): 3372-3377. |
| 68 | 徐卫煌. 挥发性有机废气治理技术研究进展[J]. 化工设计通讯, 2018, 44(10): 228-229. |
| Xu W H. Research progress of treatment technology for volatile organic waste gas[J]. Chemical Engineering Design Communications, 2018, 44(10): 228-229. | |
| 69 | 王惠平, 唐忠松. 次磷酸钠生产中“三废”的综合治理[J]. 化学世界, 1999, 40(3): 159-162. |
| Wang H P, Tang Z S. Comprehensive management of waste materials in the production process of sodium hypophosphite[J]. Chemical World, 1999, 40(3): 159-162. | |
| 70 | 周为莉, 叶明华, 余锋进, 等. 有机废气处理技术研究进展[J]. 能源工程, 2018, (5): 55-61. |
| Zhou W L, Ye M H, Yu F J, et al. Research progress of organic waste gas treatment technologies[J]. Energy Engineering, 2018, (5): 55-61. | |
| 71 | Ma Y X, Wang X Q, Ning P, et al. Simultaneous removal of PH3, H2S, and dust by corona discharge[J]. Energy & Fuels, 2016, 30(11): 9580-9588. |
| 72 | 刘树根, 苏福家, 李婷, 等. 生物滴滤法净化低浓度磷化氢及其微生物群落分析[J]. 环境工程学报, 2018, 12(12): 3406-3414. |
| Liu S G, Su F J, Li T, et al. Purification of low concentration phosphine by bio-trickling filter system and analysis of microbial community[J]. Chinese Journal of Environmental Engineering, 2018, 12(12): 3406-3414. | |
| 73 | 余硕, 刘树根, 李婷. 磷化氢生物净化体系及抑制作用机理[J]. 中国环境科学, 2021, 41(5): 2219-2225. |
| Yu S, Liu S G, Li T. Inhibition mechanism of phosphine biological purification system[J]. China Environmental Science, 2021, 41(5): 2219-2225. |
| [1] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [2] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [3] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [4] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [5] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [6] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [7] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [8] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [9] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [10] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [11] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [12] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [13] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [14] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [15] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号