化工学报 ›› 2019, Vol. 70 ›› Issue (7): 2528-2539.DOI: 10.11949/0438-1157.20190113
收稿日期:2019-02-11
修回日期:2019-04-11
出版日期:2019-07-05
发布日期:2019-07-05
通讯作者:
袁珮
作者简介:阴义轩(1993—),女,硕士研究生,<email>893584980@qq.com</email>
基金资助:
Yixuan YIN1( ),Tingting CHENG2,Xiaojun BAO1,Pei YUAN1(
),Tingting CHENG2,Xiaojun BAO1,Pei YUAN1( )
)
Received:2019-02-11
Revised:2019-04-11
Online:2019-07-05
Published:2019-07-05
Contact:
Pei YUAN
摘要:
对丁腈橡胶非均相催化加氢制备高附加值氢化丁腈橡胶过程中催化剂失活的原因进行了探究,发现造成催化剂活性下降的原因并不是贵金属纳米颗粒的流失、团聚或中毒,而是催化剂表面的活性位被聚合物覆盖而无法与反应物接触,因此将覆盖在活性位上的聚合物进行脱除才是催化剂再生和重复利用的关键。根据相似相容原理选择单溶剂或者混合溶剂对反应后催化剂进行处理,结果表明经过乙酸乙酯、丁酮、N,N-二甲基甲酰胺以及N-甲基吡咯烷酮这四种有机溶剂处理后,其催化加氢活性可以恢复至新鲜催化剂活性的90%;当用混合溶剂处理后,其催化活性可以提高至新鲜催化剂活性的95%,且循环利用4次其催化活性仍能保持不变。
中图分类号:
阴义轩, 成婷婷, 鲍晓军, 袁珮. 丁腈橡胶非均相加氢催化剂失活原因及再生性能研究[J]. 化工学报, 2019, 70(7): 2528-2539.
Yixuan YIN, Tingting CHENG, Xiaojun BAO, Pei YUAN. Deactivation and regeneration of heterogeneous catalysts for hydrogenation of nitrile butadiene rubber[J]. CIESC Journal, 2019, 70(7): 2528-2539.
| 波数/cm-1 | 基团种类 | 吸收因子 | 峰强度变化 |
|---|---|---|---|
| 3500 | —NH2 或 —NH— | — | —CN被加氢还原后新出现的峰 |
| 2236 | —CN | 1.0 | 此为内标计算加氢度 |
| 970 | 1,4-trans unit, —CH=CH— | 2.3 | 加氢度增加,峰强度减弱或消失 |
| 920 | 1,2 unit, —CH=CH2 | 2.24 | 加氢度增加,峰强度减弱或消失 |
| 750 | 1,4-cis unit, —CH=CH— | 0.36 | 加氢度增加,峰强度减弱或消失 |
| 723 | —[CH2] n — (n?4) | 0.25 | C=C被加氢还原,出现亚甲基的特征峰 |
表1 NBR及HNBR红外特征峰[33]
Table 1 Characteristic peaks for NBR and HNBR FT-IR spectra [33]
| 波数/cm-1 | 基团种类 | 吸收因子 | 峰强度变化 |
|---|---|---|---|
| 3500 | —NH2 或 —NH— | — | —CN被加氢还原后新出现的峰 |
| 2236 | —CN | 1.0 | 此为内标计算加氢度 |
| 970 | 1,4-trans unit, —CH=CH— | 2.3 | 加氢度增加,峰强度减弱或消失 |
| 920 | 1,2 unit, —CH=CH2 | 2.24 | 加氢度增加,峰强度减弱或消失 |
| 750 | 1,4-cis unit, —CH=CH— | 0.36 | 加氢度增加,峰强度减弱或消失 |
| 723 | —[CH2] n — (n?4) | 0.25 | C=C被加氢还原,出现亚甲基的特征峰 |
| 样品 | 结构类型 | H种类 | 化学位移 |
|---|---|---|---|
| NBR | 1,4 单元 | —CH=CH— | 5.50 |
| —CH2— | 2.15 | ||
| —CH(CN) — | 2.65 | ||
| —CH2CH(CN) — | 1.70 | ||
| 1,2 单元 | =CH—和=CH2 | 4.90—5.10 | |
| HNBR | —CH3 | 0.90 | |
| —CH2— | 1.30 | ||
| —CH(CN) — | 2.60 | ||
| —CH2—CH(CN) — | 1.50 |
表2 NBR及HNBR中不同结构对应的H化学位移[33]
Table 2 Chemical shifts of H protons in different microstructures of NBR or HNBR [33]
| 样品 | 结构类型 | H种类 | 化学位移 |
|---|---|---|---|
| NBR | 1,4 单元 | —CH=CH— | 5.50 |
| —CH2— | 2.15 | ||
| —CH(CN) — | 2.65 | ||
| —CH2CH(CN) — | 1.70 | ||
| 1,2 单元 | =CH—和=CH2 | 4.90—5.10 | |
| HNBR | —CH3 | 0.90 | |
| —CH2— | 1.30 | ||
| —CH(CN) — | 2.60 | ||
| —CH2—CH(CN) — | 1.50 |

图4 反应前后催化剂N2吸脱附等温线及基于吸附支的孔径分布
Fig. 4 N2 adsorption-desorption isotherms and pore diameter distribution curves obtained from adsorption branches of catalyst before and after reaction
| 催化剂 | 比表面积/(m2/g) | 孔容/(cm3/g) | 平均孔径/nm |
|---|---|---|---|
| 反应前 | 494 | 1.55 | 12.5 |
| 反应后 | 281 | 0.87 | 12.3 |
表3 反应前后催化剂的物性数据
Table 3 Physical properties of catalyst before and after reaction
| 催化剂 | 比表面积/(m2/g) | 孔容/(cm3/g) | 平均孔径/nm |
|---|---|---|---|
| 反应前 | 494 | 1.55 | 12.5 |
| 反应后 | 281 | 0.87 | 12.3 |

图9 不同单一溶剂处理后的催化剂再次用于NBR加氢得到的加氢产物的红外谱图和加氢度
Fig.9 FT-IR spectra of HNBR and hydrogenation degree of HNBR produced by regenerated catalysts treated with different single solvent

图12 不同混合溶剂处理后的催化剂再次用于NBR加氢得到的加氢产物的红外谱图和加氢度
Fig.12 FT-IR spectra of HNBR and hydrogenation degree of HNBR produced by regenerated catalysts treated with mixed solvents
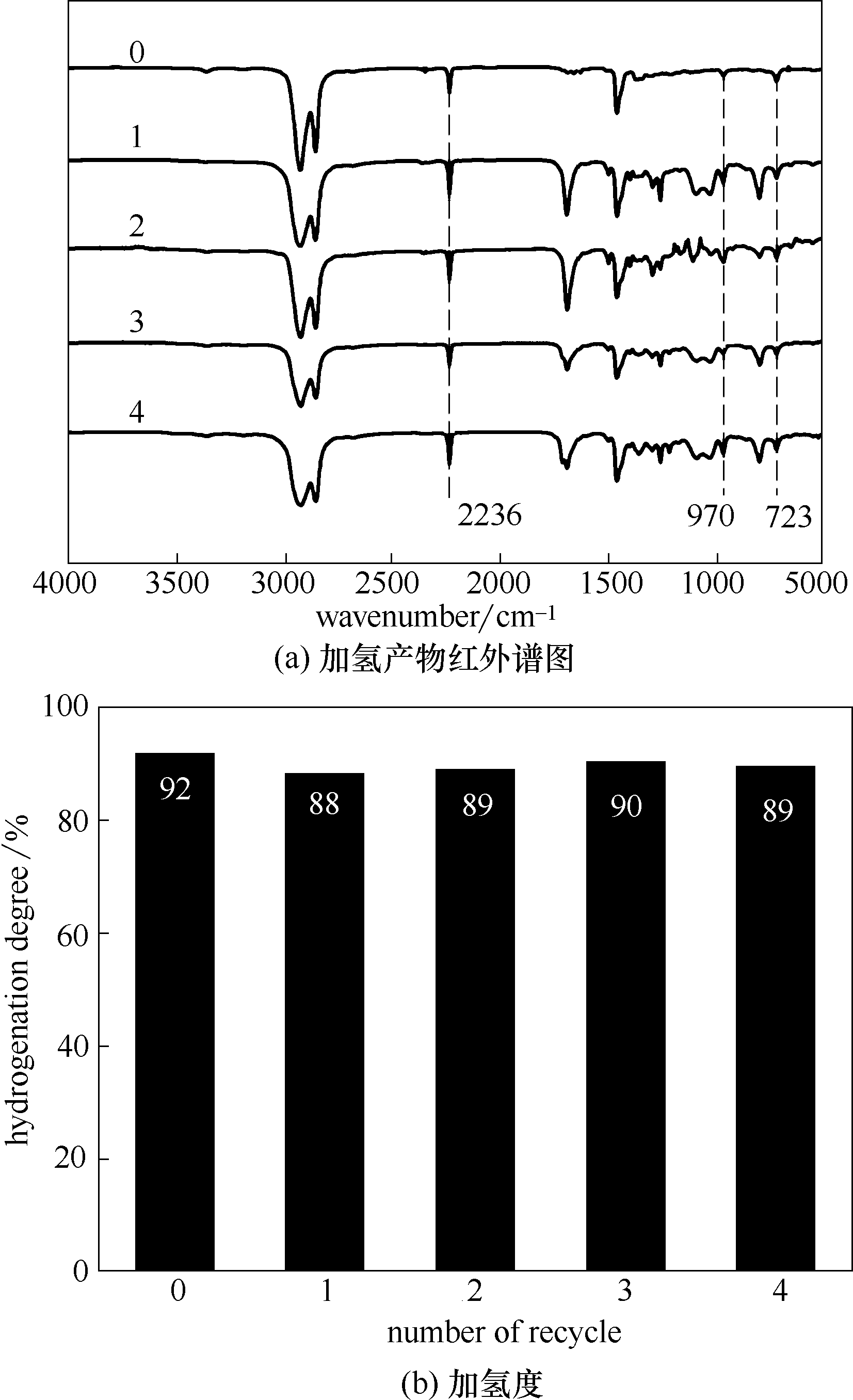
图13 混合溶剂(EA+NMP)处理的催化剂循环利用4次得到的加氢产物红外谱图和加氢度
Fig.13 FT-IR spectra of HNBR and hydrogenation degree of HNBR produced by regenerated catalysts treated with (EA+NMP) for recycling 4 times
| 1 | Mao T F , Rempel G L . Catalytic hydrogenation of nitrile-butadiene copolymers by cationic rhodium complexes [J]. Journal of Molecular Catalysis A Chemical, 1998, 135(2): 121-132. |
| 2 | Parent J S , Mcmanus N T , Rempel G L . RhCl(PPh3)3 and RhH(PPh3)4 catalyzed hydrogenation of acrylonitrile-butadiene copolymers[J]. Industrial & Engineering Chemistry Research, 1996, 35(12): 4417-4423. |
| 3 | Rempel G L , Mcmanus N T , Guo X Y . Catalytic solution hydrogenation of nitrile rubber: US5258467[P]. 1993-11-02. |
| 4 | Mcmanus N , Rempel G . Improvements in the hydrogenation of nitrile rubber using Wilkinson s catalyst [J]. Rubber Chemistry and Technology, 2008, 81(2):227-243. |
| 5 | Bhattacharjee S , Bhowmick A K , Avasthi B N . Preparation of hydrogenated nitrile rubber using palladium acetate catalyst its characterization and kinetics [J]. Journal of Polymer Science Part A-Polymer Chemistry, 1992, 30(3):471-484. |
| 6 | Bhattacharjee S , Rajagopalan P , Bhowmick A K , et al . Selective hydrogenation of olefinic bonds in styrene-isoprene-styrene triblock copolymer by palladium acetate catalyst[J]. Journal of Applied Polymer Science, 1993, 49(11): 1971-1977. |
| 7 | Schulz G A , Comin E , Souza R F . Catalytic hydrogenation of nitrile rubber using palladium and ruthenium complexes [J]. Journal of Applied Polymer Science, 2007, 106(1): 659-663. |
| 8 | Liu Q , Wei Z . Catalyst compositions and their use for hydrogenation of nitrile rubber: US9605088 [P]. 2017-03-28. |
| 9 | Hallett J P , Ford J W , Jones R S , et al . Hydroformylation catalyst recycle with gas-expanded liquids [J]. Industrial & Engineering Chemistry Research, 2008, 47(8): 2585-2589. |
| 10 | Yang L , Pan Q , Rempel G L . Recovery of Wilkinson s catalyst from polymer based matrix using carbon dioxide expanded methanol [J]. Journal of Supercritical Fluids, 2012, 68: 104-112. |
| 11 | Yang L , Pan Q , Rempel G L . Development of a green separation technique for recovery of Wilkinson s catalysts from bulk hydrogenated nitrile butadiene rubber [J]. Catalysis Today, 2013, 207(207): 153-161. |
| 12 | Hutchings G J . Heterogeneous catalysts—discovery and design [J]. Journal of Materials Chemistry, 2009, 19(9): 1222-1235. |
| 13 | Almusaiteer K A . Effect of supports on the catalytic hydrogenation of polystyrene [J]. Topics in Catalysis, 2012, 55(7/8/9/10): 498-504. |
| 14 | Buding H , Konigshofen H , Szentivanyi Z , et al . Production of hydrogenated nitrile rubbers: US4581417A[P]. 1986-04-08. |
| 15 | Kubo Y . Hydrogenation of conjugated diene polymer: JP62218403 [P]. 1987. |
| 16 | Kubo Y , Ohura K . Process for hydrogenation of conjugated diene polymers: US4337329 [P]. 1982-06-29. |
| 17 | Albers P , Pietsch P , Parker S F . Poisoning and deactivation of palladium catalysts[J]. Journal of Molecular Catalysis A Chemical, 2001, 173(1): 275-286. |
| 18 | Argyle M D , Bartholomew C H . Heterogeneous catalyst deactivation and regeneration: a review [J]. Catalysts, 2015, 5(1): 145-269. |
| 19 | 陈筱金 . Pd/C 催化剂失活原因分析与改进措施[J]. 化学反应工程与工艺, 2002, 18(3): 275-278. |
| Chen X J . Analysis of Pd/C catalyst deactivation mechanisms and improvement measures for CTA hydrogenation process [J]. Chemical Reaction Engineering and Technology, 2002, 18(3): 275-278. | |
| 20 | 张建远, 康保安 . 脂肪腈加氢胺化过程中 Pd/C 催化剂失活原因探讨[J]. 化学通报, 2006, 69(9): 696-700. |
| Zhang J Y , Kang B A . Study on Pd/C catalysts deactivation during the process of fatty nitrile hydroamination [J]. Chemistry Bulletin, 2006, 69(9): 696-700. | |
| 21 | Escandon L S , Ordonez S , Vega A , et al . Sulphur poisoning of palladium catalysts used for methane combustion: effect of the support [J]. Journal of Hazardous Materials, 2008, 153(1/2): 742-750. |
| 22 | Ahn I Y , Lee J H , Kim S K , et al . Three-stage deactivation of Pd/SiO2 and Pd-Ag/SiO2 catalysts during the selective hydrogenation of acetylene [J]. Applied Catalysis A-General, 2009, 360(1): 38-42. |
| 23 | Abate S , Perathoner S , Centi G . Deactivation mechanism of Pd supported on ordered and non-ordered mesoporous silica in the direct H2O2 synthesis using CO2-expanded methanol [J]. Catalysis Today, 2012, 179(1): 170-177. |
| 24 | Jiang D , Mao J , Fang Z , et al . Deactivation of Pd/SiO2 catalyst in the continuous liquid-phase selective hydrogenation of an unsaturated ketone [J]. Reaction Kinetics Mechanisms and Catalysis, 2015, 116(2): 451-466. |
| 25 | Zou R , Li C , Zhang L , et al . Selective hydrogenation of nitrile butadiene rubber (NBR) with rhodium nanoparticles supported on carbon nanotubes at room temperature [J]. Catalysis Communications, 2016, 81(4-9): 1566-7367. |
| 26 | Cao P , Ni Y , Zou R , et al . Enhanced catalytic properties of rhodium nanoparticles deposited on chemically modified SiO2 for hydrogenation of nitrile butadiene rubber [J]. RSC Advances, 2015, 5(5): 3417-3424. |
| 27 | Cao P , Su N , Li C , et al . Highly active and reusable rhodium catalyst for selective hydrogenation of nitrile-butadiene rubber[J]. Rubber Chemistry and Technology, 2015, 88(4): 150623072814001. |
| 28 | Chen J , Ma L , Cheng T , et al . Stable and recyclable Pd catalyst supported on modified silica hollow microspheres with microporous shells for enhanced catalytic hydrogenation of NBR [J]. Journal of Materials Science, 2018, 53(21): 15064-15080. |
| 29 | Pan D , Shi G , Zhang T , et al . New understanding and controllable synthesis of silica hollow microspheres with size-tunable penetrating macroporous shells as a superior support for polystyrene hydrogenation catalysts [J]. Journal of Materials Chemistry A, 2013, 1(34): 9597-9602. |
| 30 | Leeuwen P W N M V . Homogeneous Catalysis: Understanding the Art [M].Boston: Kluwer Academic Publishers, 2004: 6-9. |
| 31 | Brück D . IR-spectrometric determination of the proportions of acrylonitrile, butadiene and hydrogenated butadiene in hydrogenated acrylonitrile-butadiene rubbers (HNBR) (Ⅱ): Residual double bonds in commercial HNBR products [J]. Kautschuk Gummi Kunststoffe, 1989, 42(3): 194-197. |
| 32 | Bhattacharjee S , Bhowmick A K , Avasthi B N . High-pressure hydrogenation of nitrile rubber: thermodynamics and kinetics[J]. Industrial & Engineering Chemistry Research, 1991, 30(6): 1086-1092. |
| 33 | Wang H , Yang L , Rempel G L . Homogeneous hydrogenation art of nitrile butadiene rubber: a review [J]. Polymer Reviews, 2013, 53(2): 192-239. |
| [1] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [2] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [3] | 曹跃, 余冲, 李智, 杨明磊. 工业数据驱动的加氢裂化装置多工况切换过渡状态检测[J]. 化工学报, 2023, 74(9): 3841-3854. |
| [4] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [5] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [6] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [7] | 文兆伦, 李沛睿, 张忠林, 杜晓, 侯起旺, 刘叶刚, 郝晓刚, 官国清. 基于自热再生的隔壁塔深冷空分工艺设计及优化[J]. 化工学报, 2023, 74(7): 2988-2998. |
| [8] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [9] | 刘杰, 吴立盛, 李锦锦, 罗正鸿, 周寅宁. 含乙烯基胺酯键聚醚类可逆交联聚合物的制备及性能研究[J]. 化工学报, 2023, 74(7): 3051-3057. |
| [10] | 朱风, 陈凯琳, 黄小凤, 鲍银珠, 李文斌, 刘嘉鑫, 吴玮强, 高王伟. KOH改性电石渣脱除羰基硫的性能研究[J]. 化工学报, 2023, 74(6): 2668-2679. |
| [11] | 卫雪岩, 钱勇. 微米级铁粉燃料中低温氧化反应特性及其动力学研究[J]. 化工学报, 2023, 74(6): 2624-2638. |
| [12] | 周继鹏, 何文军, 李涛. 异形催化剂上乙烯催化氧化失活动力学反应工程计算[J]. 化工学报, 2023, 74(6): 2416-2426. |
| [13] | 董茂林, 陈李栋, 黄六莲, 吴伟兵, 戴红旗, 卞辉洋. 酸性助水溶剂制备木质纳米纤维素及功能应用研究进展[J]. 化工学报, 2023, 74(6): 2281-2295. |
| [14] | 龙臻, 王谨航, 任俊杰, 何勇, 周雪冰, 梁德青. 离子液体协同PVCap抑制天然气水合物生成实验研究[J]. 化工学报, 2023, 74(6): 2639-2646. |
| [15] | 杨琴, 秦传鉴, 李明梓, 杨文晶, 赵卫杰, 刘虎. 用于柔性传感的双形状记忆MXene基水凝胶的制备及性能研究[J]. 化工学报, 2023, 74(6): 2699-2707. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号