化工学报 ›› 2019, Vol. 70 ›› Issue (7): 2574-2583.DOI: 10.11949/0438-1157.20190014
收稿日期:2019-01-07
修回日期:2019-04-15
出版日期:2019-07-05
发布日期:2019-07-05
通讯作者:
唐元晖
基金资助:
Yuanhui TANG1( ),Yang HU1,Zhiqin YAN2,Chunyu LI1
),Yang HU1,Zhiqin YAN2,Chunyu LI1
Received:2019-01-07
Revised:2019-04-15
Online:2019-07-05
Published:2019-07-05
Contact:
Yuanhui TANG
摘要:
为了考察纳滤技术分离含高浓度单价盐的草甘膦溶液的可行性,采用商业化Desal-DK纳滤膜对含有高浓度NaCl的草甘膦溶液开展了分离实验研究和模拟计算。首先研究了物料浓度、跨膜压差等因素对NaCl和草甘膦的单组分溶液的体积通量及截留率的影响,并通过拟合SK(Spiegler-Kedem)方程计算得到了膜的特征参数;其次探究了Desal-DK纳滤膜对高浓度NaCl和不同浓度草甘膦混合溶液的分离效果,实验结果说明Desal-DK纳滤膜对NaCl呈现较高的透过性,而对草甘膦则呈现较高的截留性,从而能够有效实现草甘膦和NaCl的分离;最后通过对含有100 g/L NaCl和1 g/L草甘膦的混合溶液进行预浓缩-连续恒容渗滤过程的模拟计算,得到了较高浓度的草甘膦溶液和相对较纯的NaCl溶液,证明通过纳滤渗滤过程实现含草甘膦浓盐水的资源化利用是可行的。
中图分类号:
唐元晖, 扈阳, 燕至琴, 李春玉. 高浓度含盐草甘膦溶液的纳滤分离实验研究[J]. 化工学报, 2019, 70(7): 2574-2583.
Yuanhui TANG, Yang HU, Zhiqin YAN, Chunyu LI. Experimental study on nanofiltration separation of high concentrated saline glyphosate solution[J]. CIESC Journal, 2019, 70(7): 2574-2583.
| 原料液编号 | 原料液组成/(g/L) | |
|---|---|---|
| 草甘膦 | NaCl | |
| 1 | 60 | |
| 2 | 80 | |
| 3 | 100 | |
| 4 | 1 | |
| 5 | 4 | |
| 6 | 7 | |
| 7 | 1 | 100 |
| 8 | 4 | 100 |
| 9 | 7 | 100 |
表1 实验中采用的原料液组成
Table1 Concentrations of feed solution
| 原料液编号 | 原料液组成/(g/L) | |
|---|---|---|
| 草甘膦 | NaCl | |
| 1 | 60 | |
| 2 | 80 | |
| 3 | 100 | |
| 4 | 1 | |
| 5 | 4 | |
| 6 | 7 | |
| 7 | 1 | 100 |
| 8 | 4 | 100 |
| 9 | 7 | 100 |

图4 DK膜对NaCl溶液的体积透过通量和截留率
Fig.4 Relationship between volume permeate flux and rejection rate of DK membrane on NaCl solution with operating pressure and concentration
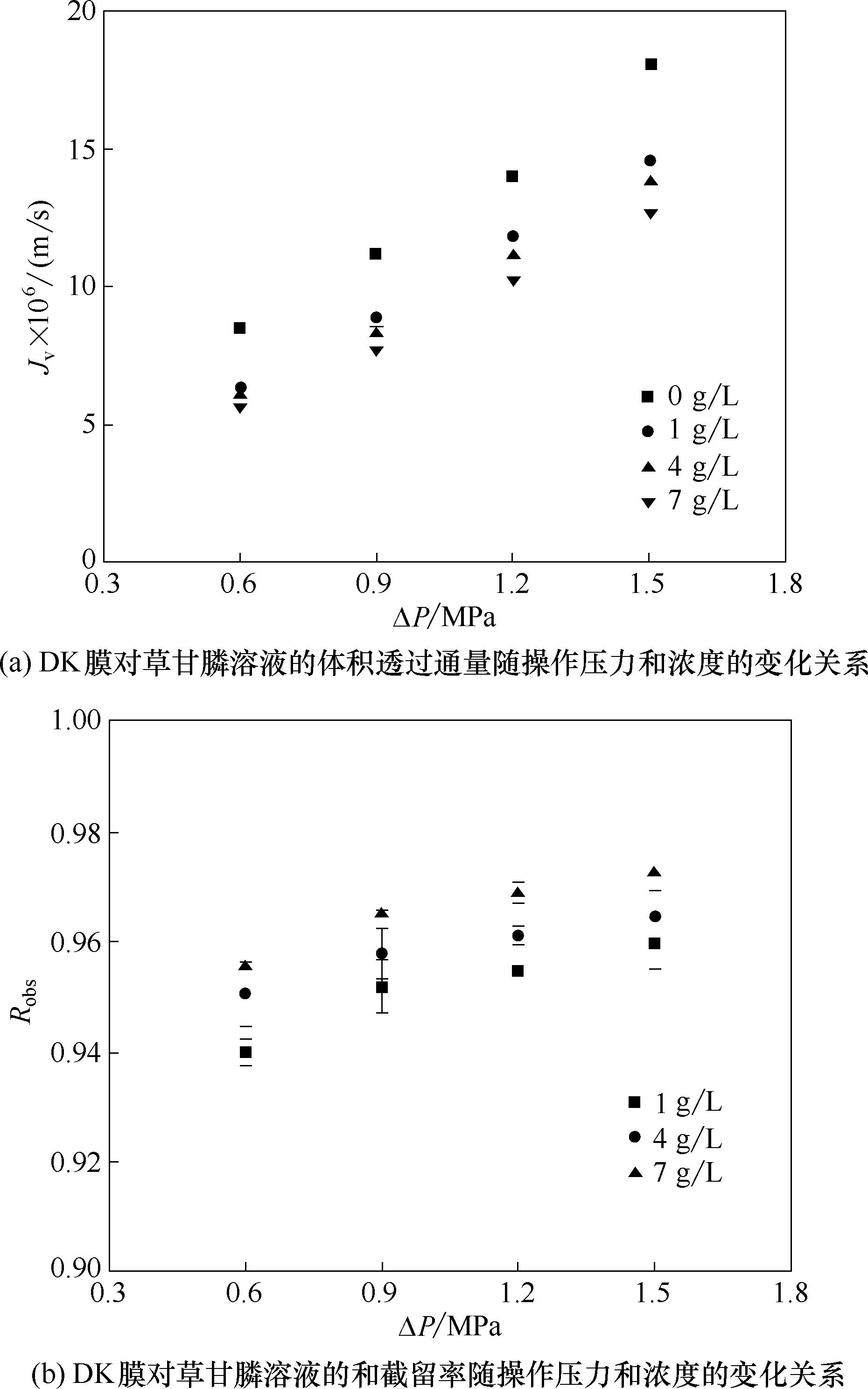
图5 DK膜对草甘膦溶液的体积透过通量和截留率
Fig.5 Volumetric flux and rejection of DK membrane to glyphosate solution as a function of operating pressure and concentration
| 溶质 | 浓度/(g/L) | σ | P×106/(m/s) |
|---|---|---|---|
| NaCl | 60 | 0.151 | 3.07 |
| 80 | 0.121 | 3.36 | |
| 100 | 0.119 | 3.89 | |
| 草甘膦 | 1 | 0.971 | 0.271 |
| 4 | 0.973 | 0.202 | |
| 7 | 0.985 | 0.198 |
表2 DK膜对实验体系的透过实验中膜特征参数和
Table 2 Fitting calculation results of membrane characteristic parameters and structural parameters of DK membrane on experimental system
| 溶质 | 浓度/(g/L) | σ | P×106/(m/s) |
|---|---|---|---|
| NaCl | 60 | 0.151 | 3.07 |
| 80 | 0.121 | 3.36 | |
| 100 | 0.119 | 3.89 | |
| 草甘膦 | 1 | 0.971 | 0.271 |
| 4 | 0.973 | 0.202 | |
| 7 | 0.985 | 0.198 |
| 操作压力/MPa | 草甘膦浓度/(g/L) | NaCl浓度/(g/L) | ||||
|---|---|---|---|---|---|---|
| 1 | 4 | 7 | 60 | 80 | 100 | |
| 0.6 | 0.048 | 0.046 | 0.044 | 0.0025 | 0.0015 | 0.00079 |
| 0.9 | 0.067 | 0.065 | 0.064 | 0.0039 | 0.0027 | 0.0017 |
| 1.2 | 0.094 | 0.091 | 0.087 | 0.0059 | 0.0042 | 0.0025 |
| 1.5 | 0.11 | 0.12 | 0.11 | 0.0079 | 0.0050 | 0.0034 |
表3 不同操作条件下的浓差极化程度表征
Table 3 Characterization of concentration polarization degree under different operating conditions
| 操作压力/MPa | 草甘膦浓度/(g/L) | NaCl浓度/(g/L) | ||||
|---|---|---|---|---|---|---|
| 1 | 4 | 7 | 60 | 80 | 100 | |
| 0.6 | 0.048 | 0.046 | 0.044 | 0.0025 | 0.0015 | 0.00079 |
| 0.9 | 0.067 | 0.065 | 0.064 | 0.0039 | 0.0027 | 0.0017 |
| 1.2 | 0.094 | 0.091 | 0.087 | 0.0059 | 0.0042 | 0.0025 |
| 1.5 | 0.11 | 0.12 | 0.11 | 0.0079 | 0.0050 | 0.0034 |

图7 DK膜对草甘膦与NaCl混合溶液的体积透过通量和截留率随操作压力和浓度的变化关系
Fig.7 Volumetric flux of DK membrane on glyphosate and NaCl mixed solution and interception rate as a function of operating pressure and concentration
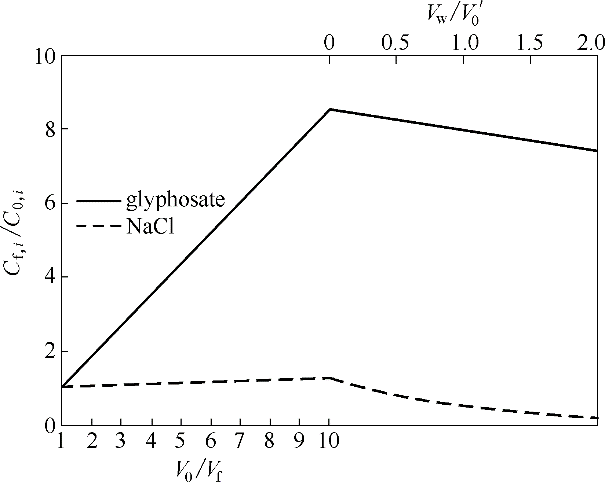
图8 预浓缩-连续恒容渗滤过程中料液中草甘膦和NaCl浓度比随浓缩倍数(V0/ Vf)与渗滤过程中水消耗量(Vw/ V0’)的变化关系
Fig.8 Relationship between concentration ratio of glyphosate and NaCl in feed solution during preconcentration-continuous constant volume percolation with concentration factor (V0/Vf ) and water consumption during percolation process (Vw/V0)
| 渗滤过程 | 水消耗量 | 料液 | 透过液 | ||||
|---|---|---|---|---|---|---|---|
| 体积 | c(草甘膦)/(g/L) | c(NaCl)/(g/L) | 体积 | c(草甘膦)/(g/L) | c(NaCl)/(g/L) | ||
| 初始 | — | V0 | 1.00 | 100.0 | — | — | — |
| 预浓缩后 | 0 | 1/10 V0 | 8.51 | 125.92 | 9/10 V0 | 0.17 | 97.12 |
| 连续恒容渗滤后 | 1/5 V0 | 1/10 V0 | 7.43 | 20.81 | 11/10 V0 | 0.24 | 89.02 |
表4 整个浓缩-渗滤过程中的水消耗量、料液及透过液的体积和浓度变化
Table 4 Variations of water consumption, volume and concentration of feed and permeate during whole concentration-infiltration process
| 渗滤过程 | 水消耗量 | 料液 | 透过液 | ||||
|---|---|---|---|---|---|---|---|
| 体积 | c(草甘膦)/(g/L) | c(NaCl)/(g/L) | 体积 | c(草甘膦)/(g/L) | c(NaCl)/(g/L) | ||
| 初始 | — | V0 | 1.00 | 100.0 | — | — | — |
| 预浓缩后 | 0 | 1/10 V0 | 8.51 | 125.92 | 9/10 V0 | 0.17 | 97.12 |
| 连续恒容渗滤后 | 1/5 V0 | 1/10 V0 | 7.43 | 20.81 | 11/10 V0 | 0.24 | 89.02 |
| 1 | ErikssonP. Nanofiltration extends the range of membrane filtration [J]. Environmental Progress, 1988, 7(1): 58-62. |
| 2 | MohammadA W, TeowY H, AngW L, et al. Nanofiltration membranes review: recent advances and future prospects[J]. Desalination, 2015, 356(S1): 226-254. |
| 3 | EsmiaC F, SchriveaL, BarreaY, et al. Using nanofiltration in a “zero-rejection” process: the removal of Ni2+ and Co2+ from salty wastewater[J]. Desalination and Water Treatment, 2013, 51(1/2/3): 476-484. |
| 4 | Perez-GonzalezA, IbanezR, GomezP, et al. Nanofiltration separation of polyvalent and monovalent anions in desalination brines[J]. Journal of Membrane Science, 2015, 473: 16-27. |
| 5 | BennaniC F, M’hiriO. Comparative study of the removal of heavy metals by two nanofiltration membranes[J]. Desalination and Water Treatment, 2015, 53(4): 1024-1030. |
| 6 | WangX L, ZhangC H, OuyangP. The possibility of separating saccharides from a NaCl solution by using nanofiltration in diafiltration mode[J]. Journal of Membrane Science, 2002, 204(1/2): 271-281. |
| 7 | ZhaoH F, HuaX, YangR J, et al. Diafiltration process on xylo-oligosaccharides syrup using nanofiltration and its modeling[J]. International Journal of Food Science and Technology, 2012, 47(1): 32-39. |
| 8 | MikulasekaP, CuhorkaJ. Desalination and concentration of liquid dyes by nanofiltration[J]. Desalination and Water Treatment, 2015, 55(10): 2711-2720. |
| 9 | HeY, LiG, WangH, et al. Diafiltration and water recovery of Reactive Brilliant Blue KN-R solution by two-stage membrane separation process[J]. Chemical Engineering and Processing, 2010, 49(5): 476-483. |
| 10 | TuC H, FangY Y, ZhuJ, et al. Free energies of the ion equilibrium partition of KCl into nanofiltration membranes based on applied electrical potential and rejection[J]. Langmuir, 2011, 27(16): 10274-10281. |
| 11 | TuC H, WangH L, WangX L. Study on transmembrane electrical potential of nanofiltration membranes in KCl and MgCl2 solutions[J]. Langmuir, 2010, 26(22): 17656-17664. |
| 12 | TuC H, WuL, WangD X, et al. Prediction of separation performance of NF membranes for mixed electrolytes solution[J]. Desalination, 2010, 260(1/2/3): 218-224. |
| 13 | WangD X, SuM, YuZ Y, et al. Separation performance of a nanofiltration membrane influenced by species and concentration of ions[J]. Desalination, 2005, 175(2): 219-225. |
| 14 | SuM, WangD X, WangX L, et al. Rejection of ions by NF membranes for binary electrolyte solutions of NaCl, NaNO3, CaCl2 and Ca(NO3)2[J]. Desalination, 2006, 191(1/2/3): 303-308. |
| 15 | MurthyZ V P, ChaudhariL B. Rejection behavior of nickel ions from synthetic wastewater containing Na2SO4, NiSO4, MgCl2 and CaCl2 salts by nanofiltration and characterization of the membrane[J]. Desalination, 2009, 247(1/2/3): 610-622. |
| 16 | WuL, SongL, WangX L, et al. Experimental study on separation performance of nanofiltration membranes for bicarbonate salts solution[J]. Desalination, 2009, 236(1/2/3): 299-305. |
| 17 | TanninenJ, ManttariM, NystromM. Effect of salt mixture concentration on fractionation with NF membranes[J]. Journal of Membrane Science, 2006, 283(1/2): 57-64. |
| 18 | Al-ZoubiH, OmarW. Rejection of salt mixtures from high saline by nanofiltration membranes[J]. Korean Journal of Chemical Engineering, 2009, 26(3): 799-805. |
| 19 | SilvaV, GeraldesV, Brites AlvesA M, et al. Multi-ionic nanofiltration of highly concentrated salt mixtures in the seawater range[J]. Desalination, 2011, 277(1/2/3): 29-39. |
| 20 | HilalN, KochkodanV, Al AbdulgaderH, et al. A combined ion exchange–nanofiltration process for water desalination(Ⅱ): Membrane selection[J]. Desalination, 2015, 363(S1): 51-57. |
| 21 | YaroshchukA E. Non-steric mechanisms of nanofiltration: superposition of Donnan and dielectric exclusion[J]. Separation and Purification Technology, 2001, 22/22/23(S1): 143-158. |
| 22 | 吴哲峰. 草甘膦生产线上副产固体盐精制的研究[D]. 浙江: 浙江理工大学, 2015. |
| WuZ F. Study on the purification of the buproduct salt from the glyphosate production process[D]. Zhejiang: Zhejiang Sci-tech University, 2015. | |
| 23 | XieM, LiuZ Y, XuY H, Removal of glyphosate in neutralization liquor from the glycine-dimethylphosphit process by nanofiltration[J]. Journal of Hazardous Materials, 2010, 181(1/2/3): 975-980. |
| 24 | 谢明, 刘志英, 赵贤广, 等. 草甘膦模拟废水的纳滤分离过程研究[J]. 环境工程学报, 2010, 4(7): 1483-1487. |
| XieM, LiuZ Y, ZhaoX G, et al. Nanofiltration separation process of model glyphosate wastewater[J]. Chinese Journal of Environmental Engineering, 2010, 4(7): 1483-1487. | |
| 25 | 黄华, 李雪梅, 潘成, 等. SPEEK涂层纳滤膜处理草甘膦废水[J]. 环境工程学报, 2012, 6(8): 2489-2494. |
| HuangH, LiX M, PanC, et al. Treatment of glyphosate containing wastewater using SPEEK coated nanofiltration membranes[J]. Chinese Journal of Environmental Engineering, 2012, 6(8): 2489-2494. | |
| 26 | SongJ F, LiX M, AlbetoF, et al. Composite hollow fiber nanofiltration membranes for recovery of glyphosate from saline wastewater[J]. Water Research, 2013, 47(6): 2065-2074. |
| 27 | YuanJ, DuanJ M, SaintC P. Removal of glyphosate and aminomethylphosphonic acid from synthetic water by nanofiltration[J]. Environmental Technology, 2018, 39(11): 1384-1392. |
| 28 | 徐演初, 吴礼光, 沈江南, 等. 一种集成膜分离浓缩草甘膦母液方法: 1775786B [P]. 2011. |
| XuY C, WuL G, ShenJ N, et al. A method for separating and separating glyphosate mother liquor by integrated membrane: 1775786B [P]. 2011. | |
| 29 | ShenJ N, HuangJ, RuanH M, et al. Techno-economic analysis of resource recovery of glyphosate liquor by membrane technology[J]. Desalination, 2014, 342(S1): 118-125. |
| 30 | ShenJ N, HuangJ, LiuL F, et al. The use of BMED for glyphosate recovery from glyphosate neutralization liquor in view of zero discharge[J]. Journal of Hazardous Materials, 2013, 260(18): 660-667. |
| 31 | 中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. 草甘膦原药: GB/T 12686—2017[S]. 北京: 中国标准出版社, 2017. |
| General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China. Glyphosate technical: GB/T 12686—2017[S]. Beijing: Standards Press of China, 2017. | |
| 32 | SpieglerK S, KedemO. Thermodynamics of hyperfiltration (reverse osmosis): criteria for efficient membranes[J]. Desalination 1966, 1(S1): 311-326. |
| 33 | SchockG, MiquelA. Mass transfer and pressure loss in spiral wound modules[J]. Desalination, 1987, 64(S1): 339-352. |
| 34 | HilalN, Al-zoubiH, MohammadA W, et al. Nanofiltration of highly concentrated salt solutions up to seawater salinity [J]. Desalination, 2005, 184(1/2/3): 315-326. |
| 35 | 燕至琴. 高浓一二价盐溶液的纳滤膜分离性能研究[D]. 北京: 清华大学, 2016. |
| YanZ Q. Research on separation performance of mono and divalent salt solution with high concentration by nanofiltration membrane [D]. Beijing: Tsinghua University, 2016. |
| [1] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [2] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [3] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [4] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [5] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [6] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [7] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [8] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| [9] | 高金明, 郭玉娇, 鄂承林, 卢春喜. 一种封闭罩内顺流多旋臂气液分离器的分离特性研究[J]. 化工学报, 2023, 74(7): 2957-2966. |
| [10] | 文兆伦, 李沛睿, 张忠林, 杜晓, 侯起旺, 刘叶刚, 郝晓刚, 官国清. 基于自热再生的隔壁塔深冷空分工艺设计及优化[J]. 化工学报, 2023, 74(7): 2988-2998. |
| [11] | 韩奎奎, 谭湘龙, 李金芝, 杨婷, 张春, 张永汾, 刘洪全, 于中伟, 顾学红. 四通道中空纤维MFI分子筛膜用于二甲苯异构体分离[J]. 化工学报, 2023, 74(6): 2468-2476. |
| [12] | 朱兴驰, 郭志远, 纪志永, 汪婧, 张盼盼, 刘杰, 赵颖颖, 袁俊生. 选择性电渗析镁锂分离过程模拟优化[J]. 化工学报, 2023, 74(6): 2477-2485. |
| [13] | 张艳梅, 袁涛, 李江, 刘亚洁, 孙占学. 高效SRB混合菌群构建及其在酸胁迫条件下的性能研究[J]. 化工学报, 2023, 74(6): 2599-2610. |
| [14] | 张兰河, 赖青燚, 王铁铮, 关潇卓, 张明爽, 程欣, 徐小惠, 贾艳萍. H2O2对SBR脱氮效率和污泥性能的影响[J]. 化工学报, 2023, 74(5): 2186-2196. |
| [15] | 王蕾, 王磊, 白云龙, 何柳柳. SA膜状锂离子筛的制备及其锂吸附性能[J]. 化工学报, 2023, 74(5): 2046-2056. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号