化工学报 ›› 2020, Vol. 71 ›› Issue (10): 4429-4444.DOI: 10.11949/0438-1157.20200612
收稿日期:2020-05-19
修回日期:2020-07-28
出版日期:2020-10-05
发布日期:2020-10-05
通讯作者:
朱昌宝
作者简介:张志波(1995—),男,博士研究生,基金资助:
Zhibo ZHANG( ),Kunyao PENG,Maoning GENG,Xinyue ZHAO,Si LIU,Changbao ZHU(
),Kunyao PENG,Maoning GENG,Xinyue ZHAO,Si LIU,Changbao ZHU( )
)
Received:2020-05-19
Revised:2020-07-28
Online:2020-10-05
Published:2020-10-05
Contact:
Changbao ZHU
摘要:
由于资源和成本优势,以及工作原理与锂离子电池的相似性,钾离子电池在未来的大规模储能应用中有着光明的发展前景。然而相比于锂、钠离子,钾离子半径较大,这不仅影响了其在电极中的输运,而且容易对电极材料的结构造成一些不可逆的破坏,进而导致较差的电化学性能。对于钾离子电池,负极可采用与锂离子电池相同的石墨负极,而正极材料是目前的研发高性能钾离子电池的关键。因此,本文在总结了最常见的四类钾离子电池正极材料的相关进展,并分别探讨了各自的优势、问题及相应的改性方法的基础上,展望了钾离子电池正极材料未来的发展。
中图分类号:
张志波, 彭琨尧, 耿茂宁, 赵昕悦, 刘思, 朱昌宝. 钾离子电池正极材料的研究进展[J]. 化工学报, 2020, 71(10): 4429-4444.
Zhibo ZHANG, Kunyao PENG, Maoning GENG, Xinyue ZHAO, Si LIU, Changbao ZHU. Recent progress on cathode materials for potassium-ion batteries[J]. CIESC Journal, 2020, 71(10): 4429-4444.
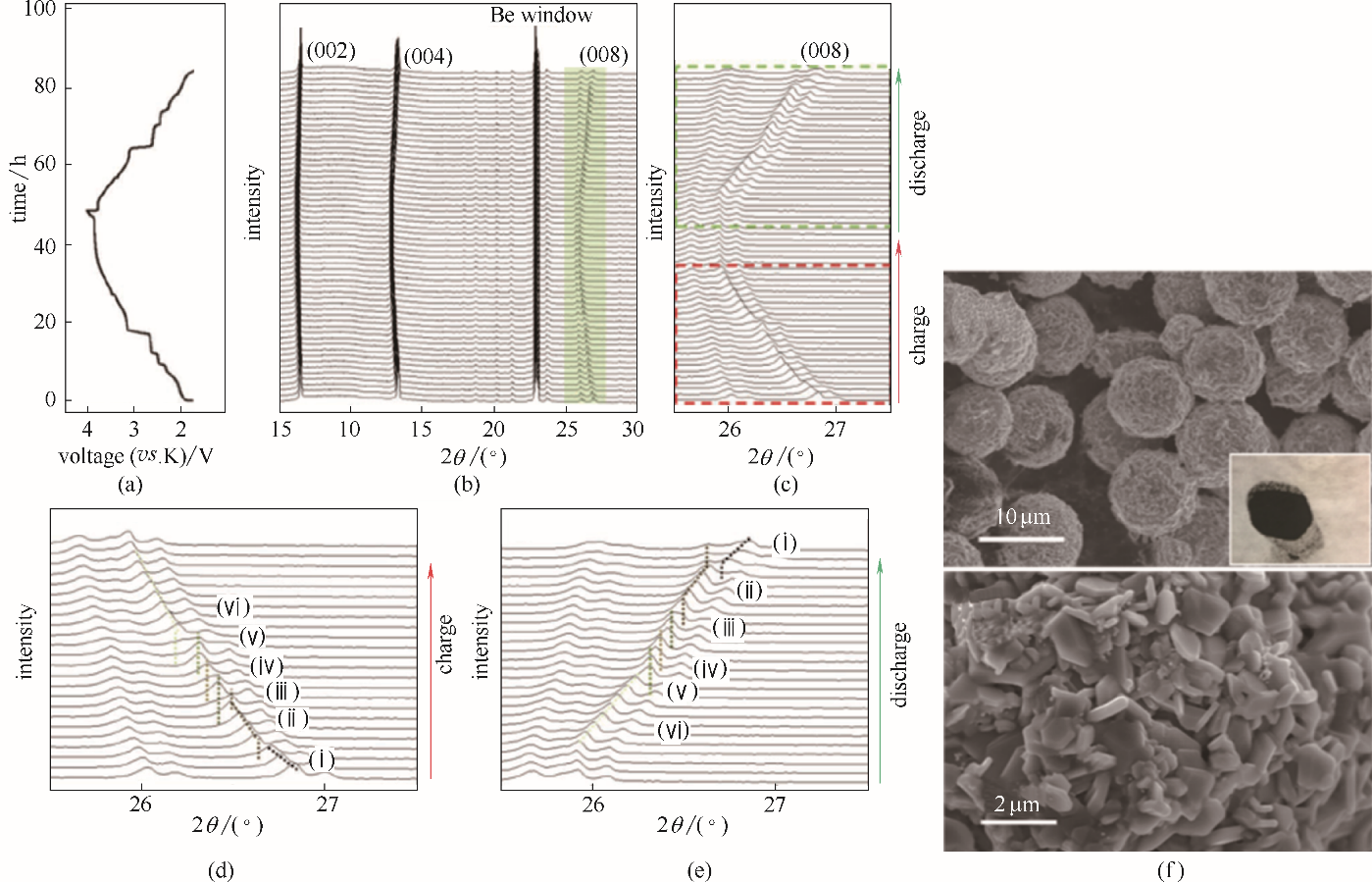
图3 P2-K0.6CoO2充放电时的原位XRD图[(a)~(e)][19]和自模板合成的P2-K0.6CoO2微球的SEM图(f)[24]
Fig.3 In situ XRD characterization of P2-type K0.6CoO2 during charge/discharge process[(a)—(e)][19] and the SEM images of P2-type K0.6CoO2 that made by self-templated method(f)[24]
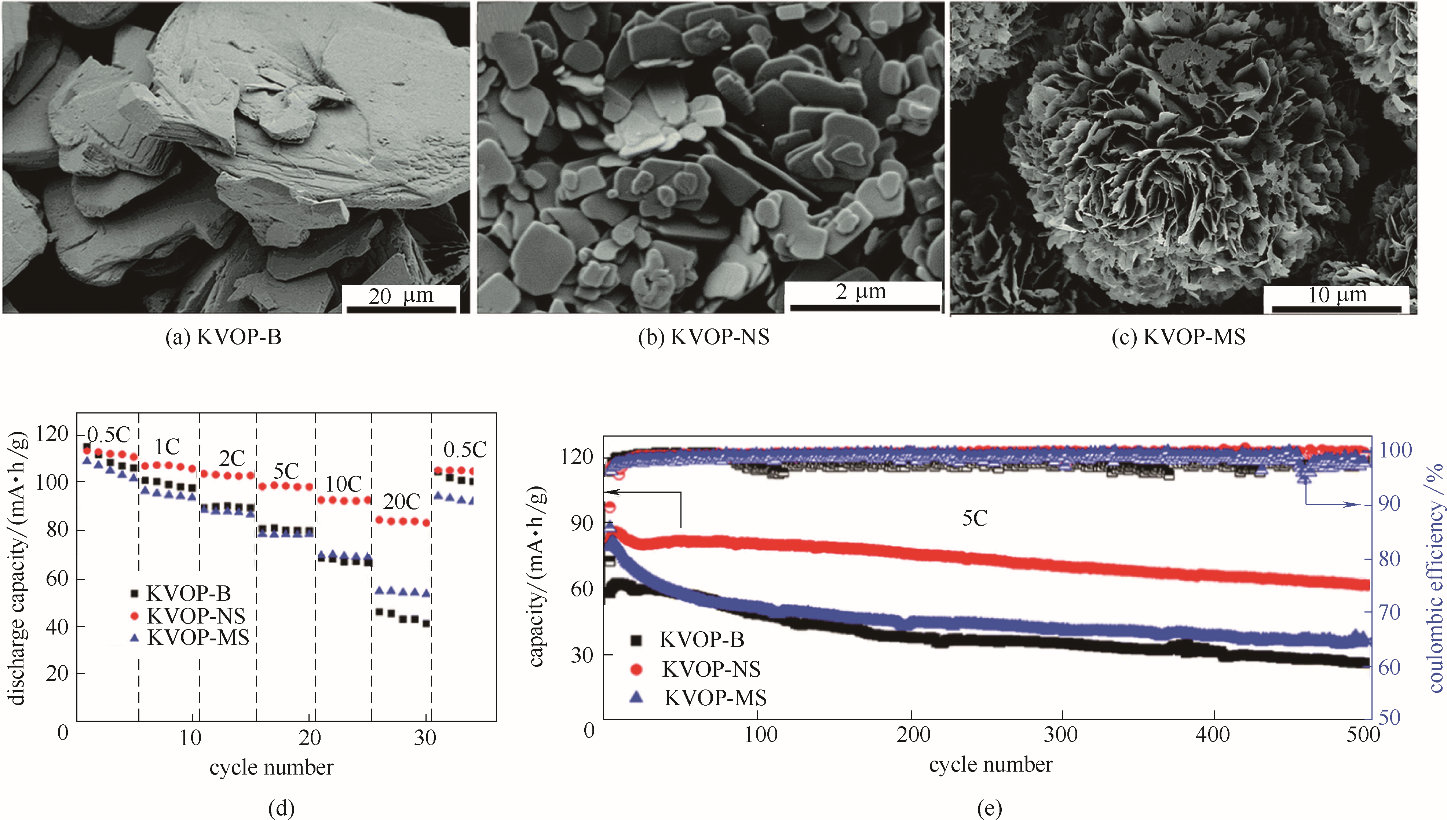
图4 KVOP-B、KVOP-NS和KVOP-MS的SEM图[(a)~(c)]和电化学性能[(d)、(e)][50]
Fig.4 SEM images[(a)—(c)], electrochemical performance[(d),(e)] of KVOP-B, KVOP-NS and KVOP-MS[50]

图6 MnHCF的形貌(a)、电化学性能[(b)、(c)][68]及其在储钾过程中的结构演变过程(d)[65]
Fig.6 STEM image(a), electrochemical performance[(b),(c)][68] and structural evolution (d) of MnHCF in process of K ions storage[65]

图7 PTCDI-DAQ 的循环伏安图(a)、不同充放电状态下的[对应(a)图中的状态]非原位FTIR光谱图(b)和其在钾离子电池中的反应机制示意图(c)[84]
Fig.7 The CV curves(a), ex situ FTIR spectroscopy under different charge and discharge states [corresponding to the states in (a)] (b) and the proposed redox mechanism (c) for PTCDI-DAQ in K-ion batteries[84]
| 材料类型 | 典型材料 | 电压 范围/V | 倍率性能 (电流密度,容量) | 循环性能 (电流密度;圈数; 保持率) | 文献 |
|---|---|---|---|---|---|
| 层状过渡金属氧化物 | P2-K0.6CoO2 | 1.7~4.0 | 10 mA/g,82 mA·h/g;100 mA/g,65 mA·h/g | 40 mA/g;300;87% | [ |
| P2-K0.65Fe0.5Mn0.5O2 | 1.5~4.2 | 20 mA/g,151 mA·h/g;100 mA/g,103 mA·h/g | 100 mA/g;350;78% | [ | |
| P′3-K0.8CrO2 | 1.5~3.8 | 11 mA/g,91 mA·h/g;436 mA/g,52 mA·h/g | 218 mA/g;300;99% | [ | |
| 聚阴离子型化合物 | KVOPO4 | 2.0~4.6 | 0.5 C,113.1 mA·h/g;20 C,83.4 mA·h/g | 5 C;500;75.6% | [ |
| KVPO4F | 2.0~5.0 | 0.5 C,101.8 mA·h/g;50 C,87.6 mA·h/g | 0.5 C;100;84.3% | [ | |
| K4Fe3(PO4)2(P2O7) | 2.1~4.1 | 0.05 C,~118 mA·h/g;5 C,~83 mA·h/g | 5 C;500;82% | [ | |
| 普鲁士蓝及其类似物 | K1.70Mn[Fe(CN)6]0.90·1.10H2O | 2.5~4.6 | 0.2 C,142.4 mA·h/g;2 C,~93 mA·h/g | 1 C;100;77% | [ |
| K1.69Fe[Fe(CN)6 ]0.90·4H2O | 2~4.5 | 10 mA/g,140 mA·h/g;100 mA/g,120 mA·h/g | 100 mA/g;300;60% | [ | |
| K1.81Ni[Fe(CN)6]0.97?0.086H2O | 2~4.5 | 10 mA/g,57 mA·h/g;500 mA/g,13.1 mA·h/g | 50 mA/g;1000;87.3% | [ | |
| 有机正极材料 | PTCDA | 1.5~3.5 | 10 mA/g,131 mA·h/g;500 mA/g,73 mA·h/g | 50 mA/g;200;66.1% | [ |
| AQDS | 1.4~3.0 | 0.1 C,95 mA·h/g;3 C,56 mA·h/g | 0.1 C;100;82.4% | [ | |
| PTCDI-DAQ | 1~3.8 | 15 C,202 mA·h/g;100 C,133 mA·h/g | 15 C;900;72.7% | [ |
表1 四类钾电正极材料中典型材料的性能总结
Table 1 The performance of typical four types of cathode electrode materials for PIBs
| 材料类型 | 典型材料 | 电压 范围/V | 倍率性能 (电流密度,容量) | 循环性能 (电流密度;圈数; 保持率) | 文献 |
|---|---|---|---|---|---|
| 层状过渡金属氧化物 | P2-K0.6CoO2 | 1.7~4.0 | 10 mA/g,82 mA·h/g;100 mA/g,65 mA·h/g | 40 mA/g;300;87% | [ |
| P2-K0.65Fe0.5Mn0.5O2 | 1.5~4.2 | 20 mA/g,151 mA·h/g;100 mA/g,103 mA·h/g | 100 mA/g;350;78% | [ | |
| P′3-K0.8CrO2 | 1.5~3.8 | 11 mA/g,91 mA·h/g;436 mA/g,52 mA·h/g | 218 mA/g;300;99% | [ | |
| 聚阴离子型化合物 | KVOPO4 | 2.0~4.6 | 0.5 C,113.1 mA·h/g;20 C,83.4 mA·h/g | 5 C;500;75.6% | [ |
| KVPO4F | 2.0~5.0 | 0.5 C,101.8 mA·h/g;50 C,87.6 mA·h/g | 0.5 C;100;84.3% | [ | |
| K4Fe3(PO4)2(P2O7) | 2.1~4.1 | 0.05 C,~118 mA·h/g;5 C,~83 mA·h/g | 5 C;500;82% | [ | |
| 普鲁士蓝及其类似物 | K1.70Mn[Fe(CN)6]0.90·1.10H2O | 2.5~4.6 | 0.2 C,142.4 mA·h/g;2 C,~93 mA·h/g | 1 C;100;77% | [ |
| K1.69Fe[Fe(CN)6 ]0.90·4H2O | 2~4.5 | 10 mA/g,140 mA·h/g;100 mA/g,120 mA·h/g | 100 mA/g;300;60% | [ | |
| K1.81Ni[Fe(CN)6]0.97?0.086H2O | 2~4.5 | 10 mA/g,57 mA·h/g;500 mA/g,13.1 mA·h/g | 50 mA/g;1000;87.3% | [ | |
| 有机正极材料 | PTCDA | 1.5~3.5 | 10 mA/g,131 mA·h/g;500 mA/g,73 mA·h/g | 50 mA/g;200;66.1% | [ |
| AQDS | 1.4~3.0 | 0.1 C,95 mA·h/g;3 C,56 mA·h/g | 0.1 C;100;82.4% | [ | |
| PTCDI-DAQ | 1~3.8 | 15 C,202 mA·h/g;100 C,133 mA·h/g | 15 C;900;72.7% | [ |
| 1 | Whittingham M S. Ultimate limits to intercalation reactions for lithium batteries[J]. Chemical Reviews, 2014, 114(23): 11414-11443. |
| 2 | Hy S, Liu H, Zhang M, et al. Performance and design considerations for lithium excess layered oxide positive electrode materials for lithium ion batteries[J]. Energy & Environmental Science, 2016, 9(6): 1931-1954. |
| 3 | Hosaka T, Shimamura T, Kubota K, et al. Polyanionic compounds for potassium-ion batteries[J]. Chemical Record, 2019, 19(4): 735-745. |
| 4 | Hwang J Y, Myung S T, Sun Y K. Sodium-ion batteries: present and future[J]. Chemical Society Reviews, 2017, 46(12): 3529-3614. |
| 5 | Liu T, Zhang Y, Jiang Z, et al. Exploring competitive features of stationary sodium ion batteries for electrochemical energy storage[J]. Energy & Environmental Science, 2019, 12(5): 1512-1533. |
| 6 | Wang T, Su D, Shanmukaraj D, et al. Electrode materials for sodium-ion batteries: considerations on crystal structures and sodium storage mechanisms[J]. Electrochemical Energy Reviews, 2018, 1(2): 200-237. |
| 7 | Matsuura N, Umemoto K, Takeuchi Z I. Standard potentials of alkali metals, silver, and thallium metal/ion couples in N, N'-dimethylformamide, dimethyl sulfoxide, and propylene carbonate[J]. Bulletin of the Chemical Society of Japan, 1974, 47(4): 813-817. |
| 8 | Komaba S, Hasegawa T, Dahbi M, et al. Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors[J]. Electrochemistry Communications, 2015, 60: 172-175. |
| 9 | Luo W, Wan J, Ozdemir B, et al. Potassium ion batteries with graphitic materials[J]. Nano Letters, 2015, 15(11): 7671-7677. |
| 10 | Ren X, Zhao Q, McCulloch W D, et al. MoS2 as a long-life host material for potassium ion intercalation[J]. Nano Research, 2017, 10(4): 1313-1321. |
| 11 | An Y, Liu Y, Tian Y, et al. Recent development and prospect of potassium-ion batteries with high energy and high safety for post-lithium batteries[J]. Functional Materials Letters, 2019, 12(4): 1930002. |
| 12 | Kubota K, Dahbi M, Hosaka T, et al. Towards K-ion and Na-ion batteries as “beyond Li-ion”[J]. The Chemical Record, 2018, 18(4): 459-479. |
| 13 | Eftekhari A, Jian Z, Ji X. Potassium secondary batteries[J]. ACS Applied Materials & Interfaces, 2017, 9(5): 4404-4419. |
| 14 | Zhao J, Zou X, Zhu Y, et al. Electrochemical intercalation of potassium into graphite[J]. Advanced Functional Materials, 2016, 26(44): 8103-8110. |
| 15 | Yang C, Feng J, Lv F, et al. Metallic graphene-like VSe2 ultrathin nanosheets: superior potassium-ion storage and their working mechanism[J]. Advanced Materials, 2018, 30(27): 1800036. |
| 16 | Zhang Q, Mao J, Pang W K, et al. Boosting the potassium storage performance of alloy-based anode materials via electrolyte salt chemistry[J]. Advanced Energy Materials, 2018, 8(15): 1703288. |
| 17 | He P, Yu H J, Li D, et al. Layered lithium transition metal oxide cathodes towards high energy lithium-ion batteries[J]. Journal of Materials Chemistry, 2012, 22(9): 3680-3695. |
| 18 | Han M H, Gonzalo E, Singh G, et al. A comprehensive review of sodium layered oxides: powerful cathodes for Na-ion batteries[J]. Energy & Environmental Science, 2015, 8(1): 81-102. |
| 19 | Kim H, Kim J C, Bo S H, et al. K-ion batteries based on a P2-type K0.6CoO2 cathode[J]. Advanced Energy Materials, 2017, 7(17): 1700098. |
| 20 | Kim H, Seo D H, Urban A, et al. Stoichiometric layered potassium transition metal oxide for rechargeable potassium batteries[J]. Chemistry of Materials, 2018, 30(18): 6532-6539. |
| 21 | Kim H, Seo D H, Kim J C, et al. Investigation of potassium storage in layered P3-type K0.5MnO2 cathode[J]. Advanced Materials, 2017, 29(37): 1702480. |
| 22 | Yabuuchi N, Hara R, Kajiyama M, et al. New O2/P2-type Li-excess layered manganese oxides as promising multi-functional electrode materials for rechargeable Li/Na batteries[J]. Advanced Energy Materials, 2014, 4(13): 1301453. |
| 23 | Hironaka Y, Kubota K, Komaba S. P2- and P3-KxCoO2 as an electrochemical potassium intercalation host[J]. Chemical Communications, 2017, 53(26): 3693-3696. |
| 24 | Deng T, Fan X L, Luo C, et al. Self-templated formation of P2-type K0.6CoO2 microspheres for high reversible potassium-ion batteries[J]. Nano Letters, 2018, 18(2): 1522-1529. |
| 25 | Sada K, Barpanda P. P3-type layered K0.48Mn0.4Co0.6O2: a novel cathode material for potassium-ion batteries[J]. Chemical Communications, 2020, 56(15): 2272-2275. |
| 26 | Liu C L, Luo S H, Huang H B, et al. Layered potassium-deficient P2- and P3-type cathode materials KxMnO2 for K-ion batteries[J]. Chemical Engineering Journal, 2019, 356: 53-59. |
| 27 | Chong S K, Wu Y F, Chen Y Z, et al. Mn-based layered oxide microspheres assembled by ultrathin nanosheets as cathode material for potassium-ion batteries[J]. Electrochimica Acta, 2019, 293: 299-306. |
| 28 | Deng T, Fan X L, Chen J, et al. Layered P2-type K0.65Fe0.5Mn0.5O2 microspheres as superior cathode for high-energy potassium-ion batteries[J]. Advanced Functional Materials, 2018, 28(28): 1800219. |
| 29 | Liu C L, Luo S H, Huang H B, et al. Fe-doped layered P3-type K0.45Mn1-xFexO2 (x ≤ 0.5) as cathode materials for low-cost potassium-ion batteries[J]. Chemical Engineering Journal, 2019, 378: 1345-1353. |
| 30 | Choi J U, Kim J, Hwang J Y, et al. K0.54[Co0.5Mn0.5]O2: new cathode with high power capability for potassium-ion batteries[J]. Nano Energy, 2019, 61: 284-294. |
| 31 | Zhang Q, Didier C, Pang W K, et al. Structural insight into layer gliding and lattice distortion in layered manganese oxide electrodes for potassium-ion batteries[J]. Advanced Energy Materials, 2019, 9(30): 1900568. |
| 32 | Zhang X Y, Yang Y B, Qu X L, et al. Layered P2-type K0.44Ni0.22Mn0.78O2 as a high-performance cathode for potassium-ion batteries[J]. Advanced Functional Materials, 2019, 29(49): 1905679. |
| 33 | Liu C L, Luo S H, Huang H B, et al. Low-cost layered K0.45Mn0.9Mg0.1O2 as a high-performance cathode material for K-ion batteries[J]. ChemElectroChem, 2019, 6(8): 2308-2315. |
| 34 | Liu C L, Luo S H, Huang H B, et al. Influence of Na-substitution on the structure and electrochemical properties of layered oxides K0.67Ni0.17Co0.17Mn0.66O2 cathode materials[J]. Electrochimica Acta, 2018, 286: 114-122. |
| 35 | Sada K, Senthilkumar B, Barpanda P. Potassium-ion intercalation mechanism in layered Na2Mn3O7[J]. ACS Applied Energy Materials, 2018, 1(10): 5410-5416. |
| 36 | Lin B W, Zhu X H, Fang L Z, et al. Birnessite nanosheet arrays with high K content as a high-capacity and ultrastable cathode for K-ion batteries[J]. Advanced Materials, 2019, 31(24): 1900060. |
| 37 | Hwang J Y, Kim J, Yu T Y, et al. Development of P3-K0.69CrO2 as an ultra-high-performance cathode material for K-ion batteries[J]. Energy & Environmental Science, 2018, 11(10): 2821-2827. |
| 38 | Naveen N, Park W B, Singh S P, et al. KCrS2 cathode with considerable cyclability and high rate performance: the first K+ stoichiometric layered compound for potassium-ion batteries[J]. Small, 2018, 14(49): 1803495. |
| 39 | Naveen N, Han S C, Singh S P, et al. Highly stable P'3-K0.8CrO2 cathode with limited dimensional changes for potassium ion batteries[J]. Journal of Power Sources, 2019, 430: 137-144. |
| 40 | Deng L Q, Niu X G, Ma G S, et al. Layered potassium vanadate K0.5V2O5 as a cathode material for nonaqueous potassium ion batteries[J]. Advanced Functional Materials, 2018, 28(49): 1800670. |
| 41 | Kim H, Park I, Seo D H, et al. New iron-based mixed-polyanion cathodes for lithium and sodium rechargeable batteries: combined first principles calculations and experimental study[J]. Journal of the American Chemical Society, 2012, 134(25): 10369-10372. |
| 42 | Han J, Li G N, Liu F, et al. Investigation of K3V2(PO4)3/C nanocomposites as high-potential cathode materials for potassium-ion batteries[J]. Chemical Communications, 2017, 53(11): 1805-1808. |
| 43 | Zhang L, Zhang B, Wang C, et al. Constructing the best symmetric full K-ion battery with the NASICON-type K3V2(PO4)3[J]. Nano Energy, 2019, 60: 432-439. |
| 44 | Zheng S, Cheng S, Xiao S, et al. Partial replacement of K by Rb to improve electrochemical performance of K3V2(PO4)3 cathode material for potassium-ion batteries[J]. Journal of Alloys and Compounds, 2020, 815: 152379. |
| 45 | Hyoung J, Heo J W, Chae M S, et al. Electrochemical exchange reaction mechanism and the role of additive water to stabilize the structure of VOPO4·2H2O as a cathode material for potassium-ion batteries[J]. ChemSusChem, 2019, 12(5): 1069-1075. |
| 46 | Mathew V, Kim S, Kang J, et al. Amorphous iron phosphate: potential host for various charge carrier ions[J]. NPG Asia Materials, 2014, 6(10): e138. |
| 47 | Sultana I, Rahman M M, Mateti S, et al. Approaching leactive KFePO4 phase for potassium storage by adopting an advanced design strategy[J]. Batteries & Supercaps, 2020, 3(5): 450-455. |
| 48 | Chihara K, Katogi A, Kubota K, et al. KVPO4F and KVOPO4 toward 4 volt-class potassium-ion batteries[J]. Chemical Communications, 2017, 53(37): 5208-5211. |
| 49 | Lian R, Wang D, Ming X, et al. Phase transformation, ionic diffusion, and charge transfer mechanisms of KVOPO4 in potassium ion batteries: first-principles calculations[J]. Journal of Materials Chemistry A, 2018, 6(33): 16228-16234. |
| 50 | Liao J, Hu Q, Che B, et al. Competing with other polyanionic cathode materials for potassium-ion batteries via fine structure design: new layered KVOPO4 with a tailored particle morphology[J]. Journal of Materials Chemistry A, 2019, 7(25): 15244-15251. |
| 51 | Liao J, Hu Q, He X, et al. A long lifespan potassium-ion full battery based on KVPO4F cathode and VPO4 anode[J]. Journal of Power Sources, 2020, 451: 227739. |
| 52 | Kim H, Seo D H, Bianchini M, et al. A new strategy for high-voltage cathodes for K-ion batteries: stoichiometric KVPO4F[J]. Advanced Energy Materials, 2018, 8(26): 1801591. |
| 53 | Lin X, Huang J, Tan H, et al. K3V2(PO4)2F3 as a robust cathode for potassium-ion batteries[J]. Energy Storage Materials, 2019, 16: 97-101. |
| 54 | Kim H, Ishado Y, Tian Y, et al. Investigation of alkali-ion (Li, Na, and K) intercalation in KxVPO4F (x∼0) cathode[J]. Advanced Functional Materials, 2019, 29(34): 1902392. |
| 55 | Park W B, Han S C, Park C, et al. KVP2O7 as a robust high-energy cathode for potassium-ion batteries: pinpointed by a full screening of the inorganic registry under specific search conditions[J]. Advanced Energy Materials, 2018, 8(13): 1703099. |
| 56 | Nose M, Nakayama H, Nobuhara K, et al. Na4Co3(PO4)2P2O7: a novel storage material for sodium-ion batteries[J]. Journal of Power Sources, 2013, 234: 175-179. |
| 57 | Kim H, Park I, Lee S, et al. Understanding the electrochemical mechanism of the new iron-based mixed-phosphate Na4Fe3(PO4)2(P2O7) in a Na rechargeable battery[J]. Chemistry of Materials, 2013, 25(18): 3614-3622. |
| 58 | Park H, Kim H, Ko W, et al. Development of K4Fe3(PO4)2(P2O7) as a novel Fe-based cathode with high energy densities and excellent cyclability in rechargeable potassium batteries[J]. Energy Storage Materials, 2020, 28: 47-54. |
| 59 | Recham N, Rousse G, Sougrati M T, et al. Preparation and characterization of a stable FeSO4F-based framework for alkali ion insertion electrodes[J]. Chemistry of Materials, 2012, 24(22): 4363-4370. |
| 60 | Ko W, Park H, Jo J H, et al. Unveiling yavapaiite-type KFe(SO4)2 as a new Fe-based cathode with outstanding electrochemical performance for potassium-ion batteries[J]. Nano Energy, 2019, 66: 104184. |
| 61 | Tokoro H, Ohkoshi S. Novel magnetic functionalities of Prussian blue analogs[J]. Dalton Transactions, 2011, 40(26): 6825-6833. |
| 62 | Eftekhari A. Potassium secondary cell based on Prussian blue cathode[J]. Journal of Power Sources, 2004, 126(1/2): 221-228. |
| 63 | Ling C, Chen J J, Mizuno F. First-principles study of alkali and alkaline earth ion intercalation in iron hexacyanoferrate: the important role of ionic radius[J]. Journal of Physical Chemistry C, 2013, 117(41): 21158-21165. |
| 64 | Morant-Giner M, Sanchis-Gual R, Romero J, et al. Prussian blue@MoS2 layer composites as highly efficient cathodes for sodium- and potassium-ion batteries[J]. Advanced Functional Materials, 2018, 28(27): 1706125. |
| 65 | Bie X F, Kubota K, Hosaka T, et al. A novel K-ion battery: hexacyanoferrate(Ⅱ)/graphite cell[J]. Journal of Materials Chemistry A, 2017, 5(9): 4325-4330. |
| 66 | You Y, Wu X L, Yin Y X, et al. High-quality Prussian blue crystals as superior cathode materials for room-temperature sodium-ion batteries[J]. Energy & Environmental Science, 2014, 7(5): 1643-1647. |
| 67 | Zhang C, Xu Y, Zhou M, et al. Potassium Prussian blue nanoparticles: a low-cost cathode material for potassium‐ion batteries[J]. Advanced Functional Materials, 2017, 27(4): 1604307. |
| 68 | Xue L G, Li Y T, Gao H C, et al. Low-cost high-energy potassium cathode[J]. Journal of the American Chemical Society, 2017, 139(6): 2164-2167. |
| 69 | He G, Nazar L F. Crystallite size control of Prussian white analogues for nonaqueous potassium-ion batteries[J]. ACS Energy Letters, 2017, 2(5): 1122-1127. |
| 70 | Zhu Y H, Yang X, Bao D, et al. High-energy-density flexible potassium-ion battery based on patterned electrodes[J]. Joule, 2018, 2(4): 736-746. |
| 71 | Chong S K, Wu Y F, Guo S W, et al. Potassium nickel hexacyanoferrate as cathode for high voltage and ultralong life potassium-ion batteries[J]. Energy Storage Materials, 2019, 22: 120-127. |
| 72 | Huang B, Liu Y C, Lu Z Y, et al. Prussian blue [K2FeFe(CN)6] doped with nickel as a superior cathode: an efficient strategy to enhance potassium storage performance[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(19): 16659-16667. |
| 73 | Huang B, Shao Y J, Liu Y C, et al. Improving potassium-ion batteries by optimizing the composition of Prussian blue cathode[J]. ACS Applied Energy Materials, 2019, 2(9): 6528-6535. |
| 74 | Heo J W, Chae M S, Hyoung J, et al. Rhombohedral potassium-zinc hexacyanoferrate as a cathode material for nonaqueous potassium-ion batteries[J]. Inorganic Chemistry, 2019, 58(5): 3065-3072. |
| 75 | Targholi E, Mousavi-Khoshdel S M, Rahmanifara M, et al. Cu- and Fe-hexacyanoferrate as cathode materials for potassium ion battery: a first-principles study[J]. Chemical Physics Letters, 2017, 687: 244-249. |
| 76 | Jiang X, Zhang T R, Yang L Q, et al. A Fe/Mn-based Prussian blue analogue as a K-rich cathode material for potassium-ion batteries[J]. ChemElectroChem, 2017, 4(9): 2237-2242. |
| 77 | Xue L, Li L, Huang Y X, et al. Polypyrrole-modified Prussian blue cathode material for potassium ion batteries viain situ polymerization coating[J]. ACS Applied Materials & Interfaces, 2019, 11(25): 22339-22345. |
| 78 | Chen Y, Luo W, Carter M, et al. Organic electrode for non-aqueous potassium-ion batteries[J]. Nano Energy, 2015, 18: 205-211. |
| 79 | Xing Z, Jian Z, Luo W, et al. A perylene anhydride crystal as a reversible electrode for K-ion batteries[J]. Energy Storage Materials, 2016, 2: 63-68. |
| 80 | Fan L, Ma R, Wang J, et al. An ultrafast and highly stable potassium–organic battery[J]. Advanced Materials, 2018, 30(51): 1805486. |
| 81 | Tian B, Zheng J, Zhao C, et al. Carbonyl-based polyimide and polyquinoneimide for potassium-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(16): 9997-10003. |
| 82 | Zhao J, Yang J, Sun P, et al. Sodium sulfonate groups substituted anthraquinone as an organic cathode for potassium batteries[J]. Electrochemistry Communications, 2018, 86: 34-37. |
| 83 | Li D, Tang W, Wang C, et al. A polyanionic organic cathode for highly efficient K-ion full batteries[J]. Electrochemistry Communications, 2019, 105: 106509. |
| 84 | Hu Y, Tang W, Yu Q, et al. Novel insoluble organic cathodes for advanced organic K‐ion batteries[J]. Advanced Functional Materials, 2020, 30(17): 2000675. |
| 85 | Hu Y, Ding H, Bai Y, et al. Rational design of a polyimide cathode for a stable and high-rate potassium-ion battery[J]. ACS Applied Materials & Interfaces, 2019, 11(45): 42078-42085. |
| 86 | Xiong M, Tang W, Cao B, et al. A small-molecule organic cathode with fast charge-discharge capability for K-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(35): 20127-20131. |
| 87 | Wang M, Tang Y B. A review on the features and progress of dual-ion batteries[J]. Advanced Energy Materials, 2018, 8(19): 1703320. |
| 88 | Zhang M, Song X H, Ou X W, et al. Rechargeable batteries based on anion intercalation graphite cathodes[J]. Energy Storage Materials, 2019, 16: 65-84. |
| 89 | Read J A, Cresce A V, Ervin M H, et al. Dual-graphite chemistry enabled by a high voltage electrolyte[J]. Energy & Environmental Science, 2014, 7(2): 617-620. |
| 90 | Ji B F, Zhang F, Wu N Z, et al. A dual-carbon battery based on potassium-ion electrolyte[J]. Advanced Energy Materials, 2017, 7(20): 1700920. |
| 91 | Fan L, Liu Q, Chen S H, et al. Potassium-based dual ion battery with dual-graphite electrode[J]. Small, 2017, 13(30): 1701011. |
| 92 | Ji B, Zhang F, Song X, et al. A novel potassium-ion-based dual-ion battery[J]. Advanced Materials, 2017, 29(19): 1700519. |
| 93 | Fan L, Liu Q, Xu Z, et al. An organic cathode for potassium dual-ion full battery[J]. ACS Energy Letters, 2017, 2(7): 1614-1620. |
| 94 | Li C, Xue J, Huang A, et al. Poly(N-vinylcarbazole) as an advanced organic cathode for potassium-ion-based dual-ion battery[J]. Electrochimica Acta, 2019, 297: 850-855. |
| [1] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [2] | 刘远超, 关斌, 钟建斌, 徐一帆, 蒋旭浩, 李耑. 单层XSe2(X=Zr/Hf)的热电输运特性研究[J]. 化工学报, 2023, 74(9): 3968-3978. |
| [3] | 胡兴枝, 张皓焱, 庄境坤, 范雨晴, 张开银, 向军. 嵌有超小CeO2纳米粒子的碳纳米纤维的制备及其吸波性能[J]. 化工学报, 2023, 74(8): 3584-3596. |
| [4] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| [5] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [6] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [7] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [8] | 邢美波, 张中天, 景栋梁, 张洪发. 磁调控水基碳纳米管协同多孔材料强化相变储/释能特性[J]. 化工学报, 2023, 74(7): 3093-3102. |
| [9] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [10] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [11] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [12] | 张澳, 罗英武. 低模量、高弹性、高剥离强度丙烯酸酯压敏胶[J]. 化工学报, 2023, 74(7): 3079-3092. |
| [13] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [14] | 张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| [15] | 蔡斌, 张效林, 罗倩, 党江涛, 左栗源, 刘欣梅. 导电薄膜材料的研究进展[J]. 化工学报, 2023, 74(6): 2308-2321. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号