化工学报 ›› 2021, Vol. 72 ›› Issue (7): 3658-3667.DOI: 10.11949/0438-1157.20210050
收稿日期:2021-01-09
修回日期:2021-04-12
出版日期:2021-07-05
发布日期:2021-07-05
通讯作者:
康金灿
作者简介:程挥戈(1996—),男,硕士研究生,基金资助:
CHENG Huige( ),NIU Wei,TANG Xinglei,YUE Liangxu,KANG Jincan(
),NIU Wei,TANG Xinglei,YUE Liangxu,KANG Jincan( ),ZHANG Qinghong,WANG Ye
),ZHANG Qinghong,WANG Ye
Received:2021-01-09
Revised:2021-04-12
Online:2021-07-05
Published:2021-07-05
Contact:
KANG Jincan
摘要:
设计乙烷经氯氧化制备乙烯再与苯烷基化一步法制备乙苯的接力催化路线。研制铈基氧化物作为活化乙烷生成中间产物乙烯的催化剂,并耦合H-ZSM-5沸石分子筛与苯进一步烷基化生成乙苯。在Mn/CeO2氧化物与H-ZSM-5沸石分子筛以研磨混合形成的双功能催化剂上,实现了乙烷与苯催化制备乙苯的可控接力催化。考察了氧化物的组成、氧化物与沸石分子筛的耦合方式与最适质量配比、沸石分子筛的硅铝比对接力催化反应的影响,并进行了催化剂稳定性研究。结合X射线衍射(XRD)、NH3程序升温脱附(NH3-TPD)、透射电子显微镜(TEM)、X射线荧光光谱分析(XRF)等表征手段分析了催化剂结构及其与催化性能的构效关系。提出后续催化剂研究的关键在于分子筛烷基化能力以及抗流失能力的提高。
中图分类号:
程挥戈, 牛韦, 汤兴蕾, 岳亮旭, 康金灿, 张庆红, 王野. 乙烷与苯经接力催化路线制备乙苯[J]. 化工学报, 2021, 72(7): 3658-3667.
CHENG Huige, NIU Wei, TANG Xinglei, YUE Liangxu, KANG Jincan, ZHANG Qinghong, WANG Ye. Synthesis of ethylbenzene from ethane and benzene by tandem catalysis[J]. CIESC Journal, 2021, 72(7): 3658-3667.
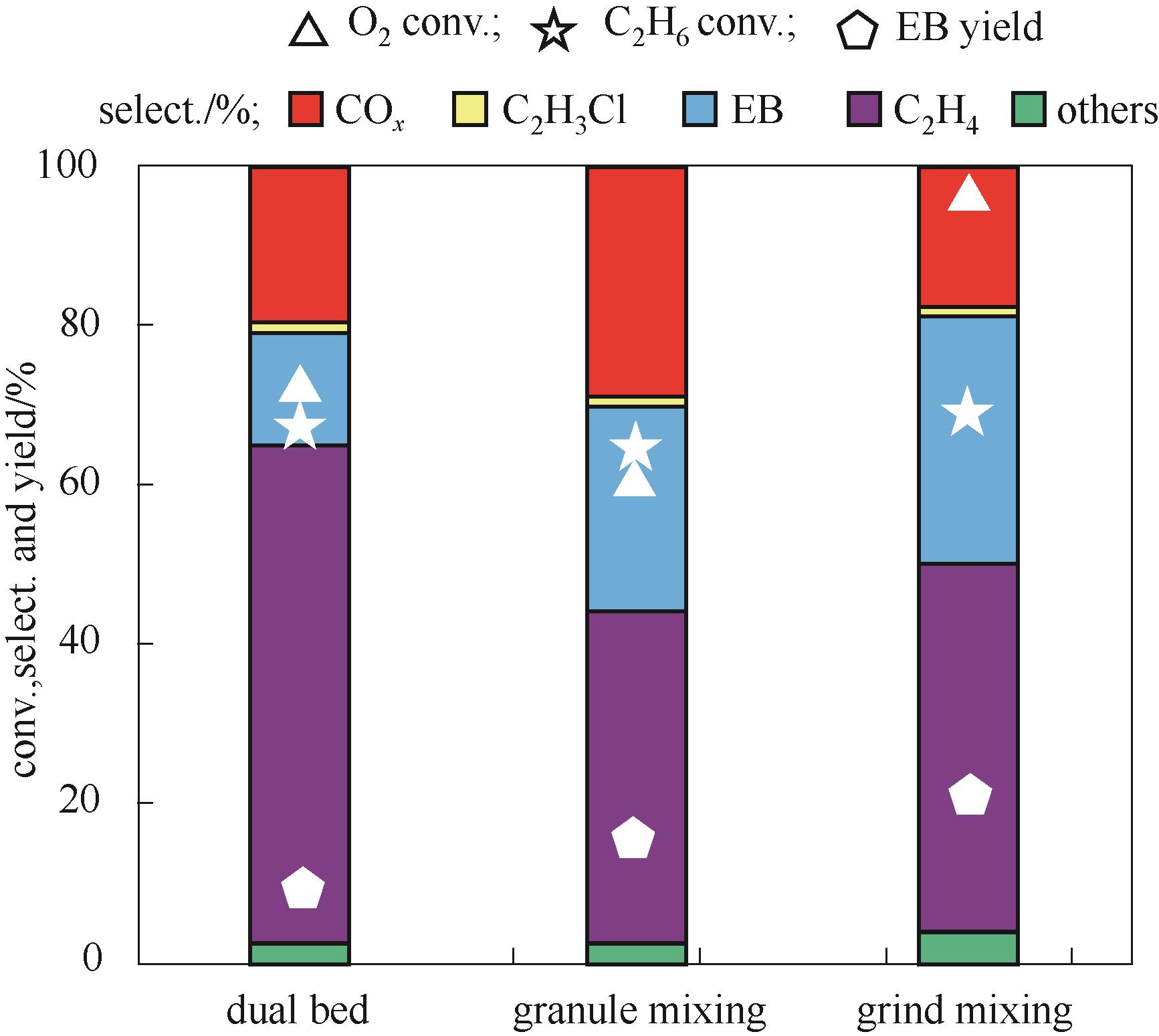
图2 Mn/CeO2氧化物与H-ZSM-5(25)分子筛的耦合方式对反应性能的影响(反应条件:W(Mn/CeO2) = 0.5 g, T = 450℃, P = 0.1 MPa, F(total) = 40 ml/min, time on stream 2.5 h, C2H6/benzene/HCl/O2/N2/He = 1/3.2/3/1/1.5/3.5, Mn/CeO2∶H-ZSM-5= 1∶2)
Fig.2 Effect of contact manner of Mn/CeO2 oxide and H-ZSM-5(25) zeolite on catalytic performances others—C2H4Cl2 and hydrocarbons
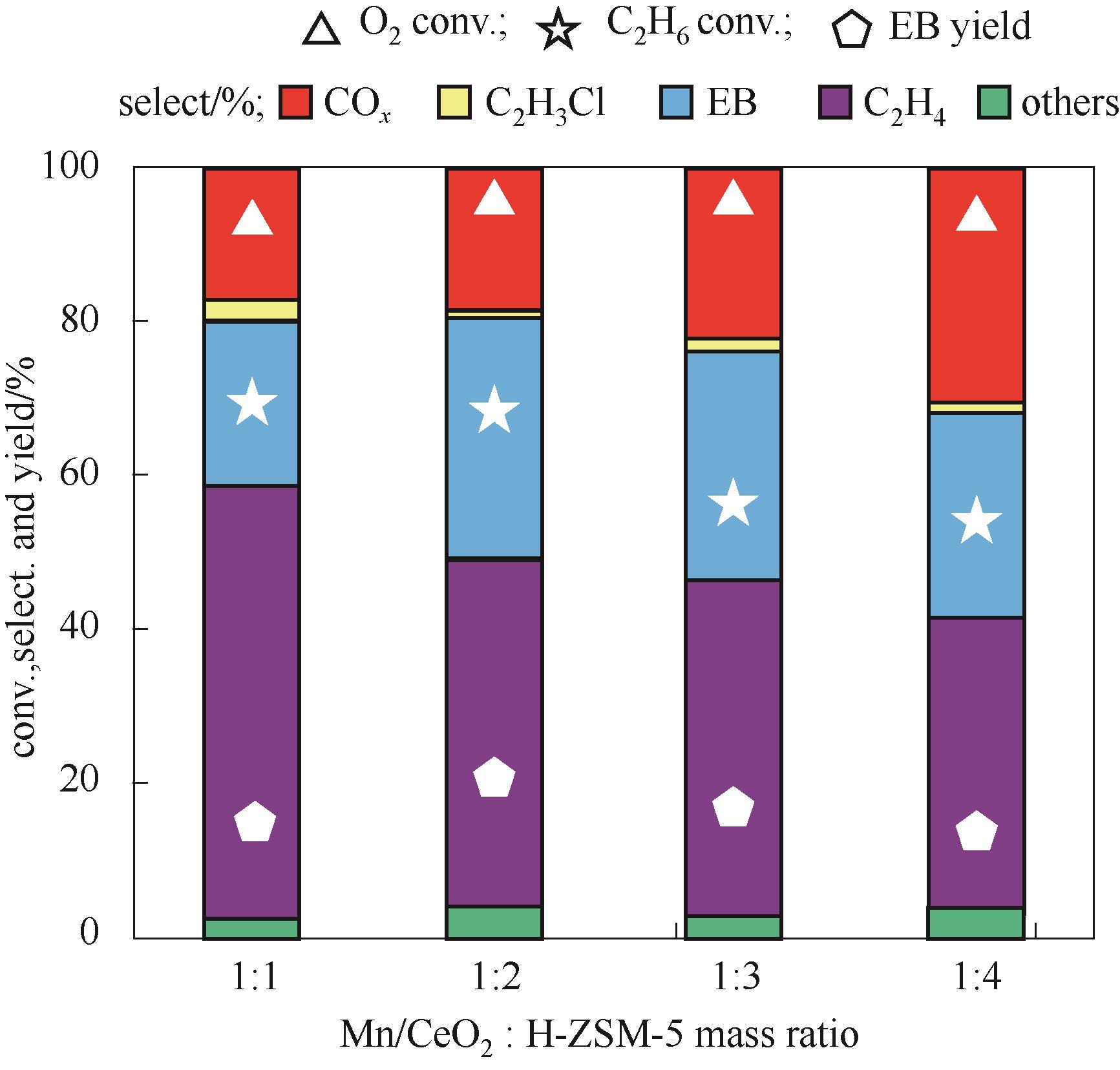
图3 Mn/CeO2氧化物与H-ZSM-5分子筛的质量比对催化性能的影响(反应条件:W = 1.5 g, T = 450℃, P = 0.1 MPa, F(total) = 40 ml/min, time on stream 2.5 h, C2H6/benzene/HCl/O2/N2/He = 1/3.2/3/1/1.5/3.5)
Fig.3 Catalytic performances of Mn/CeO2-H-ZSM-5-grind with different mass ratios of Mn/CeO2 oxide to H-ZSM-5 zeolite
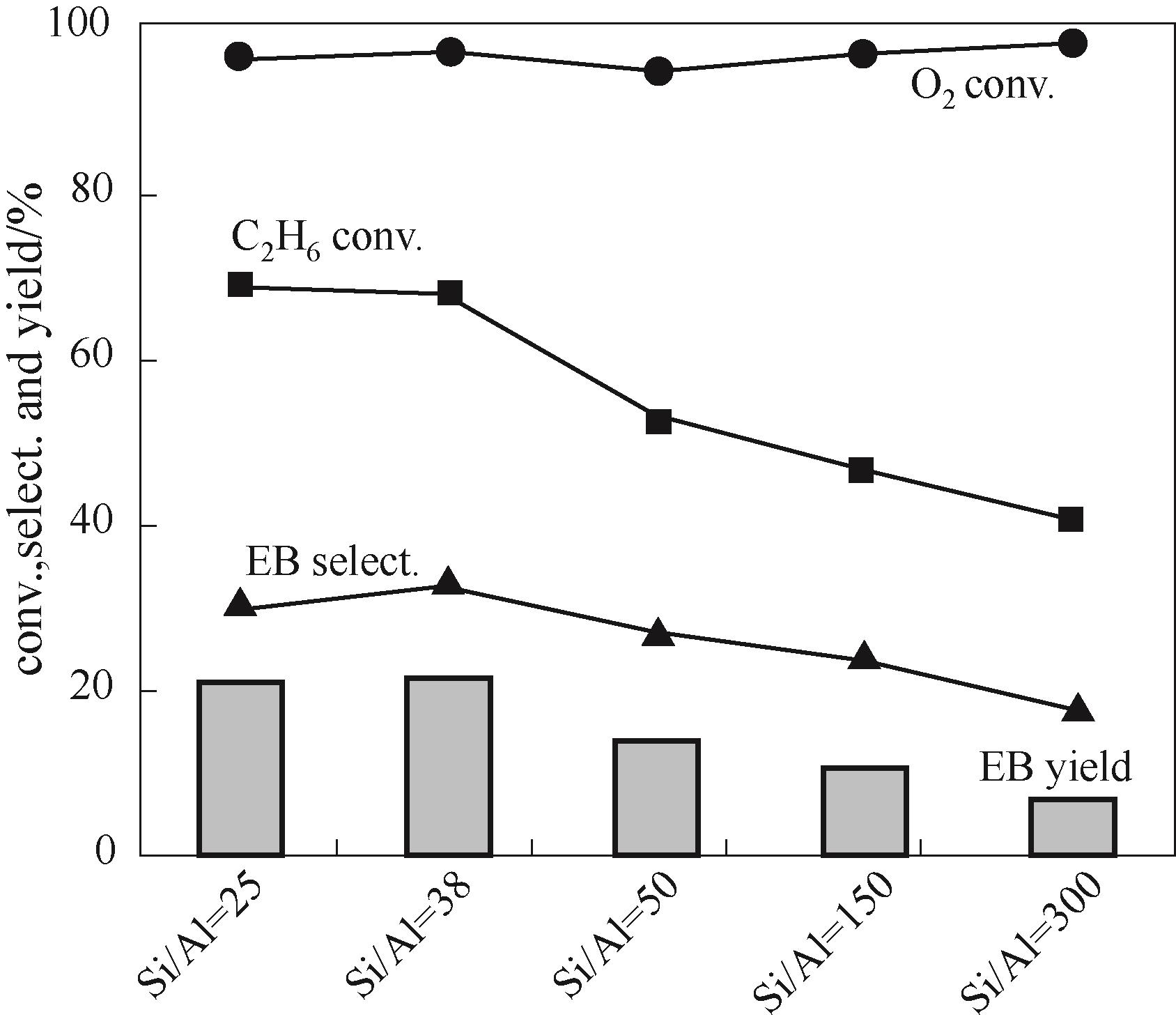
图4 H-ZSM-5分子筛硅铝比对双功能催化剂反应性能的影响(反应条件:W = 1.5 g, T = 450℃, P = 0.1 MPa, F(total) = 40 ml/min, Mn/CeO2∶H-ZSM-5 = 1∶2, time on stream 2.5 h, C2H6/benzene/HCl/O2/N2/He = 1/3.2/3/1/1.5/3.5)
Fig.4 Effect of Si/Al ratio of H-ZSM-5 on catalytic performances over Mn/CeO2-H-ZSM-5 bifunctional catalyst
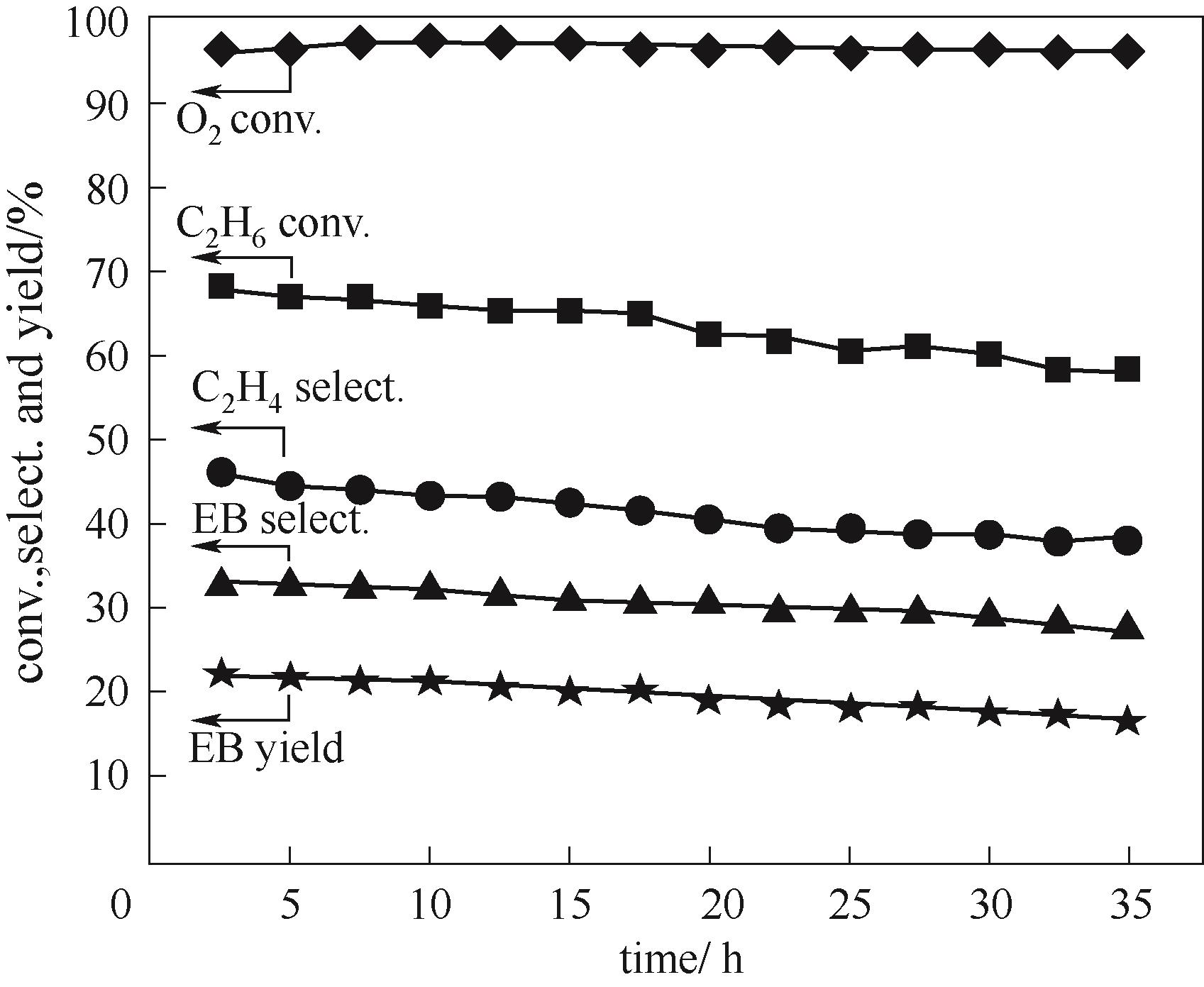
图5 Mn/CeO2-H-ZSM-5(38)- grind催化剂上反应性能随时间变化(反应条件:W = 1.5 g, T = 450℃, P = 0.1 MPa, F(total) = 40 ml/min, C2H6/benzene/HCl/O2/N2/He = 1/3.2/3/1/1.5/3.5)
Fig.5 Catalytic performances with time on stream over Mn/CeO2-H-ZSM-5-grindcatalyst
| Reaction time/h | Element content/% (mass) | |||
|---|---|---|---|---|
| Ce | Mn | Al | Si | |
| 0 | 15.86 | 2.16 | 0.84 | 34.3 |
| 2.5 | 15.91 | 2.07 | 0.81 | 34.0 |
| 35 | 16.43 | 1.62 | 0.75 | 39.7 |
表1 催化剂反应前后的XRF分析结果
Table 1 XRF analysis of Mn/CeO2-H-ZSM-5 before and after catalytic reaction
| Reaction time/h | Element content/% (mass) | |||
|---|---|---|---|---|
| Ce | Mn | Al | Si | |
| 0 | 15.86 | 2.16 | 0.84 | 34.3 |
| 2.5 | 15.91 | 2.07 | 0.81 | 34.0 |
| 35 | 16.43 | 1.62 | 0.75 | 39.7 |
| 1 | Cavani F, Trifirò F. Alternative processes for the production of styrene[J]. Applied Catalysis A: General, 1995, 133(2): 219-239. |
| 2 | Vrieland G E, Menon P G. Nature of the catalytically active carbonaceous sites for the oxydehydrogenation of ethylbenzene to styrene: a brief review[J]. Applied Catalysis, 1991, 77(1): 1-8. |
| 3 | Kainthla I, Bhanushali J T, Keri R S, et al. Activity studies of vanadium, iron, carbon and mixed oxides based catalysts for the oxidative dehydrogenation of ethylbenzene to styrene: a review[J]. Catalysis Science & Technology, 2015, 5(12): 5062-5076. |
| 4 | Bokade V V, Yadav G D. Heteropolyacid supported on acidic clay: a novel efficient catalyst for alkylation of ethylbenzene with dilute ethanol to diethylbenzene in presence of C8 aromatics[J]. Journal of Molecular Catalysis A: Chemical, 2008, 285(1/2): 155-161. |
| 5 | Yang W M, Wang Z D, Sun H M, et al. Advances in development and industrial applications of ethylbenzene processes[J]. Chinese Journal of Catalysis, 2016, 37(1): 16-26. |
| 6 | Liu S L, Chen F C, Xie S J, et al. Highly selective ethylbenzene production through alkylation of dilute ethylene with gas phase-liquid phase benzene and transalkylation feed[J]. Journal of Natural Gas Chemistry, 2009, 18(1): 21-24. |
| 7 | Zhong L, Yu F, An Y, et al. Cobalt carbide nanoprisms for direct production of lower olefins from syngas[J]. Nature, 2016, 538(7623): 84-87. |
| 8 | Li Y J, Zhang T T. Integration and optimized utilization of naphtha resources[J]. China Petroleum Processing and Petrochemical Technology, 2010, 12(2): 51-56. |
| 9 | Haribal V P, Chen Y, Neal L, et al. Intensification of ethylene production from naphtha via a redox oxy-cracking scheme: process simulations and analysis[J]. Engineering, 2018, 4(5): 714-721. |
| 10 | 曹杰, 迟东训. 中国乙烯工业发展现状与趋势[J]. 国际石油经济, 2019, 27(12): 53-59. |
| Cao J, Chi D X. Development status and trend of ethylene industry in China[J]. International Petroleum Economics, 2019, 27(12): 53-59. | |
| 11 | Zhao Z T, Chong K T, Jiang J Y, et al. Low-carbon roadmap of chemical production: a case study of ethylene in China[J]. Renewable and Sustainable Energy Reviews, 2018, 97: 580-591. |
| 12 | 黄格省, 师晓玉, 张彦, 等. 国内外乙烷裂解制乙烯发展现状及思考[J]. 现代化工, 2018, 38(10): 1-5. |
| Huang G S, Shi X Y, Zhang Y, et al. Situation of ethylene production via ethane cracking and considerations[J]. Modern Chemical Industry, 2018, 38(10): 1-5. | |
| 13 | 徐海丰. 2018年世界乙烯行业发展状况与趋势[J]. 国际石油经济, 2019, 27(1): 82-88. |
| Xu H F. Global ethylene industry in 2018 and its development trend[J]. International Petroleum Economics, 2019, 27(1): 82-88. | |
| 14 | Dasani D, Wang Y, Tsotsis T T, et al. Laboratory-scale investigation of sorption kinetics of methane/ethane mixtures in shale[J]. Industrial & Engineering Chemistry Research, 2017, 56(36): 9953-9963. |
| 15 | Scott A R. Composition of coalbed gases [J]. In Situ, 1994, 18(2): 185-208. |
| 16 | Nakano S, Yamamoto K, Ohgaki K. Natural gas exploitation by carbon dioxide from gas hydrate fields—high-pressure phase equilibrium for an ethane hydrate system[J]. Proceedings of the Institution of Mechanical Engineers, Part A: Journal of Power and Energy, 1998, 212(3): 159-163. |
| 17 | Choudhary V R, Uphade B S, Mulla S A R. Coupling of endothermic thermal cracking with exothermic oxidative dehydrogenation of ethane to ethylene using a diluted SrO/La2O3 catalyst[J]. Angewandte Chemie International Edition in English, 1995, 34(6): 665-666. |
| 18 | Yang J I, Kim J N, Cho S H, et al. Catalytic composites based on yttria stabilized zirconia for oxidative dehydrogenation of ethane[J]. Korean Journal of Chemical Engineering, 2004, 21(2): 381-384. |
| 19 | 温翯, 郭晓莉, 苟尕莲, 等. 乙烷裂解制乙烯的工艺研究进展[J]. 现代化工, 2020, 40(5): 47-51. |
| Wen H, Guo X L, Gou G L, et al. Process research advances in ethane cracking to ethylene[J]. Modern Chemical Industry, 2020, 40(5): 47-51. | |
| 20 | Olah G A, Schilling P, Staral J S, et al. Electrophilic reactions at single bonds(ⅪⅤ): Anhydrous fluoroantimonic acid catalyzed alkylation of benzene with alkanes and alkane-alkene and alkane-alkylbenzene mixtures[J]. Journal of the American Chemical Society, 1975, 97(23): 6807-6810. |
| 21 | Isaev S A, Vasina T V, Bragin O V. Alkylation of benzene by propane over zeolite-containing pentasil-alumina compositions and dealuminated pentasils[J]. Bulletin of the Russian Academy of Sciences, Division of Chemical Science, 1992, 41(12): 2143-2146. |
| 22 | Kato S, Nakagawa K, Ikenaga N O, et al. Alkylation of benzene with ethane over platinum-loaded zeolite catalyst[J]. Catalysis Letters, 2001, 73(2/3/4): 175-180. |
| 23 | Lukyanov D, Vazhnova T. A kinetic study of benzene alkylation with ethane into ethylbenzene over bifunctional PtH-MFI catalyst[J]. Journal of Catalysis, 2008, 257(2): 382-389. |
| 24 | Zhou W, Kang J, Cheng K, et al. Direct conversion of syngas into methyl acetate, ethanol, and ethylene by relay catalysis via the intermediate dimethyl ether[J]. Angew. Chem. Int. Ed., 2018, 57(37): 12012-12016. |
| 25 | Zhou W, Cheng K, Kang J C, et al. New horizon in C1 chemistry: breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels[J]. Chemical Society Reviews, 2019, 48(12): 3193-3228. |
| 26 | Kang J, He S, Zhou W, et al. Single-pass transformation of syngas into ethanol with high selectivity by triple tandem catalysis[J]. Nature Communications, 2020, 11(1): 827. |
| 27 | Yu F C, Wu X J, Zhang Q H, et al. Oxidative dehydrogenation of ethane to ethylene in the presence of HCl over CeO2-based catalysts[J]. Chinese Journal of Catalysis, 2014, 35(8): 1260-1266. |
| 28 | Shavaleev D A, Pavlov M L, Basimova R A, et al. Synthesis of a zeolite-containing catalyst for gas-phase alkylation of benzene with ethylene[J]. Petroleum Chemistry, 2020, 60(10): 1164-1169. |
| 29 | Zhang Z Q, Ding J, Chai R J, et al. Oxidative dehydrogenation of ethane to ethylene: a promising CeO2-ZrO2-modified NiO-Al2O3/Ni-foam catalyst[J]. Applied Catalysis A: General, 2018, 550: 151-159. |
| 30 | Cheng K, Zhou W, Kang J C, et al. Bifunctional catalysts for one-step conversion of syngas into aromatics with excellent selectivity and stability[J]. Chem, 2017, 3(2): 334-347. |
| 31 | Cuo Z X, Wang D D, Gong Y, et al. A novel porous ceramic membrane supported monolithic Cu-doped Mn–Ce catalysts for benzene combustion[J]. Catalysts, 2019, 9(8): 652. |
| 32 | Gärtner C A, Van Veen A C, Lercher J A. Oxidative dehydrogenation of ethane: common principles and mechanistic aspects[J]. ChemCatChem, 2013, 5(11): 3196-3217. |
| 33 | Li C W, Sun Y, Hess F, et al. Catalytic HCl oxidation reaction: stabilizing effect of Zr-doping on CeO2 nano-rods[J]. Applied Catalysis B: Environmental, 2018, 239: 628-635. |
| 34 | Katada N, Igi H, Kim J H. Determination of the acidic properties of zeolite by theoretical analysis of temperature-programmed desorption of ammonia based on adsorption equilibrium[J]. The Journal of Physical Chemistry B, 1997, 101(31): 5969-5977. |
| 35 | 徐如人, 庞文琴, 霍启升, 等. 分子筛与多孔材料化学[M]. 2版. 北京: 科学出版社, 2015. |
| Xu R R, Pang W Q, Huo Q S. Molecular Sieves and Porous Materials Chemistry [M]. 2nd ed. Beijing: Science Press, 2015. | |
| 36 | Shirazi L, Jamshidi E, Ghasemi M R. The effect of Si/Al ratio of ZSM-5 zeolite on its morphology, acidity and crystal size[J]. Crystal Research and Technology, 2008, 43(12): 1300-1306. |
| 37 | Zhu H B, Liu Z C, Kong D J, et al. Synthesis and catalytic performances of mesoporous zeolites templated by polyvinyl butyral gel as the mesopore directing agent[J]. The Journal of Physical Chemistry C, 2008, 112(44): 17257-17264. |
| 38 | Janssens T V W. A new approach to the modeling of deactivation in the conversion of methanol on zeolite catalysts[J]. Journal of Catalysis, 2009, 264(2): 130-137. |
| 39 | 刘巧玲. 含氯化氢废气的处理与回收利用[J]. 化工管理, 2017,(23): 226-228. |
| Liu Q L. Treatment and recovery of waste gas containing hydrogen chloride [J]. Chemical Enterprise Management, 2017,(23): 226-228. | |
| 40 | 黄冬兰. 膜法分离丙烯和氯化氢混合气[D]. 大连: 大连理工大学, 2004. |
| Huang D L. Study on separation of propylene/hydrogen chloride mixture gas by membrane technology[D]. Dalian: Dalian University of Technology, 2004. |
| [1] | 王阳, 戴永强, 曾炜. 2,5-二羟基苯磺酸增强离子水凝胶材料热电性能的研究[J]. 化工学报, 2023, 74(9): 3946-3955. |
| [2] | 韩奎奎, 谭湘龙, 李金芝, 杨婷, 张春, 张永汾, 刘洪全, 于中伟, 顾学红. 四通道中空纤维MFI分子筛膜用于二甲苯异构体分离[J]. 化工学报, 2023, 74(6): 2468-2476. |
| [3] | 王辰, 史秀锋, 武鲜凤, 魏方佳, 张昊虹, 车寅, 吴旭. 氧化还原法制备Mn3O4催化剂及其甲苯催化氧化性能与机理研究[J]. 化工学报, 2023, 74(6): 2447-2457. |
| [4] | 王子健, 柯明, 李佳涵, 李舒婷, 孙巾茹, 童燕兵, 赵治平, 刘加英, 任璐. 短b轴ZSM-5分子筛制备方法及应用研究进展[J]. 化工学报, 2023, 74(4): 1457-1473. |
| [5] | 李木金, 胡松, 施德磐, 赵鹏, 高瑞, 李进龙. 环氧丁烷尾气溶剂吸收及精制工艺[J]. 化工学报, 2023, 74(4): 1607-1618. |
| [6] | 王荣, 王永洪, 张新儒, 李晋平. 6FDA型聚酰亚胺炭分子筛气体分离膜的构筑及其应用[J]. 化工学报, 2023, 74(4): 1433-1445. |
| [7] | 肖川宝, 李林洋, 刘武锋, 钟年丙, 解泉华, 钟登杰, 常海星. 光催化与离子交换吸附耦合有效去除2,4,6-三氯苯酚[J]. 化工学报, 2023, 74(4): 1587-1597. |
| [8] | 刘润竹, 储甜甜, 张孝阿, 王成忠, 张军营. α,ω-端羟基亚苯基氟硅聚合物的合成及性能[J]. 化工学报, 2023, 74(3): 1360-1369. |
| [9] | 胡月, 马守骏, 蹇锡高, 翁志焕. 新型聚芳醚腈固化邻苯二甲腈树脂的研究[J]. 化工学报, 2023, 74(2): 871-882. |
| [10] | 李敏, 阎雪茹, 刘新磊. 苯并咪唑连接聚合物吸附剂和膜研究进展[J]. 化工学报, 2023, 74(2): 599-616. |
| [11] | 白宇恩, 张彬瑞, 刘东阳, 赵亮, 高金森, 徐春明. ZSM-5分子筛酸性能和孔结构的协同作用对C5烯烃催化裂解性能的影响[J]. 化工学报, 2023, 74(1): 438-448. |
| [12] | 刘潜, 张香兰, 李志平, 李玉龙, 韩梦醒. 油酚分离过程低共熔溶剂的筛选及萃取性能研究[J]. 化工学报, 2022, 73(9): 3915-3928. |
| [13] | 韩双, 张楠, 王慧, 张璇, 杨金栾, 张蔓琳, 张志超. 金霉素分子印迹电化学传感器的制备与应用[J]. 化工学报, 2022, 73(8): 3758-3767. |
| [14] | 郭丹, 方雨洁, 许一寒, 李致远, 黄守莹, 王胜平, 马新宾. 乙烷和二氧化碳催化转化的研究进展[J]. 化工学报, 2022, 73(8): 3406-3416. |
| [15] | 王沛, 魏荣阔. 光热驱动多孔氧化铈热化学循环解水制氢非热质平衡模型[J]. 化工学报, 2022, 73(7): 2885-2894. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号