化工学报 ›› 2022, Vol. 73 ›› Issue (8): 3406-3416.DOI: 10.11949/0438-1157.20220518
郭丹( ), 方雨洁, 许一寒, 李致远, 黄守莹(
), 方雨洁, 许一寒, 李致远, 黄守莹( ), 王胜平, 马新宾
), 王胜平, 马新宾
收稿日期:2022-04-11
修回日期:2022-07-09
出版日期:2022-08-05
发布日期:2022-09-06
通讯作者:
黄守莹
作者简介:郭丹(1993—),女,博士研究生,guodan207@tju.edu.cn
基金资助:
Dan GUO( ), Yujie FANG, Yihan XU, Zhiyuan LI, Shouying HUANG(
), Yujie FANG, Yihan XU, Zhiyuan LI, Shouying HUANG( ), Shengping WANG, Xinbin MA
), Shengping WANG, Xinbin MA
Received:2022-04-11
Revised:2022-07-09
Online:2022-08-05
Published:2022-09-06
Contact:
Shouying HUANG
摘要:
CO2与乙烷反应是实现碳减排目标、利用非常规能源的重要手段,符合国家重大需求和国际学术前沿。其中,二氧化碳通过“活性氧”机理、“晶格氧”机理以及“反应耦合”机理促进乙烷的活化。通过催化剂设计选择性地断裂C—H/C—C键,可以实现反应定向地按照两条路径进行——乙烷干重整反应(DRE)和乙烷氧化脱氢(ODH)。综述了DRE和ODH两类反应的热力学、反应物活化机制和催化剂研究进展,分析了催化剂均存在产物选择性低、易烧结、积炭问题的主要影响因素以及催化剂设计和改进策略,并对该研究未来的发展方向进行展望。
中图分类号:
郭丹, 方雨洁, 许一寒, 李致远, 黄守莹, 王胜平, 马新宾. 乙烷和二氧化碳催化转化的研究进展[J]. 化工学报, 2022, 73(8): 3406-3416.
Dan GUO, Yujie FANG, Yihan XU, Zhiyuan LI, Shouying HUANG, Shengping WANG, Xinbin MA. Research progress of the catalytic conversion of ethane and carbon dioxide[J]. CIESC Journal, 2022, 73(8): 3406-3416.
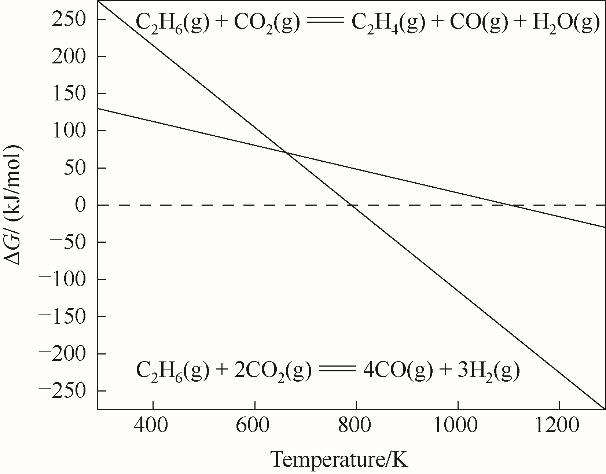
图2 乙烷干重整和乙烷氧化脱氢反应Gibbs自由能随温度的变化[7]
Fig.2 The variation of Gibbs free energy with temperature for dry reforming of ethane and oxidative dehydrogenation of ethane[7]

图4 负载型单金属催化剂DRE的反应性能[17,31]以及载体酸碱性对Ni基催化剂性能的影响[35]
Fig.4 The catalytic performance of DRE on supported single-metal catalyst[17,31] and influence of support acidity and alkalinity on performance of Ni-based catalyst[35]
| 活性 组分 | 载体 | 负载量(质量分数) | 反应 温度/K | 空速/ (ml/(g∙h)) | 转化率/% | CO选择性/% | 反应时间/10-4s | 文献 | |
|---|---|---|---|---|---|---|---|---|---|
| CO2 | C2H6 | ||||||||
| NiPt | CeO2 | 1.51%Ni,1.67%Pt | 873 | 24000 | 53.4 | 22.8 | 92.8 | 3.4~4.2 | [ |
| NiPt | TiO2 | 1.51%Ni,1.67%Pt | 873 | 24000 | 27.9 | 11.1 | 85.5 | 3.4~4.2 | [ |
| NiPt | γ-Al2O3 | 1.51%Ni,1.67%Pt | 873 | 24000 | 26.5 | 9.2 | 93.0 | 3.4~4.2 | [ |
| NiPt | SiO2 | 1.51%Ni,1.67%Pt | 873 | 24000 | 28.2 | 9.2 | 97.0 | 3.4~4.2 | [ |
| PdCo | CeO2 | 1%Pd,9%Co | 923 | 18000 | 31.5 | 31.0 | 82.0 | >1.2 | [ |
| PdNi | CeO2 | 1%Pd,1%Ni | 923 | 18000 | 53.0 | 39.8 | 86.0 | >1.2 | [ |
| PdCu | CeO2 | 1%Pd,1%Cu | 923 | 18000 | 13.0 | 12.5 | 51.0 | >1.2 | [ |
| NiFe | CeO2-C2 (25 nm) | 4.0%Ni,1.6%Fe | 873 | 24000 | 8.0 | 3.9 | 53.0 | 4.3 | [ |
| NiFe | CeO2 | 0.48%Ni,1.51%Fe | 873 | 150000 | 41.3 | 16.7 | 98.6 | 4.0~4.7 | [ |
| NiFe | SiO2 | 0.5%Ni,1.4%Fe | 873 | 24000 | 0.9 | 0.4 | — | 4.0~4.7 | [ |
| NiFe | ZrO2 | 0.5%Ni,1.4%Fe | 873 | 24000 | 5.6 | 2.6 | 37.9 | 4.0~4.7 | [ |
| NiFe | CeO2 | 2%Ni,5.6%Fe | 873 | 24000 | 13.1 | 3.5 | 67.7 | 4.0~4.7 | [ |
表1 典型乙烷干重整双金属催化剂研究进展
Table 1 Research progress of typical bimetallic catalysts for DRE
| 活性 组分 | 载体 | 负载量(质量分数) | 反应 温度/K | 空速/ (ml/(g∙h)) | 转化率/% | CO选择性/% | 反应时间/10-4s | 文献 | |
|---|---|---|---|---|---|---|---|---|---|
| CO2 | C2H6 | ||||||||
| NiPt | CeO2 | 1.51%Ni,1.67%Pt | 873 | 24000 | 53.4 | 22.8 | 92.8 | 3.4~4.2 | [ |
| NiPt | TiO2 | 1.51%Ni,1.67%Pt | 873 | 24000 | 27.9 | 11.1 | 85.5 | 3.4~4.2 | [ |
| NiPt | γ-Al2O3 | 1.51%Ni,1.67%Pt | 873 | 24000 | 26.5 | 9.2 | 93.0 | 3.4~4.2 | [ |
| NiPt | SiO2 | 1.51%Ni,1.67%Pt | 873 | 24000 | 28.2 | 9.2 | 97.0 | 3.4~4.2 | [ |
| PdCo | CeO2 | 1%Pd,9%Co | 923 | 18000 | 31.5 | 31.0 | 82.0 | >1.2 | [ |
| PdNi | CeO2 | 1%Pd,1%Ni | 923 | 18000 | 53.0 | 39.8 | 86.0 | >1.2 | [ |
| PdCu | CeO2 | 1%Pd,1%Cu | 923 | 18000 | 13.0 | 12.5 | 51.0 | >1.2 | [ |
| NiFe | CeO2-C2 (25 nm) | 4.0%Ni,1.6%Fe | 873 | 24000 | 8.0 | 3.9 | 53.0 | 4.3 | [ |
| NiFe | CeO2 | 0.48%Ni,1.51%Fe | 873 | 150000 | 41.3 | 16.7 | 98.6 | 4.0~4.7 | [ |
| NiFe | SiO2 | 0.5%Ni,1.4%Fe | 873 | 24000 | 0.9 | 0.4 | — | 4.0~4.7 | [ |
| NiFe | ZrO2 | 0.5%Ni,1.4%Fe | 873 | 24000 | 5.6 | 2.6 | 37.9 | 4.0~4.7 | [ |
| NiFe | CeO2 | 2%Ni,5.6%Fe | 873 | 24000 | 13.1 | 3.5 | 67.7 | 4.0~4.7 | [ |
| 催化剂 | 反应温度/K | 空速/(ml/(g·h)) | 反应时间/h | 文献 | |||
|---|---|---|---|---|---|---|---|
| CrO x /Al2O3 | 873 | 48000 | — | 6.9 | 96 | 2 | [ |
| CrO x /ZrO2 | 873 | 48000 | — | 10 | 91 | 2 | [ |
| CrO x /Ce x Zr1-x O2 | 873 | 48000 | — | 2.8 | 77 | 2 | [ |
| Cr/TiO2-ZrO2 | 973 | 9000 | — | 48.5 | 95 | — | [ |
| (K-Ca)50/(Cr10/H-ZSM-5)5 | 973 | — | 18 | 17 | 96 | — | [ |
| Mo2C | 873 | 24000 | — | 12 | 20 | 2 | [ |
| 1%(质量) Fe/Mo2C | 873 | 24000 | — | 8 | 71 | 2 | [ |
表2 CO2-ODH反应催化剂性能
Table 2 Performance of CO2-ODH catalysts
| 催化剂 | 反应温度/K | 空速/(ml/(g·h)) | 反应时间/h | 文献 | |||
|---|---|---|---|---|---|---|---|
| CrO x /Al2O3 | 873 | 48000 | — | 6.9 | 96 | 2 | [ |
| CrO x /ZrO2 | 873 | 48000 | — | 10 | 91 | 2 | [ |
| CrO x /Ce x Zr1-x O2 | 873 | 48000 | — | 2.8 | 77 | 2 | [ |
| Cr/TiO2-ZrO2 | 973 | 9000 | — | 48.5 | 95 | — | [ |
| (K-Ca)50/(Cr10/H-ZSM-5)5 | 973 | — | 18 | 17 | 96 | — | [ |
| Mo2C | 873 | 24000 | — | 12 | 20 | 2 | [ |
| 1%(质量) Fe/Mo2C | 873 | 24000 | — | 8 | 71 | 2 | [ |
| 47 | Das S, Sengupta M, Patel J, et al. A study of the synergy between support surface properties and catalyst deactivation for CO2 reforming over supported Ni nanoparticles[J]. Applied Catalysis A: General, 2017, 545: 113-126. |
| 48 | Porosoff M D, Chen J G. Trends in the catalytic reduction of CO2 by hydrogen over supported monometallic and bimetallic catalysts[J]. Journal of Catalysis, 2013, 301: 30-37. |
| 49 | Iwasaki N O, Miyake T, Yagasaki E, et al. Partial oxidation of ethane to synthesis gas over Co-loaded catalysts[J]. Catalysis Today, 2006, 111(3/4): 391-397. |
| 50 | Liu Y, Wu Y, Akhtamberdinova Z, et al. Dry reforming of shale gas and carbon dioxide with Ni-Ce-Al2O3 catalyst: syngas production enhanced over Ni-CeO x formation[J]. ChemCatChem, 2018, 10(20): 4689-4698. |
| 51 | Yoon S, Kang I, Bae J. Effects of ethylene on carbon formation in diesel autothermal reforming[J]. International Journal of Hydrogen Energy, 2008, 33(18): 4780-4788. |
| 52 | Joensen F, Rostrup-Nielsen J R. Conversion of hydrocarbons and alcohols for fuel cells[J]. Journal of Power Sources, 2002, 105(2): 195-201. |
| 53 | Choudhary V R, Mondal K C, Mulla S A R. Non-catalytic pyrolysis of ethane to ethylene in the presence of CO2 with or without limited O2 [J]. Journal of Chemical Sciences, 2006, 118(3): 261-267. |
| 54 | Bugrova T A, Dutov V V, Svetlichnyi V A, et al. Oxidative dehydrogenation of ethane with CO2 over CrO x catalysts supported on Al2O3, ZrO2, CeO2 and Ce x Zr1- x O2 [J]. Catalysis Today, 2019, 333: 71-80. |
| 55 | Talati A, Haghighi M, Rahmani F. Oxidative dehydrogenation of ethane to ethylene by carbon dioxide over Cr/TiO2-ZrO2 nanocatalyst: effect of active phase and support composition on catalytic properties and performance[J]. Advanced Powder Technology, 2016, 27(4): 1195-1206. |
| 56 | Al-Mamoori A, Lawson S, Rownaghi A A, et al. Oxidative dehydrogenation of ethane to ethylene in an integrated CO2 capture-utilization process[J]. Applied Catalysis B: Environmental, 2020, 278: 119329. |
| 57 | Yao S Y, Yan B H, Jiang Z, et al. Combining CO2 reduction with ethane oxidative dehydrogenation by oxygen-modification of molybdenum carbide[J]. ACS Catalysis, 2018, 8(6): 5374-5381. |
| 58 | Jeong M H, Sun J, Young H G, et al. Successive reduction-oxidation activity of FeO x /TiO2 for dehydrogenation of ethane and subsequent CO2 activation[J]. Applied Catalysis B: Environmental, 2020, 270: 118887. |
| 59 | Koirala R, Safonova O V, Pratsinis S E, et al. Effect of cobalt loading on structure and catalytic behavior of CoO x /SiO2 in CO2-assisted dehydrogenation of ethane[J]. Applied Catalysis A: General, 2018, 552: 77-85. |
| 60 | Nowicka E, Reece C, Althahban S M, et al. Elucidating the role of CO2 in the soft oxidative dehydrogenation of propane over ceria-based catalysts[J]. ACS Catalysis, 2018, 8(4): 3454-3468. |
| 61 | Koirala R, Buechel R, Pratsinis S E, et al. Silica is preferred over various single and mixed oxides as support for CO2-assisted cobalt-catalyzed oxidative dehydrogenation of ethane[J]. Applied Catalysis A: General, 2016, 527: 96-108. |
| 62 | Zhou Y L, Wei F F, Lin J, et al. Sulfate-modified NiAl mixed oxides as effective C—H bond-breaking agents for the sole production of ethylene from ethane[J]. ACS Catalysis, 2020, 10(14): 7619-7629. |
| 63 | Zhu H B, Rosenfeld D C, Harb M, et al. Ni–M–O (M = Sn, Ti, W) catalysts prepared by a dry mixing method for oxidative dehydrogenation of ethane[J]. ACS Catalysis, 2016, 6(5): 2852-2866. |
| 64 | Lei T Q, Miao C X, Hua W M, et al. Oxidative dehydrogenation of ethane with CO2 over Au/CeO2 nanorod catalysts[J]. Catalysis Letters, 2018, 148(6): 1634-1642. |
| 65 | Ruiz Puigdollers A, Schlexer P, Tosoni S, et al. Increasing oxide reducibility: the role of metal/oxide interfaces in the formation of oxygen vacancies[J]. ACS Catalysis, 2017, 7(10): 6493-6513. |
| 66 | Li X Y, Liu S F, Chen H R, et al. Improved catalytic performance of ethane dehydrogenation in the presence of CO2 over Zr-promoted Cr/SiO2 [J]. ACS Omega, 2019, 4(27): 22562-22573. |
| 67 | Sagar T V, Surendar M, Padmakar D, et al. Selectivity reversal in oxidative dehydrogenation of ethane with CO2 on CaO–NiO/Al2O3 catalysts[J]. Catalysis Letters, 2017, 147(1): 82-89. |
| 68 | Liu G, Zeng L, Zhao Z J, et al. Platinum-modified ZnO/Al2O3 for propane dehydrogenation: minimized platinum usage and improved catalytic stability[J]. ACS Catalysis, 2016, 6(4): 2158-2162. |
| 69 | Wang S B, Zhu Z H. Catalytic conversion of alkanes to olefins by carbon dioxide oxidative dehydrogenation: a review[J]. Energy & Fuels, 2004, 18(4): 1126-1139. |
| 70 | Porosoff M D, Yang X F, Boscoboinik J A, et al. Molybdenum carbide as alternative catalysts to precious metals for highly selective reduction of CO2 to CO[J]. Angewandte Chemie, 2014, 126(26): 6823-6827. |
| 1 | 刘磊, 金晶, 赵庆庆, 等. 中国及世界一次能源消费结构现状分析[J]. 能源研究与信息, 2014, 30(1): 7-11. |
| Liu L, Jin J, Zhao Q Q, et al. Study on the structure of China and world primary energy consumption[J]. Energy Research and Information, 2014, 30(1): 7-11. | |
| 2 | Jales M. Commodities at a Glance: Special Issue on Gum Arabic[M]. Geneva: UNCTAD, 2018. |
| 3 | Melzer D, Xu P H, Hartmann D, et al. Atomic-scale determination of active facets on the MoVTeNb oxide M1 phase and their intrinsic catalytic activity for ethane oxidative dehydrogenation[J]. Angewandte Chemie International Edition, 2016, 55(31): 8873-8877. |
| 4 | Dang D, Chen X, Yan B H, et al. Catalytic performance of phase-pure M1 MoVNbTeO x /CeO2 composite for oxidative dehydrogenation of ethane[J]. Journal of Catalysis, 2018, 365: 238-248. |
| 5 | Yan B H, Yang X F, Yao S Y, et al. Dry reforming of ethane and butane with CO2 over PtNi/CeO2 bimetallic catalysts[J]. ACS Catalysis, 2016, 6(11): 7283-7292. |
| 6 | Xie Z H, Yan B H, Lee J H, et al. Effects of oxide supports on the CO2 reforming of ethane over Pt-Ni bimetallic catalysts[J]. Applied Catalysis B: Environmental, 2019, 245: 376-388. |
| 7 | Myint M, Yan B H, Wan J, et al. Reforming and oxidative dehydrogenation of ethane with CO2 as a soft oxidant over bimetallic catalysts[J]. Journal of Catalysis, 2016, 343: 168-177. |
| 8 | Wang Y L, Hu P, Yang J, et al. C—H bond activation in light alkanes: a theoretical perspective[J]. Chemical Society Reviews, 2021, 50(7): 4299-4358. |
| 9 | Mateos Pedrero C, González Carrazán S, Ruiz P. Preliminary results on the role of the deposition of small amounts of ZrO2 on Al2O3 support on the partial oxidation of methane and ethane over Rh and Ni supported catalysts[J]. Catalysis Today, 2021, 363: 111-121. |
| 10 | Savchenko V I, Zimin Y S, Nikitin A V, et al. Utilization of CO2 in non-catalytic dry reforming of C1—C4 hydrocarbons[J]. Journal of CO2 Utilization, 2021, 47: 101490. |
| 11 | Porosoff M D, Myint M N Z, Kattel S, et al. Identifying different types of catalysts for CO2 reduction by ethane through dry reforming and oxidative dehydrogenation[J]. Angewandte Chemie, 2015, 127(51): 15721-15725. |
| 12 | Zhao B H, Yan B H, Yao S Y, et al. LaFe0.9Ni0.1O3 perovskite catalyst with enhanced activity and coke-resistance for dry reforming of ethane[J]. Journal of Catalysis, 2018, 358: 168-178. |
| 71 | Posada-Pérez S, Viñes F, Ramirez P J, et al. The bending machine: CO2 activation and hydrogenation on δ-MoC(001) and β-Mo2C(001) surfaces[J]. Physical Chemistry Chemical Physics, 2014, 16(28): 14912-14921. |
| 13 | Tsiotsias A I, Charisiou N D, Sebastian V, et al. A comparative study of Ni catalysts supported on Al2O3, MgO-CaO-Al2O3 and La2O3-Al2O3 for the dry reforming of ethane[J]. International Journal of Hydrogen Energy, 2022, 47(8): 5337-5353. |
| 14 | Li Y C, Li L Y, Sun W J, et al. Porous silica coated ceria as a switch in tandem oxidative dehydrogenation and dry reforming of ethane with CO2 [J]. ChemCatChem, 2021, 13(15): 3501-3509. |
| 15 | Kattel S, Chen J G, Liu P. Mechanistic study of dry reforming of ethane by CO2 on a bimetallic PtNi(111) model surface[J]. Catalysis Science & Technology, 2018, 8(15): 3748-3758. |
| 16 | Lv J N, Wang D C, Wang M Y, et al. Integrated coal pyrolysis with dry reforming of low carbon alkane over Ni/La2O3 to improve tar yield[J]. Fuel, 2020, 266: 117092. |
| 17 | Li X Q, Yang Z Q, Zhang L, et al. Effect of Pd doping in (Fe/Ni)/CeO2 catalyst for the reaction path in CO2 oxidative ethane dehydrogenation/reforming[J]. Energy, 2021, 234: 121261. |
| 18 | Xu X D, Moulijn J A. Mitigation of CO2 by chemical conversion: plausible chemical reactions and promising products[J]. Energy & Fuels, 1996, 10(2): 305-325. |
| 19 | Ayari F, Charrad R, Asedegbega-Nieto E, et al. Ethane oxidative dehydrogenation over ternary and binary mixtures of alkaline and alkaline earth chlorides supported on zeolites[J]. Microporous and Mesoporous Materials, 2017, 250: 65-71. |
| 20 | Song Y F, Lin L, Feng W C, et al. Interfacial enhancement by γ-Al2O3 of electrochemical oxidative dehydrogenation of ethane to ethylene in solid oxide electrolysis cells[J]. Angewandte Chemie, 2019, 131(45): 16189-16192. |
| 21 | Yao R, Herrera J E, Chen L H, et al. Generalized mechanistic framework for ethane dehydrogenation and oxidative dehydrogenation on molybdenum oxide catalysts[J]. ACS Catalysis, 2020, 10(12): 6952-6968. |
| 22 | Bian Y X, Kim M, Li T, et al. Facile dehydrogenation of ethane on the IrO2(110) surface[J]. Journal of the American Chemical Society, 2018, 140(7): 2665-2672. |
| 23 | Xu X, Kumar Megarajan S, Xia X F, et al. Effect of reduction temperature on the structure and catalytic performance of mesoporous Ni-Fe-Al2O3 in oxidative dehydrogenation of ethane[J]. New Journal of Chemistry, 2020, 44(44): 18994-19001. |
| 24 | Park J L, Canizales K A, Argyle M D, et al. The effects of doping alumina with silica in alumina-supported NiO catalysts for oxidative dehydrogenation of ethane[J]. Microporous and Mesoporous Materials, 2020, 293: 109799. |
| 25 | 吴小平, 王晨光, 张琦, 等. PtSn-Mg(Zn)AlO催化剂应用于乙烷脱氢反应研究[J]. 化工学报, 2019, 70(11): 4268-4277. |
| Wu X P, Wang C G, Zhang Q, et al. Study on ethane dehydrogenation over PtSn-Mg(Zn)AlO catalyst[J]. CIESC Journal, 2019, 70(11): 4268-4277. | |
| 26 | 李倩倩, 唐思扬, 岳海荣, 等. Pd-Rh/TiO2光催化CO2氧化乙烷脱氢研究[J]. 化工学报, 2020, 71(8): 3556-3564. |
| Li Q Q, Tang S Y, Yue H R, et al. Study on the photocatalytic oxidative dehydrogenation of ethane with CO2 over Pd-Ph/TiO2 catalyst[J]. CIESC Journal, 2020, 71(8): 3556-3564. | |
| 27 | Yan B H, Yao S Y, Chen J G. Effect of oxide support on catalytic performance of FeNi-based catalysts for CO2-assisted oxidative dehydrogenation of ethane[J]. ChemCatChem, 2020, 12(2): 494-503. |
| 28 | Guo M, Feng K, Wang Y N, et al. Unveiling the role of active oxygen species in oxidative dehydrogenation of ethane with CO2 over NiFe/CeO2 [J]. ChemCatChem, 2021, 13(13): 3119-3131. |
| 29 | Ye L T, Duan X Y, Xie K. Electrochemical oxidative dehydrogenation of ethane to ethylene in a solid oxide electrolyzer[J]. Angewandte Chemie International Edition, 2021, 60(40): 21746-21750. |
| 30 | Gambo Y, Adamu S, Tanimu G, et al. CO2-mediated oxidative dehydrogenation of light alkanes to olefins: advances and perspectives in catalyst design and process improvement[J]. Applied Catalysis A: General, 2021, 623: 118273. |
| 31 | Xie Z H, Tian D, Xie M, et al. Interfacial active sites for CO2 assisted selective cleavage of C—C/C—H bonds in ethane[J]. Chem, 2020, 6(10): 2703-2716. |
| 32 | 李桂英, 鲁大学, 董淑娟, 等. CO2催化氧化乙烷的研究进展[J]. 天然气化工(C1化学与化工), 2011, 36(2): 50-54. |
| Li G Y, Lu D X, Dong S J, et al. Advances in oxidation of ethane using CO2 as oxidant[J]. Natural Gas Chemical Industry, 2011, 36(2): 50-54. | |
| 33 | Varghese J J, Mushrif S H. Insights into the C—H bond activation on NiO surfaces: the role of nickel and oxygen vacancies and of low valent dopants on the reactivity and energetics[J]. The Journal of Physical Chemistry C, 2017, 121(33): 17969-17981. |
| 34 | Zhao Z J, Liu S H, Zha S J, et al. Theory-guided design of catalytic materials using scaling relationships and reactivity descriptors[J]. Nature Reviews Materials, 2019, 4(12): 792-804. |
| 35 | Kim K H, You Y W, Jeong M H, et al. Influence of support acidity on CO2 reforming of ethane at high temperature[J]. Journal of CO2 Utilization, 2021, 53: 101713. |
| 36 | Saadi S, Abild-Pedersen F, Helveg S, et al. On the role of metal step-edges in graphene growth[J]. The Journal of Physical Chemistry C, 2010, 114(25): 11221-11227. |
| 37 | Pakhare D, Spivey J. A review of dry (CO2) reforming of methane over noble metal catalysts[J]. Chemical Society Reviews, 2014, 43(22): 7813-7837. |
| 38 | Jang W J, Shim J O, Kim H M, et al. A review on dry reforming of methane in aspect of catalytic properties[J]. Catalysis Today, 2019, 324: 15-26. |
| 39 | Abdulrasheed A, Jalil A A, Gambo Y, et al. A review on catalyst development for dry reforming of methane to syngas: recent advances[J]. Renewable and Sustainable Energy Reviews, 2019, 108: 175-193. |
| 40 | Yan B H, Yao S Y, Kattel S, et al. Active sites for tandem reactions of CO2 reduction and ethane dehydrogenation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(33): 8278-8283. |
| 41 | de Miguel S R, Vilella I M J, Maina S P, et al. Influence of Pt addition to Ni catalysts on the catalytic performance for long term dry reforming of methane[J]. Applied Catalysis A: General, 2012, 435/436: 10-18. |
| 42 | García-Diéguez M, Pieta I S, Herrera M C, et al. Improved Pt-Ni nanocatalysts for dry reforming of methane[J]. Applied Catalysis A: General, 2010, 377(1/2): 191-199. |
| 43 | Lonergan W W, Vlachos D G, Chen J G. Correlating extent of Pt-Ni bond formation with low-temperature hydrogenation of benzene and 1, 3-butadiene over supported Pt/Ni bimetallic catalysts[J]. Journal of Catalysis, 2010, 271(2): 239-250. |
| 44 | Lu S L, Lonergan W W, Zhu Y X, et al. Support effect on the low-temperature hydrogenation of benzene over PtCo bimetallic and the corresponding monometallic catalysts[J]. Applied Catalysis B: Environmental, 2009, 91(3/4): 610-618. |
| 45 | Appel L G, Eon J, Schmal M. The CO2–CeO2 interaction and its role in the CeO2 reactivity[J]. Catalysis Letters, 1998, 56: 199-202. |
| 46 | Raseale S, Marquart W, Jeske K, et al. Supported Fe x Ni y catalysts for the co-activation of CO2 and small alkanes[J]. Faraday Discussions, 2021, 229: 208-231. |
| [1] | 代宝民, 王启龙, 刘圣春, 张佳宁, 李鑫海, 宗凡迪. 非共沸工质辅助过冷CO2冷热联供系统的热力学性能分析[J]. 化工学报, 2023, 74(S1): 64-73. |
| [2] | 杨天阳, 邹慧明, 周晖, 王春磊, 田长青. -30℃电动汽车补气式CO2热泵制热性能实验研究[J]. 化工学报, 2023, 74(S1): 272-279. |
| [3] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [4] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [5] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [6] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [7] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [8] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [9] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [10] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [11] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [12] | 李贵贤, 曹阿波, 孟文亮, 王东亮, 杨勇, 周怀荣. 耦合固体氧化物电解槽的CO2制甲醇过程设计与评价研究[J]. 化工学报, 2023, 74(7): 2999-3009. |
| [13] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [14] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [15] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号