化工学报 ›› 2025, Vol. 76 ›› Issue (12): 6376-6386.DOI: 10.11949/0438-1157.20250288
彭郅众( ), 郎学磊, 房强, 井慧芳, 钟达忠(
), 郎学磊, 房强, 井慧芳, 钟达忠( ), 李晋平, 赵强(
), 李晋平, 赵强( )
)
收稿日期:2025-03-24
修回日期:2025-04-25
出版日期:2025-12-31
发布日期:2026-01-23
通讯作者:
钟达忠,赵强
作者简介:彭郅众(1998—),男,硕士研究生,pengzhizhong1054@link.tyut.edu.cn
基金资助:
Zhizhong PENG( ), Xuelei LANG, Qiang FANG, Huifang JING, dazhong ZHONG(
), Xuelei LANG, Qiang FANG, Huifang JING, dazhong ZHONG( ), Jinping LI, Qiang ZHAO(
), Jinping LI, Qiang ZHAO( )
)
Received:2025-03-24
Revised:2025-04-25
Online:2025-12-31
Published:2026-01-23
Contact:
dazhong ZHONG, Qiang ZHAO
摘要:
酸性条件下的电催化二氧化碳还原(CO2RR)因其能够提高CO2利用率并抑制碳酸盐形成而备受关注。然而,酸性环境由于H+浓度升高导致竞争性析氢反应(HER)加剧,从而对催化剂的稳定性和选择性提出了更高的要求。在此,报道了一种Ag-Sn双金属催化剂,在pH=2的酸性膜电极中表现出了优异的CO2RR性能。该催化剂在400 mA/cm2的电流密度下CO选择性高达99.4%,且在1 A/cm2的条件下,CO的法拉第效率(FECO)仍能维持在83.5%,CO的分电流密度最高达到了834.8 mA/cm2。研究发现,少量Sn的引入能够调节Ag的电子结构,从而加速CO2活化以及向*COOH中间体的转变。这项工作为开发适用于酸性条件的高效稳定CO2RR催化剂提供了新的思路。
中图分类号:
彭郅众, 郎学磊, 房强, 井慧芳, 钟达忠, 李晋平, 赵强. Ag-Sn间电子结构调控在酸性环境中实现1 A/cm2下高效CO2还原[J]. 化工学报, 2025, 76(12): 6376-6386.
Zhizhong PENG, Xuelei LANG, Qiang FANG, Huifang JING, dazhong ZHONG, Jinping LI, Qiang ZHAO. Ag-Sn interfacial electronic structure modulation for high-efficiency CO2 electroreduction at 1 A/cm2 under acidic conditions[J]. CIESC Journal, 2025, 76(12): 6376-6386.
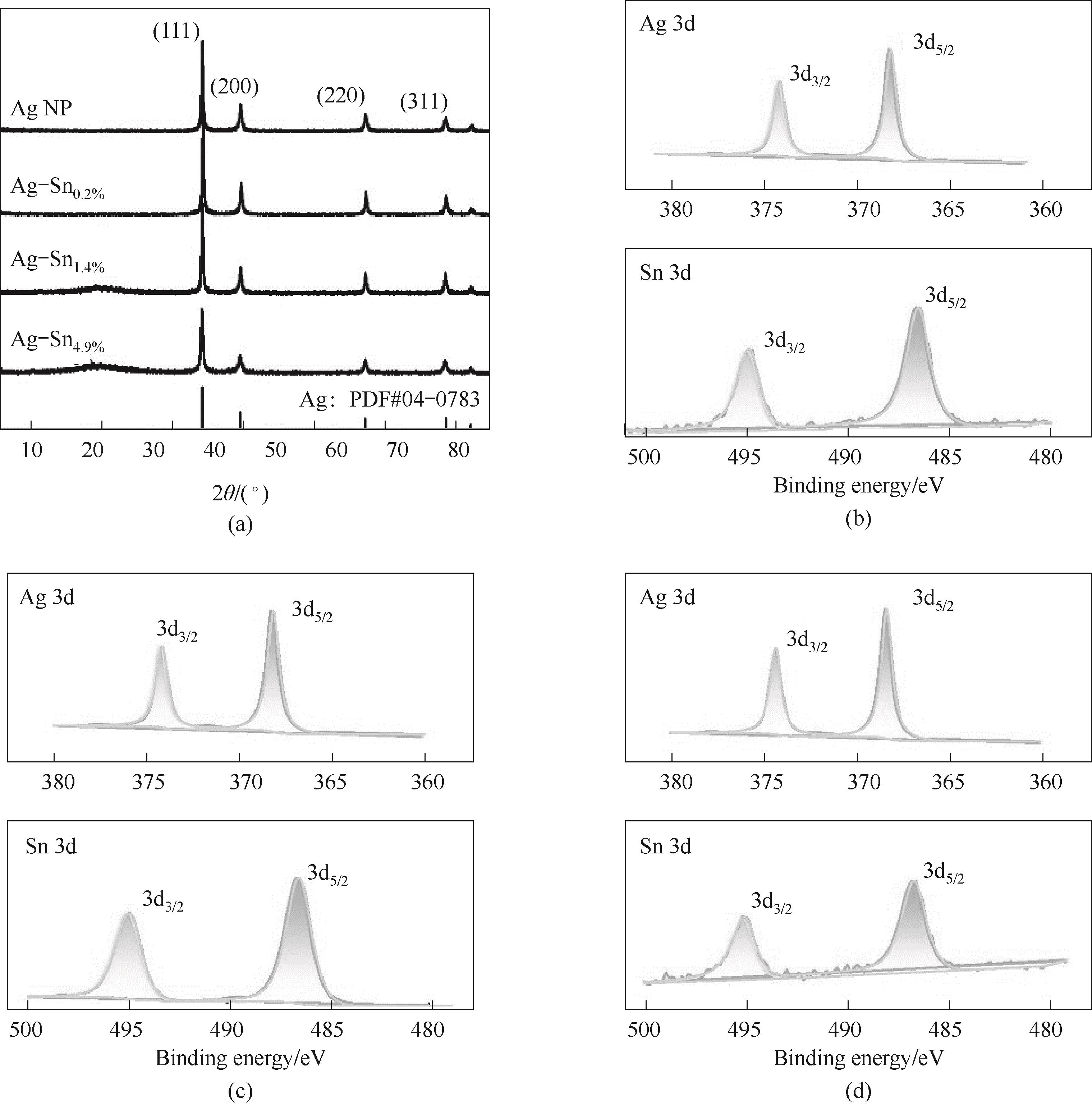
图3 (a) Ag-Sn及Ag NP的XRD谱图;(b~d) Ag-Sn4.9%,Ag-Sn1.4%,Ag-Sn0.2%的XPS谱图
Fig.3 (a) XRD of Ag-Sn and Ag NP; XPS for (b) Ag-Sn4.9%; (c) Ag-Sn1.4%; (d) Ag-Sn0.2%
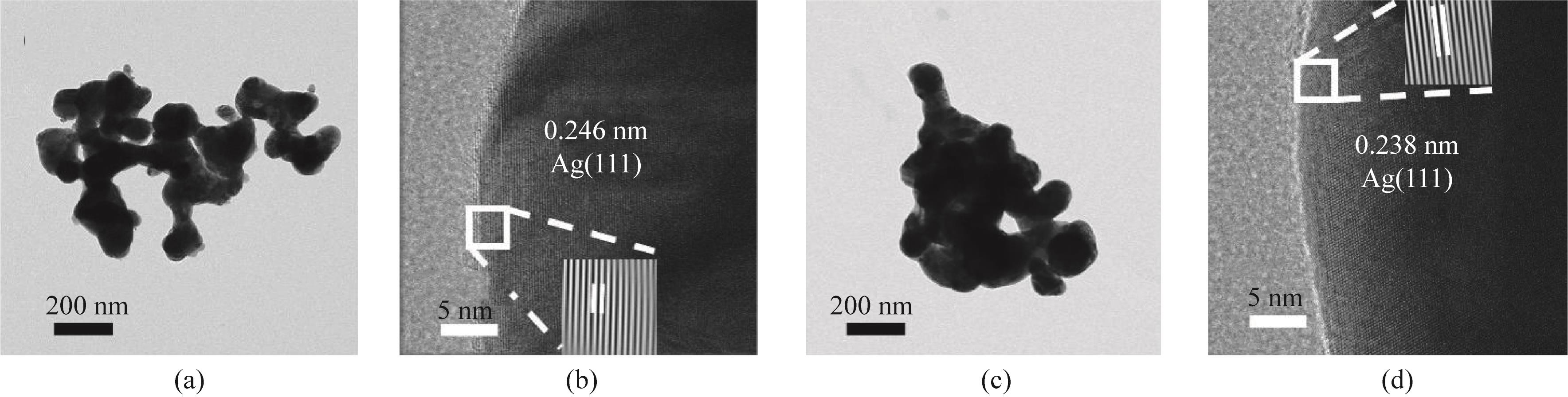
图5 (a)Ag-Sn1.4%的透射电镜图;(b)Ag-Sn1.4%的高分辨率透射电镜图;(c)Ag NP的透射电镜图;(d)Ag NP的高分辨率透射电镜图
Fig.5 (a) TEM of Ag-Sn1.4%; (b) HRTEM of Ag-Sn1.4%; (c) TEM of Ag NP; (d) HRTEM of Ag NP
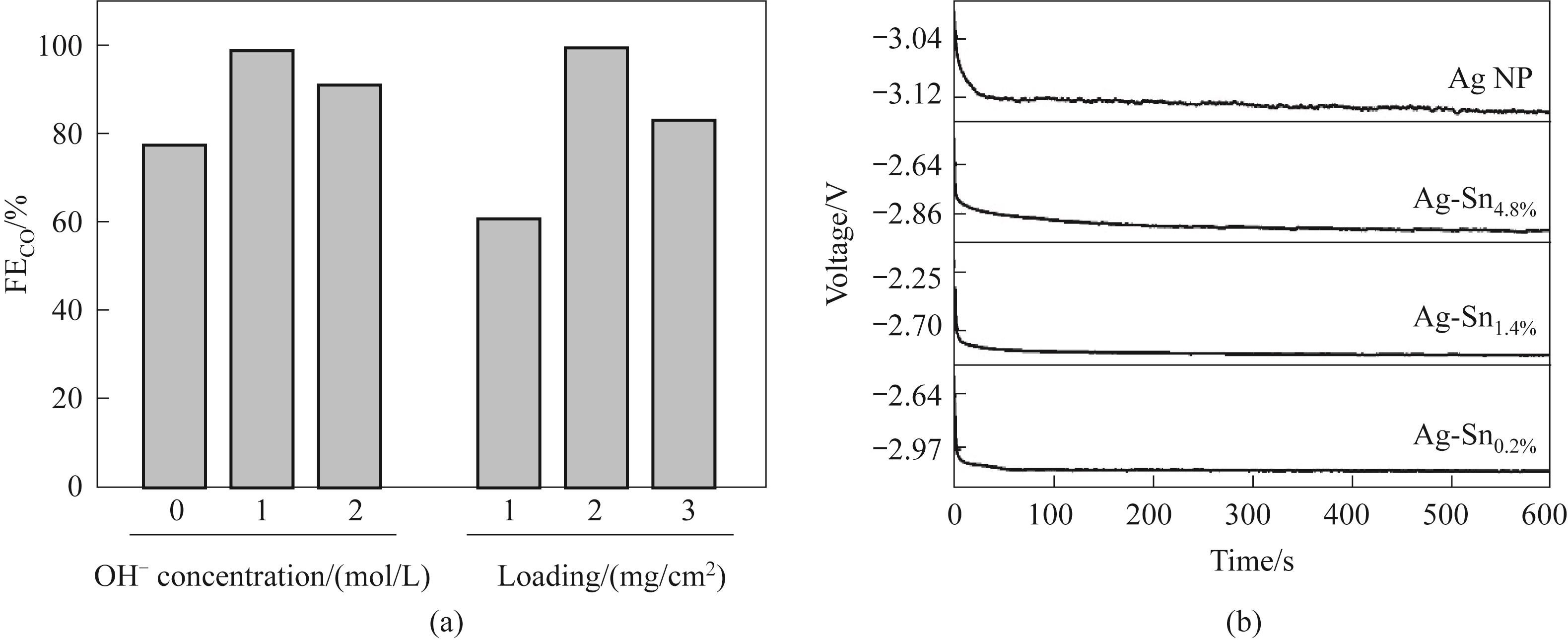
图9 (a)合成过程中反应液的pH及Ag-Sn催化剂的负载量对催化性能的影响;(b)Ag-Sn催化剂在50 mA/cm2下进行的预还原过程
Fig.9 (a) Effect of pH of reaction solution during synthesis and loading of Ag-Sn catalyst on catalytic performance; (b) Pre-reduction process of Ag-Sn at 50 mA/cm2
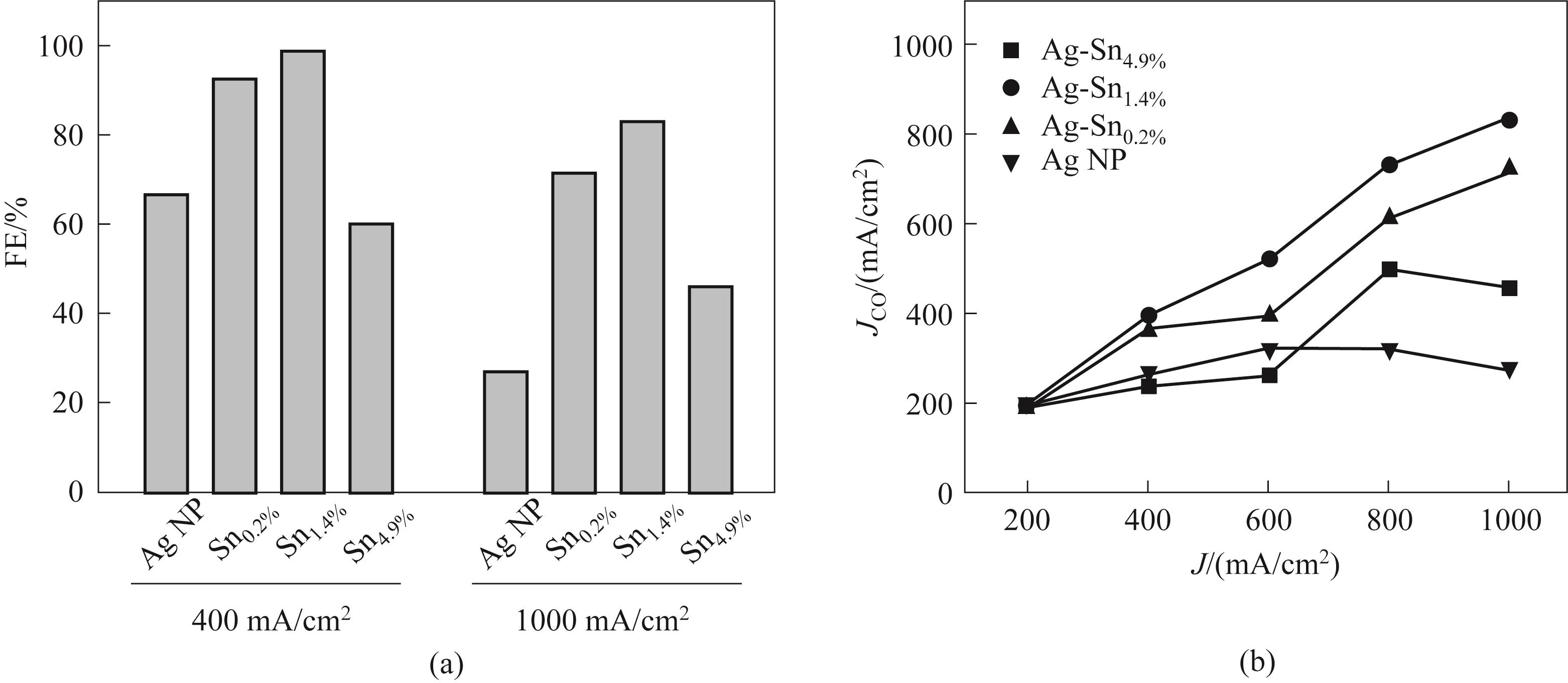
图11 (a)在400 mA/cm2和1 A/cm2下不同Sn含量样品的对比图;(b)CO分电流密度
Fig.11 (a) Comparison of samples with different Sn contents at 400 mA/cm2 and 1 A/cm2; (b) CO partial current density
| Catalyst | JTotal/(mA/cm2) | Electrolyte | FECO/% | Ref. |
|---|---|---|---|---|
| Ag-Sn1.4% | 1000 | K2SO4 + H2SO4 | 83.5 | this work |
| Ag-Sn1.4% | 400 | K2SO4 + H2SO4 | 99.4 | this work |
| Ni-N-C | 500 | K2SO4 + H2SO4 | 约80 | [ |
| Ag | 60 | Cs2SO4 + H2SO4 | 约80 | [ |
| Ag@C-d | 200 | K2SO4 + H2SO4 | 91.6 | [ |
| Ag∶Cs-DC | 400 | KHCO3 | 81.6 | [ |
| Ni1-NSC | 225 | KOH | 约100 | [ |
表1 膜电极CO2RR活性对比
Table 1 Comparison of catalytic activity in MEA
| Catalyst | JTotal/(mA/cm2) | Electrolyte | FECO/% | Ref. |
|---|---|---|---|---|
| Ag-Sn1.4% | 1000 | K2SO4 + H2SO4 | 83.5 | this work |
| Ag-Sn1.4% | 400 | K2SO4 + H2SO4 | 99.4 | this work |
| Ni-N-C | 500 | K2SO4 + H2SO4 | 约80 | [ |
| Ag | 60 | Cs2SO4 + H2SO4 | 约80 | [ |
| Ag@C-d | 200 | K2SO4 + H2SO4 | 91.6 | [ |
| Ag∶Cs-DC | 400 | KHCO3 | 81.6 | [ |
| Ni1-NSC | 225 | KOH | 约100 | [ |
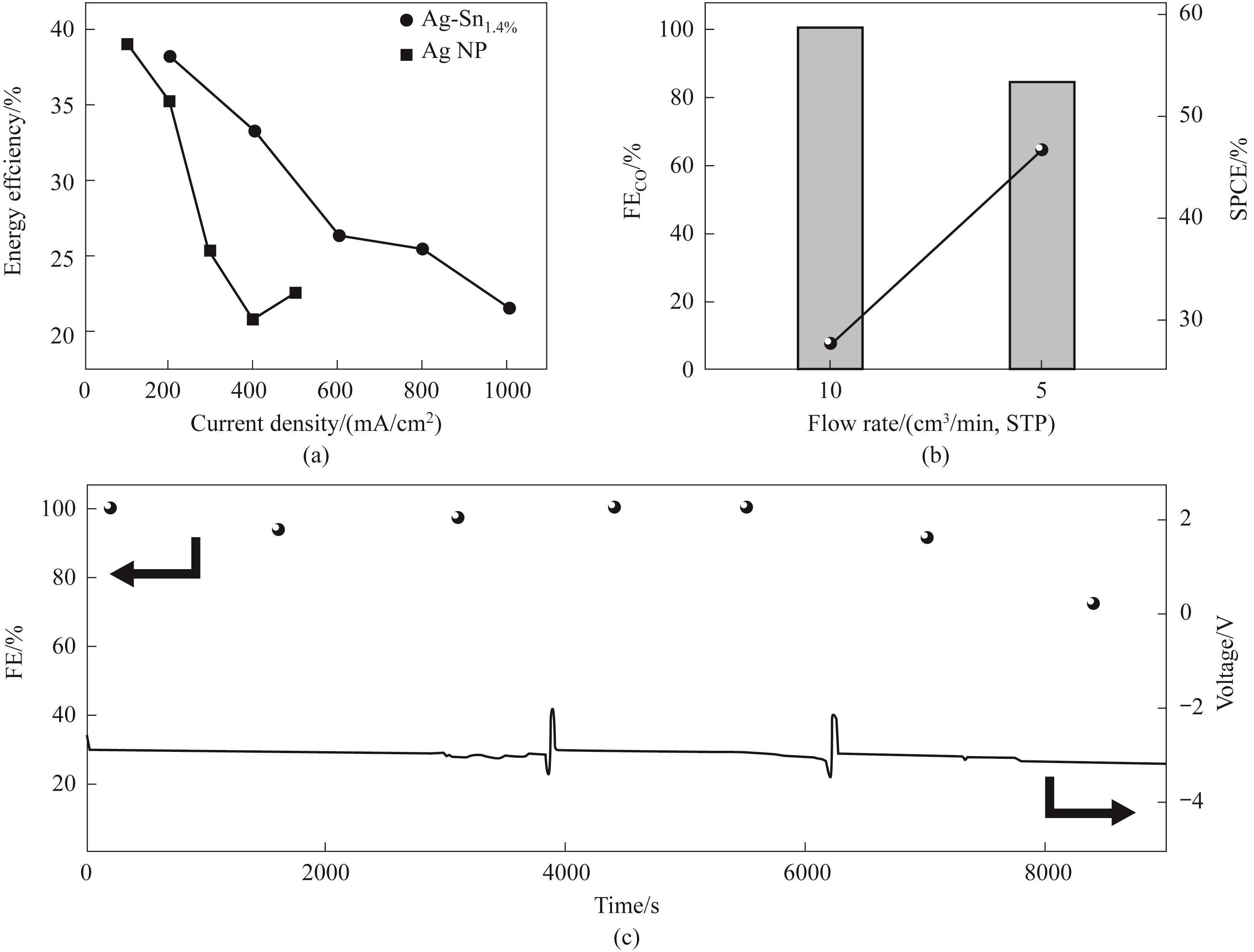
图12 (a)Ag-Sn1.4%与Ag NP的能量效率;(b)Ag-Sn1.4%的单程碳效率;(c)Ag-Sn1.4%在pH=2下的稳定性测试
Fig.12 (a) Energy efficiency of Ag-Sn1.4% and Ag NP; (b) SPCE of Ag-Sn1.4%; (c) Stability test of Ag-Sn1.4% at pH=2
| [1] | Leveni M, Bielicki J M. A potential for climate benign direct air CO2 capture with CO2-driven geothermal utilization and storage (DACCUS)[J]. Environmental Research Letters, 2023, 19(1): 014007. |
| [2] | Li C, Zhang T F, Qiu Z, et al. Plasma-assisted fabrication of multiscale materials for electrochemical energy conversion and storage[J]. Carbon Energy, 2025, 7(2): e641. |
| [3] | Yun H, Yoo S, Son J, et al. Strong cation concentration effect of Ni-N-C electrocatalysts in accelerating acidic CO2 reduction reaction[J]. Chem, 2025. DOI: 10.10161j.Chempr.2025.102461 |
| [4] | Terholsen D H, Huerta-Zerón H D, Möller C, et al. Photocatalytic CO2 reduction using CO2-binding enzymes[J]. Angewandte Chemie International Edition, 2024, 63(16): e202319313. |
| [5] | Bai S T, De Smet G, Liao Y H, et al. Homogeneous and heterogeneous catalysts for hydrogenation of CO2 to methanol under mild conditions[J]. Chemical Society Reviews, 2021, 50(7): 4259-4298. |
| [6] | Diehl T, Lanzerath P, Franciò D G, et al. A self-separating multiphasic system for catalytic hydrogenation of CO2 and CO2-derivatives to methanol[J]. ChemSusChem, 2022, 15(22): e202201250. |
| [7] | Pang Q Q, Fan X Z, Sun K H, et al. Nickel-nitrogen-carbon (Ni-N-C) electrocatalysts toward CO2 electroreduction to CO: advances, optimizations, challenges, and prospects[J]. Energy & Environmental Materials, 2024, 7(5): e12731. |
| [8] | Weng C C, Wang C, Song Y, et al. In-situ reconstruction of active bismuth for enhanced CO2 electroreduction to formate[J]. Chemical Engineering Journal, 2025, 505: 159732. |
| [9] | Zhang N, Zhang Y L. Recent advances in electrocatalytic conversion of CO2-to-ethylene: from reaction mechanisms to tuning strategies[J]. Applied Catalysis B: Environment and Energy, 2025, 363: 124822. |
| [10] | Zhou J, He B L, Huang P, et al. Regulating interfacial hydrogen-bonding networks by implanting Cu sites with perfluorooctane to accelerate CO2 electroreduction to ethanol[J]. Angewandte Chemie International Edition, 2024, 137(6): e202418459. |
| [11] | Gong S H, Han X, Li W S, et al. Paired electrolysis for efficient coproduction of CO and S8 with techno-economic analysis[J]. Chemical Engineering Journal, 2025, 507: 160286. |
| [12] | Huang J E, Li F W, Ozden A, et al. CO2 electrolysis to multicarbon products in strong acid[J]. Science, 2021, 372(6546): 1074-1078. |
| [13] | Gu J, Liu S, Ni W Y, et al. Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium[J]. Nature Catalysis, 2022, 5: 268-276. |
| [14] | Su L N, Hua Q F, Yang Y N, et al. Regulating competing reaction pathways for efficient CO2 electroreduction in acidic conditions[J]. Journal of Energy Chemistry, 2025, 105: 326-351. |
| [15] | Hernandez-Aldave S, Andreoli E. Fundamentals of gas diffusion electrodes and electrolysers for carbon dioxide utilisation: challenges and opportunities[J]. Catalysts, 2020, 10(6): 713. |
| [16] | Jouny M, Luc W, Jiao F,et al. General techno-economic analysis of CO2 electrolysis systems[J]. Industrial & Engineering Chemistry Research, 2018, 57(6): 2165-2177. |
| [17] | Gabardo C M, O'Brien C P, Edwards J P, et al. Continuous carbon dioxide electroreduction to concentrated multi-carbon products using a membrane electrode assembly[J]. Joule, 2019, 3(11): 2777-2791. |
| [18] | De Luna P, Hahn C, Higgins D, et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes?[J]. Science, 2019, 364(6438): eaav3506. |
| [19] | Jiang Y L, Huang L S, Chen C J, et al. Catalyst-electrolyte interface engineering propels progress in acidic CO2 electroreduction[J]. Energy & Environmental Science, 2025, 18(5): 2025-2049. |
| [20] | Goyal A, Marcandalli G, Mints V A, et al. Competition between CO2 reduction and hydrogen evolution on a gold electrode under well-defined mass transport conditions[J]. Journal of the American Chemical Society, 2020, 142(9): 4154-4161. |
| [21] | Liu Z K, Yan T, Shi H, et al. Acidic electrocatalytic CO2 reduction using space-confined nanoreactors[J]. ACS Applied Materials & Interfaces, 2022, 14(6): 7900-7908. |
| [22] | Sheng X D, Ge W X, Jiang H L, et al. Engineering the Ni-N-C catalyst microenvironment enabling CO2 electroreduction with nearly 100% CO selectivity in acid[J]. Advanced Materials, 2022, 34(38): 2201295. |
| [23] | Singh M R, Goodpaster J D, Weber A Z, et al. Mechanistic insights into electrochemical reduction of CO2 over Ag using density functional theory and transport models[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(42): E8812-E8821. |
| [24] | Wu X H, Guo Y N, Sun Z S, et al. Fast operando spectroscopy tracking in situ generation of rich defects in silver nanocrystals for highly selective electrochemical CO2 reduction[J]. Nature Communications, 2021, 12(1): 660. |
| [25] | Ren D W, Xu D A, Chan P K, et al. A cation concentration gradient approach to tune the selectivity and activity of CO2 electroreduction[J]. Angewandte Chemie International Edition, 2022, 134(49): e202214173. |
| [26] | Cai C, Liu B, Liu K, et al. Heteroatoms induce localization of the electric field and promote a wide potential-window selectivity towards CO in the CO2 electroreduction[J]. Angewandte Chemie International Edition, 2022, 61(44): e202212640. |
| [27] | Dai R Y, Sun K A, Shen R A, et al. Direct microenvironment modulation of CO2 electroreduction: negatively charged Ag sites going beyond catalytic surface reactions[J]. Angewandte Chemie International Edition, 2024, 63(37): e202408580. |
| [28] | Bokuniaeva A O, Vorokh A S. Estimation of particle size using the Debye equation and the Scherrer formula for polyphasic TiO2 powder[J]. Journal of Physics: Conference Series, 2019, 1410(1): 012057. |
| [29] | Sandoval M G, Walia J, Houache M S E, et al. CO2 adsorption and activation on Ag(111) surfaces in the presence of surface charge density: a static gas phase DFT study[J]. Applied Surface Science, 2023, 610: 155498. |
| [30] | Li H F, Li H B, Wei P F, et al. Tailoring acidic microenvironments for carbon-efficient CO2 electrolysis over a Ni-N-C catalyst in a membrane electrode assembly electrolyzer[J]. Energy & Environmental Science, 2023, 16(4): 1502-1510. |
| [31] | Pan B B, Fan J, Zhang J, et al. Close to 90% single-pass conversion efficiency for CO2 electroreduction in an acid-fed membrane electrode assembly[J]. ACS Energy Letters, 2022, 7(12): 4224-4231. |
| [32] | Zhang B, Zou J H, Chen Z H, et al. Defect-engineered carbon-confined silver for enhanced CO2 electrocatalytic reduction to CO in acidic media[J]. Next Nanotechnology, 2023, 2: 100014. |
| [33] | Sun Y H, Chen P J, Du X M, et al. Anchoring Cs+ ions on carbon vacancies for selective CO2 electroreduction to CO at high current densities in membrane electrode assembly electrolyzers[J]. Angewandte Chemie International Edition, 2024, 63(40): e202410802. |
| [34] | Chen Z Y, Wang C H, Zhong X, et al. Achieving efficient CO2 electrolysis to CO by local coordination manipulation of nickel single-atom catalysts[J]. Nano Letters, 2023, 23(15): 7046-7053. |
| [35] | Han J Y, Bai X, Xu X Q, et al. Advances and challenges in the electrochemical reduction of carbon dioxide[J]. Chemical Science, 2024, 15(21): 7870-7907. |
| [36] | Bohra D, Ledezma-Yanez I, Li G N, et al. Lateral adsorbate interactions inhibit HCOO- while promoting CO selectivity for CO2 electrocatalysis on silver[J]. Angewandte Chemie International Edition, 2019, 58(5): 1345-1349. |
| [37] | Chernyshova I V, Somasundaran P, Ponnurangam S. On the origin of the elusive first intermediate of CO2 electroreduction[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(40): E9261-E9270. |
| [38] | Lin Y X, Wang S C, Liu H J, et al. Regulating the electrocatalytic active centers for accelerated proton transfer towards efficient CO2 reduction[J]. National Science Review, 2025, 12(3): nwaf010. |
| [39] | Feng S J, Wang X J, Cheng D F, et al. Stabilizing *CO2 intermediates at the acidic interface using molecularly dispersed cobalt phthalocyanine as catalysts for CO2 reduction[J]. Angewandte Chemie International Edition, 2024, 63(8): e202317942. |
| [40] | Liu H M, Yan T, Tan S D, et al. Observation on microenvironment changes of dynamic catalysts in acidic CO2 reduction[J]. Journal of the American Chemical Society, 2024, 146(8): 5333-5342. |
| [41] | Zamora Zeledón J A, Stevens M B, Gunasooriya G T K K, et al. Tuning the electronic structure of Ag-Pd alloys to enhance performance for alkaline oxygen reduction[J]. Nature Communications, 2021, 12(1): 620. |
| [1] | 廖兵, 祝鑫宇, 黄倩倩, 胥雯, 寇梦瑶, 郭娜. 盐酸羟胺强化芬顿体系在近中性条件下去除2,4-DCP的性能及机理研究[J]. 化工学报, 2025, 76(8): 4273-4283. |
| [2] | 李同辉, 回天力, 郑涛, 张睿, 刘海燕, 刘植昌, 徐春明, 孟祥海. 氢氧化物协同钯双活性位点用于大电流和pH通用析氢反应[J]. 化工学报, 2025, 76(7): 3671-3685. |
| [3] | 吴雷, 胡紫璇, 高渊, 刘长波, 曹虎生, 刘田田, 朱瑞玉, 周军. 微波联合生物炭活化过硫酸盐氧化修复多环芳烃污染土壤研究[J]. 化工学报, 2025, 76(7): 3659-3670. |
| [4] | 赵清萍, 张敏, 赵辉, 王刚, 邱永福. 乙烯氢甲酯化合成丙酸甲酯的氢键作用机制及反应动力学研究[J]. 化工学报, 2025, 76(6): 2701-2713. |
| [5] | 何军, 李勇, 赵楠, 何孝军. 碳负载硒掺杂硫化钴在锂硫电池中的性能研究[J]. 化工学报, 2025, 76(6): 2995-3008. |
| [6] | 高越, 李丁, 高玉苗. 有机污染场地土壤催化氧化修复技术研究[J]. 化工学报, 2025, 76(3): 1297-1304. |
| [7] | 陈仲卿, 刘家旭, 王艳语, 井红权, 侯翠红, 屈凌波. K-B-Al体系对磷矿熔融特性及玻璃结构的影响[J]. 化工学报, 2025, 76(3): 1323-1333. |
| [8] | 杨田, 郭惠霞, 孙梦慈. CoFe2O4/CeO2复合材料活化PMS降解盐酸四环素[J]. 化工学报, 2025, 76(12): 6680-6695. |
| [9] | 王煜晨, 王万宗, 张鑫, 郭茂强, 周晓明, 盛利志. 高负载梓树豆荚壳衍生多孔碳材料的电化学性能研究[J]. 化工学报, 2025, 76(12): 6748-6760. |
| [10] | 尹胜强, 钟湘宇, 龚漫雨, 李露, 刘远征, 周寿斌, 肖俊兵, 刘昌会, 贾传坤. 活化桃胶碳基复合相变材料性能表征及导热增强研究[J]. 化工学报, 2025, 76(12): 6614-6625. |
| [11] | 邱家齐, 杨仲卿, 张志刚, 甘海龙, 霍春秀, 窦志帅, 冉景煜. Mn/Ce共掺杂强化氧物种转化与稀薄甲烷催化燃烧机制研究[J]. 化工学报, 2025, 76(11): 5604-5616. |
| [12] | 邹吉军, 刘宝宏, 史成香, 潘伦, 张香文. 综纤维素衍生物转化合成生物航空燃料的非均相催化剂研究进展[J]. 化工学报, 2025, 76(1): 1-17. |
| [13] | 张佳颖, 王聪, 王雅君. CNT-Co/Bi2O3催化剂光催化协同过硫酸盐活化高效降解四环素[J]. 化工学报, 2024, 75(9): 3163-3175. |
| [14] | 罗莉, 陈文尧, 张晶, 钱刚, 周兴贵, 段学志. 氧化铝结构与表面性质调控及其催化甲醇脱水制二甲醚性能研究[J]. 化工学报, 2024, 75(7): 2522-2532. |
| [15] | 刘旭升, 李泽洋, 杨宇森, 卫敏. 电催化二氧化碳还原制备气态产物的研究进展[J]. 化工学报, 2024, 75(7): 2385-2408. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号