化工学报 ›› 2025, Vol. 76 ›› Issue (8): 4239-4247.DOI: 10.11949/0438-1157.20250181
收稿日期:2025-02-26
修回日期:2025-05-22
出版日期:2025-08-25
发布日期:2025-09-17
通讯作者:
严密
作者简介:史松伟(1977—),男,高级工程师,syhxrd_ssw@163.com
基金资助:
Songwei SHI1( ), Cheng ZHAO1, Shuai LIU2, Yuxuan YING2, Mi YAN2(
), Cheng ZHAO1, Shuai LIU2, Yuxuan YING2, Mi YAN2( )
)
Received:2025-02-26
Revised:2025-05-22
Online:2025-08-25
Published:2025-09-17
Contact:
Mi YAN
摘要:
Fe-Zn/Al2O3吸附剂在H2S脱除方面展现了良好的性能,但其高昂的原料成本和复杂的制备过程增加了在大型沼气净化设施中的应用成本。为降低成本,本研究将富铁飞灰(FA)部分替代耦合Fe-Zn/Al2O3吸附剂,以改善沼气脱硫效果。研究结果表明,Zn/Fe摩尔比为3∶1的Zn-Fe/Al2O3复合吸附剂在室温条件下具有最佳的H2S脱除性能,其穿透容量达到3.234 mg/g。进一步加入FA作为粗脱硫剂,通过二段式吸附过程,显著提高了整体H2S穿透容量,增幅达到7.4%。结果表明,FA的添加能够有效降低沼气脱硫的成本,具有较好的应用前景。
中图分类号:
史松伟, 赵诚, 刘帅, 应雨轩, 严密. 富铁飞灰耦合Fe-Zn/Al2O3脱除沼气H2S研究[J]. 化工学报, 2025, 76(8): 4239-4247.
Songwei SHI, Cheng ZHAO, Shuai LIU, Yuxuan YING, Mi YAN. Removal of biogas H2S using iron-rich fly ash coupled with Fe-Zn/Al2O3[J]. CIESC Journal, 2025, 76(8): 4239-4247.
| 成分 | 含量/%(质量) |
|---|---|
| Na2O | 2.29 |
| Al2O3 | 14.28 |
| CaO | 4.64 |
| TiO2 | 1.2 |
| Fe2O3 | 53.85 |
| ZnO | 1.5 |
| 其他 | 22.24 |
表1 富铁FA中主要金属氧化物的含量
Table 1 Concentration of major metal oxides in iron-rich FA
| 成分 | 含量/%(质量) |
|---|---|
| Na2O | 2.29 |
| Al2O3 | 14.28 |
| CaO | 4.64 |
| TiO2 | 1.2 |
| Fe2O3 | 53.85 |
| ZnO | 1.5 |
| 其他 | 22.24 |

图3 不同Zn/Fe摩尔比的Zn-Fe/Al2O3吸附剂的N2吸脱附曲线(a)和计算得到的BJH孔径分布(b),不同Zn/Fe摩尔比的Zn-Fe/Al2O3吸附剂的FTIR光谱(c)
Fig.3 N2 adsorption/desorption curves (a) and calculated BJH pore distributions (b) of Zn-Fe/Al2O3 adsorbents with different Zn/Fe ratios, FTIR spectra of Zn-Fe/Al2O3 adsorbents with different Zn/Fe ratios (c)
| 样品 | 比表面积/(m2/g) | 孔容/(cm3/g) | 平均孔径/nm |
|---|---|---|---|
| Al2O3 | 165.94 | 0.26 | 6.74 |
| 1ZnFe | 182.85 | 0.44 | 7.12 |
| 2ZnFe | 182.82 | 0.45 | 7.06 |
| 3ZnFe | 181.53 | 0.44 | 7.10 |
表2 不同Zn/Fe摩尔比的Zn-Fe/Al2O3的孔隙特性
Table 2 Pore characteristics of Zn-Fe/Al2O3 with different Zn/Fe ratio
| 样品 | 比表面积/(m2/g) | 孔容/(cm3/g) | 平均孔径/nm |
|---|---|---|---|
| Al2O3 | 165.94 | 0.26 | 6.74 |
| 1ZnFe | 182.85 | 0.44 | 7.12 |
| 2ZnFe | 182.82 | 0.45 | 7.06 |
| 3ZnFe | 181.53 | 0.44 | 7.10 |
| 元素 | 含量/%(质量) | |
|---|---|---|
| 新鲜吸附剂 | 废吸附剂 | |
| Zn | 11.24 | 10.78 |
| Fe | 3.56 | 3.02 |
| Al | 44.96 | 43.56 |
| O | 40.06 | 41.81 |
| S | 0.08 | 0.73 |
表3 新鲜吸附剂和穿透后吸附剂的元素分析
Table 3 Concentration of each element in fresh and spent adsorbent
| 元素 | 含量/%(质量) | |
|---|---|---|
| 新鲜吸附剂 | 废吸附剂 | |
| Zn | 11.24 | 10.78 |
| Fe | 3.56 | 3.02 |
| Al | 44.96 | 43.56 |
| O | 40.06 | 41.81 |
| S | 0.08 | 0.73 |
| 吸附剂 | 比表面积/(m2/g) | 孔容/(cm3/g) | 平均孔径/nm |
|---|---|---|---|
| 新鲜吸附剂 | 181.53 | 0.44 | 7.10 |
| 废吸附剂 | 148.39 | 0.41 | 7.31 |
表4 穿透前后吸附剂的孔隙特性
Table 4 Pore characteristics of adsorbents before and after breakthrough
| 吸附剂 | 比表面积/(m2/g) | 孔容/(cm3/g) | 平均孔径/nm |
|---|---|---|---|
| 新鲜吸附剂 | 181.53 | 0.44 | 7.10 |
| 废吸附剂 | 148.39 | 0.41 | 7.31 |
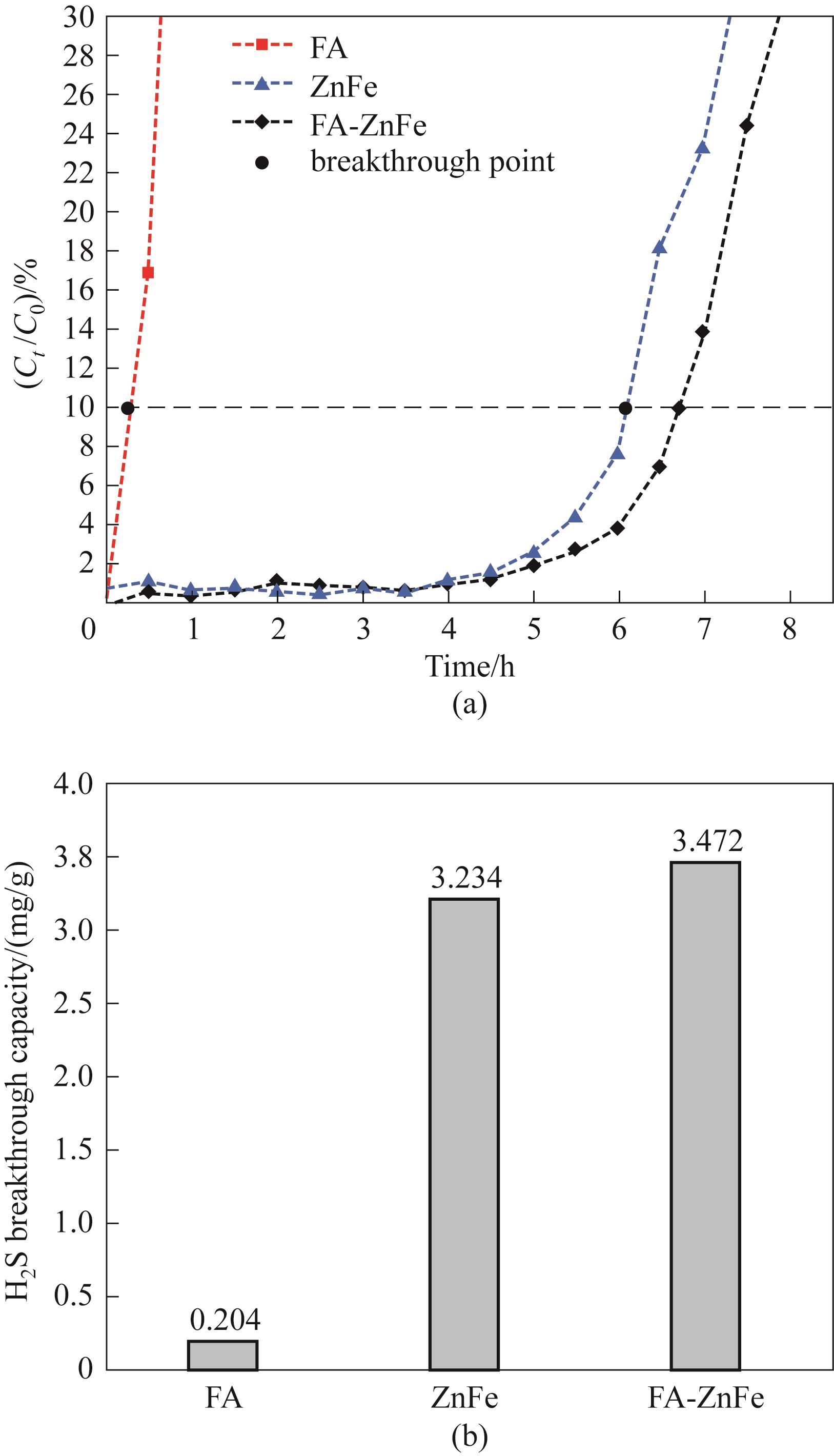
图6 FA&Zn-Fe/Al2O3二段式吸附的H2S穿透曲线(a)和H2S穿透容量(b)
Fig.6 H2S breakthrough curves (a) and H2S breakthrough capacity (b) of two-step FA&Zn-Fe/Al2O3 adsorption
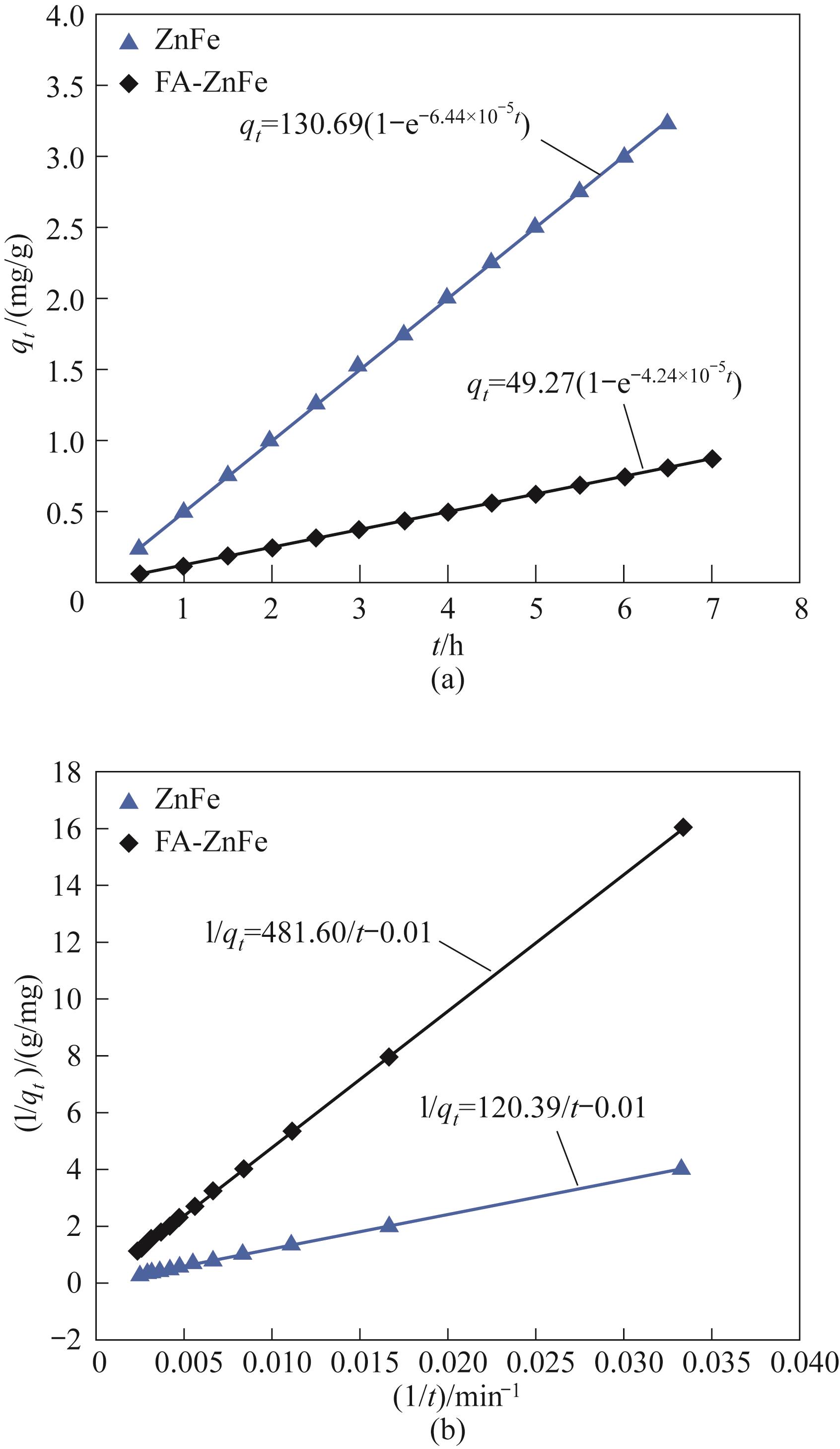
图7 单独使用ZnFe吸附剂及FA-ZnFe二段式吸附的拟一阶动力学拟合(a)和拟二阶动力学拟合(b)
Fig.7 Proposed first-order kinetic fit (a) and proposed second-order kinetic fit (b) for ZnFe adsorbent alone and FA-ZnFe binary adsorption
| 项目 | 拟一阶动力学 | 拟二阶动力学 | ||||
|---|---|---|---|---|---|---|
| k1 | qe | R2 | k2 | qe | R2 | |
| ZnFe | 6.44×10-5 | 130.69 | 0.9999 | 8.31×10-7 | -100 | 0.9999 |
| FA-ZnFe | 4.24×10-5 | 49.27 | 0.9999 | 2.08×10-7 | -100 | 0.9999 |
表5 动力学拟合结果汇总
Table 5 Summary of the results of the kinetic fitting
| 项目 | 拟一阶动力学 | 拟二阶动力学 | ||||
|---|---|---|---|---|---|---|
| k1 | qe | R2 | k2 | qe | R2 | |
| ZnFe | 6.44×10-5 | 130.69 | 0.9999 | 8.31×10-7 | -100 | 0.9999 |
| FA-ZnFe | 4.24×10-5 | 49.27 | 0.9999 | 2.08×10-7 | -100 | 0.9999 |
| [1] | Chan Y H, Lock S S M, Wong M K, et al. A state-of-the-art review on capture and separation of hazardous hydrogen sulfide (H2S): Recent advances, challenges and outlook[J]. Environmental Pollution, 2022, 314: 120219. |
| [2] | Cristiano D M, de A Mohedano R, Nadaleti W C, et al. H2S adsorption on nanostructured iron oxide at room temperature for biogas purification: application of renewable energy[J]. Renewable Energy, 2020, 154: 151-160. |
| [3] | Feng Y, Wang J C, Hu Y F, et al. Microwave heating motivated performance promotion and kinetic study of iron oxide sorbent for coal gas desulfurization[J]. Fuel, 2020, 267: 117215. |
| [4] | Haider J, Saeed S, Qyyum M A, et al. Simultaneous capture of acid gases from natural gas adopting ionic liquids: challenges, recent developments, and prospects[J]. Renewable and Sustainable Energy Reviews, 2020, 123: 109771. |
| [5] | Cho S H, Lee S, Kim Y, et al. Applications of agricultural residue biochars to removal of toxic gases emitted from chemical plants: a review[J]. Science of the Total Environment, 2023, 868: 161655. |
| [6] | Ma Y L, Guo H F, Selyanchyn R, et al. Hydrogen sulfide removal from natural gas using membrane technology: a review[J]. Journal of Materials Chemistry A, 2021, 9(36): 20211-20240. |
| [7] | Zheng X H, Li Y L, Zhang L Y, et al. Insight into the effect of morphology on catalytic performance of porous CeO2 nanocrystals for H2S selective oxidation[J]. Applied Catalysis B: Environmental, 2019, 252: 98-110. |
| [8] | Chen J N, Xu W T, Zhu J, et al. Highly effective direct decomposition of H2S by microwave catalysis on core-shell Mo2N-MoC@SiO2 microwave catalyst[J]. Applied Catalysis B: Environmental, 2020, 268: 118454. |
| [9] | Li L, Sun T H, Shu C H, et al. Low temperature H2S removal with 3-D structural mesoporous molecular sieves supported ZnO from gas stream[J]. Journal of Hazardous Materials, 2016, 311: 142-150. |
| [10] | Baird T, Denny P J, Hoyle R, et al. Modified zinc oxide absorbents for low-temperature gas desulfurisation[J]. Journal of the Chemical Society, Faraday Transactions, 1992, 88(22): 3375-3382. |
| [11] | Jiang D H, Su L H, Ma L, et al. Cu–Zn–Al mixed metal oxides derived from hydroxycarbonate precursors for H2S removal at low temperature[J]. Applied Surface Science, 2010, 256(10): 3216-3223. |
| [12] | Pahalagedara L R, Poyraz A S, Song W Q, et al. Low temperature desulfurization of H2S: high sorption capacities by mesoporous cobalt oxide via increased H2S diffusion[J]. Chemistry of Materials, 2014, 26(22): 6613-6621. |
| [13] | Cecilia J A, Soriano M D, Marques Correia L, et al. Fe2O3 supported on hollow micro/mesospheres silica for the catalytic partial oxidation of H2S to sulfur[J]. Microporous and Mesoporous Materials, 2020, 294: 109875. |
| [14] | Wu M M, Guo E H, Li Q C, et al. Mesoporous Zn-Fe-based binary metal oxide sorbent with sheet-shaped morphology: synthesis and application for highly efficient desulfurization of hot coal gas[J]. Chemical Engineering Journal, 2020, 389: 123750. |
| [15] | Li Z S, Liu T, Sun Y J, et al. Well-dispersed CuFe doping nanoparticles with mixed valence in carbon aerogel as effective adsorbent for H2S removal at low temperature[J]. Fuel Processing Technology, 2023, 245: 107744. |
| [16] | Yang C, Florent M, de Falco G, et al. ZnFe2O4/activated carbon as a regenerable adsorbent for catalytic removal of H2S from air at room temperature[J]. Chemical Engineering Journal, 2020, 394: 124906. |
| [17] | Mostbauer P, Lombardi L, Olivieri T, et al. Pilot scale evaluation of the BABIU process—upgrading of landfill gas or biogas with the use of MSWI bottom ash[J]. Waste Management, 2014, 34(1): 125-133. |
| [18] | Pham C H, Saggar S, Berben P, et al. Removing hydrogen sulfide contamination in biogas produced from animal wastes[J]. Journal of Environmental Quality, 2019, 48(1): 32-38. |
| [19] | Gasquet V, Kim B, Bonhomme A, et al. Sewage sludge ash-derived materials for H2S removal from a landfill biogas[J]. Waste Management, 2021, 136: 230-237. |
| [20] | Rahim D A, Fang W, Zhu G J, et al. Microwave-assisted synthesis of Zn-Fe adsorbent supported on alumina: effect of Zn to Fe ratio on syngas desulfurization performance[J]. Chemical Engineering and Processing - Process Intensification, 2021, 168: 108565. |
| [21] | Thinakaran N, Baskaralingam P, Pulikesi M, et al. Removal of Acid Violet 17 from aqueous solutions by adsorption onto activated carbon prepared from sunflower seed hull[J]. Journal of Hazardous Materials, 2008, 151(2/3): 316-322. |
| [22] | Khamizov R K. A pseudo-second order kinetic equation for sorption processes[J]. Russian Journal of Physical Chemistry A, 2020, 94(1): 171-176. |
| [23] | Wu M M, Shi L, Lim T T, et al. Ordered mesoporous Zn-based supported sorbent synthesized by a new method for high-efficiency desulfurization of hot coal gas[J]. Chemical Engineering Journal, 2018, 353: 273-287. |
| [24] | Thommes M, Kaneko K, Neimark A V, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure and Applied Chemistry, 2015, 87(9/10): 1051-1069. |
| [25] | Samokhvalov A, Tatarchuk B J. Characterization of active sites, determination of mechanisms of H2S, COS and CS2 sorption and regeneration of ZnO low-temperature sorbents: past, current and perspectives[J]. Physical Chemistry Chemical Physics, 2011, 13(8): 3197-3209. |
| [26] | 苏子兵, 武蒙蒙, 贾磊, 等. 固相法制备半焦负载铁酸锌脱硫剂的脱硫行为 [J]. 化工进展, 2017, 36(7): 2684-2690. |
| Su Z B, Wu M M, Jia L, et al. Preparation of zinc ferrite sorbent by mechanochemical method for the removal of H2S from hot coal gas [J]. Chemical Industry and Engineering Progress, 2017, 36(7): 2684-2690. | |
| [27] | Yang C, de Falco G, Florent M, et al. Support features govern the properties of the active phase and the performance of bifunctional ZnFe2O4-based H2S adsorbents[J]. Carbon, 2020, 169: 327-337. |
| [28] | 李俏春. SBA-15负载锌基氧化物的煤气脱硫与再生行为研究 [D]. 太原: 太原理工大学, 2021. |
| Li Q C. Study on desulfurization and regeneration behavior of coal gas with SBA-15 loaded zinc-based oxides [D]. Taiyuan: Taiyuan University of Technology, 2021. | |
| [29] | Hernández S P, Chiappero M, Russo N, et al. A novel ZnO-based adsorbent for biogas purification in H2 production systems[J]. Chemical Engineering Journal, 2011, 176: 272-279. |
| [30] | Seredych M, Strydom C, Bandosz T J. Effect of fly ash addition on the removal of hydrogen sulfide from biogas and air on sewage sludge-based composite adsorbents[J]. Waste Management, 2008, 28(10): 1983-1992. |
| [31] | Jepleting A, Mecha A C, Sombei D, et al. Potential of low-cost materials for biogas purification, a review of recent developments[J]. Renewable and Sustainable Energy Reviews, 2025, 210: 115152. |
| [32] | Bao J C, Sun X, Ning P, et al. Industrial solid wastes to environmental protection materials for removal of gaseous pollutants: a review[J]. Green Energy & Environment, 2025, 10(1): 34-83. |
| [1] | 潘煜, 王子航, 王佳韵, 王如竹, 张华. 基于可得然-氯化锂复合吸附剂的除湿换热器热湿性能研究[J]. 化工学报, 2023, 74(3): 1352-1359. |
| [2] | 邓超和, 王佳韵, 李金凤, 刘业凤, 王如竹. 可低温驱动的凝胶复合吸附剂的制备及吸/脱附性能研究[J]. 化工学报, 2021, 72(8): 4401-4409. |
| [3] | 李威, 王秋旺, 曾敏. 水合盐基中低温热化学储热材料性能测试及数值研究[J]. 化工学报, 2021, 72(5): 2763-2772. |
| [4] | 田军鹏, 沈圆辉, 张东辉, 唐忠利. 规整复合吸附剂真空变压吸附分离CH4/N2工艺模拟与分析[J]. 化工学报, 2021, 72(11): 5675-5685. |
| [5] | 赵惠忠, 雷敏, 黄天厚, 刘涛, 张敏. 复合吸附剂MWCNT/MgCl2的水蒸气吸附性能[J]. 化工学报, 2020, 71(S1): 272-281. |
| [6] | 赵惠忠, 程俊峰, 唐祥虎, 张少波. 多壁碳纳米管嵌入13X/MgCl2复合吸附剂的性能实验[J]. 化工学报, 2017, 68(5): 1860-1865. |
| [7] | 朱芳啟, 江龙, 王丽伟, 王如竹. MnCl2/CaCl2-NH3再吸附系统的制冷性能[J]. 化工学报, 2016, 67(S2): 32-37. |
| [8] | 高娇, 王丽伟, 周志松, 王如竹. 多盐复合吸附剂的非平衡吸附/解吸特性[J]. 化工学报, 2016, 67(S2): 184-190. |
| [9] | 赖艳华, 吴涛, 赵琳妍, 董震, 郝宗华, 陈常念, 吕明新. 低温吸湿复合吸附剂的制备及吸湿性能[J]. 化工学报, 2015, 66(S1): 154-158. |
| [10] | 王令宝1,张 刚2,卜宪标1,李华山1,3. 制冷用木屑基质复合吸附剂的制备及性能测试[J]. 化工进展, 2013, 32(06): 1357-1362. |
| [11] | 龚丽霞,王如竹,陈传涓. 复合吸附剂的基质选择与动态吸附性能测试 [J]. CIESC Journal, 2010, 61(S2): 25-29. |
| [12] | 李程, 王如竹, 王丽伟, 李廷贤, 陈宇. 不同驱动温度下间歇性太阳能吸附制冰机的实验研究 [J]. 化工学报, 2010, 61(S2): 112-115. |
| [13] | 陈恒, 李廷贤, 王丽伟, 吴静怡, 王如竹, Oliveira R G. 太阳能吸附式空调固化复合吸附剂性能 [J]. 化工学报, 2009, 60(5): 1097-1103. |
| [14] | 李廷贤;王如竹;陈恒;王丽伟;吴静怡. 采用固化复合吸附剂的热化学吸附式低温冷冻系统的性能 [J]. CIESC Journal, 2008, 59(S2): 192-198. |
| [15] | 陈砺, 王丽, 王红林, 董鹏飞. 化学吸附制冷用氯化锶复合吸附剂的制冷性能 [J]. 化工学报, 2008, 59(4): 836-842. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号