化工学报 ›› 2019, Vol. 70 ›› Issue (11): 4437-4448.DOI: 10.11949/0438-1157.20190558
收稿日期:2019-05-23
修回日期:2019-07-31
出版日期:2019-11-05
发布日期:2019-11-05
通讯作者:
尤东江
作者简介:尤东江(1981—),男,博士,高级工程师,基金资助:
Dongjiang YOU( ),Jianyun WEI,Xuejing LI,Jingyuan LOU
),Jianyun WEI,Xuejing LI,Jingyuan LOU
Received:2019-05-23
Revised:2019-07-31
Online:2019-11-05
Published:2019-11-05
Contact:
Dongjiang YOU
摘要:
液流电池通常采用对角平推流流场,会形成电解液滞留区,造成电池局部浓差极化大,影响综合性能。鉴于此,提出了一种基于框架设计的流场优化方法,通过设计电极框架,可以得到“蛇形流道”和“平行流道”两类流场。以全钒液流电池为例,通过数学建模,研究了不同流场结构和参数对于多孔电极内电解液流动特性、电化学反应和温度变化特性的影响规律。计算结果与实验结果一致性良好,结果表明:电解液在“平行流场”内的流动均匀性比在“蛇形流场”内好,且不存在滞留区,同时在“平行流场”内浓差极化也较“蛇形流场”低;此外,对于同样的电极面积,在电极内部的“平行流道”越多,电解液的流速分布越均匀,反应特性越好。
中图分类号:
尤东江, 魏建云, 李雪菁, 娄景媛. 基于框架设计的液流电池流场优化模拟研究[J]. 化工学报, 2019, 70(11): 4437-4448.
Dongjiang YOU, Jianyun WEI, Xuejing LI, Jingyuan LOU. Simulation study on flow field optimization of flow battery based on flow frame design[J]. CIESC Journal, 2019, 70(11): 4437-4448.
| 名称 | 控制方程 | 源项 | 边界条件 |
|---|---|---|---|
| 质量守恒方程 | | ||
| 动量守恒方程 | | | p=p in p out =0 |
| 物质守恒方程 | | V2 + : V3 + : V4 + : V5 + : | |
| 电荷守恒方程 | | | 电极和集流体界面上: 电极和隔膜界面上: |
| 能量守恒方程 | | | 入口: 出口: |
| Butler-Volmer 定律 | | ||
| 浓差过电位 | | ||
| Nernst方程 | |
表1 模型控制方程和边界条件
Table 1 Governing equations and boundary conditions
| 名称 | 控制方程 | 源项 | 边界条件 |
|---|---|---|---|
| 质量守恒方程 | | ||
| 动量守恒方程 | | | p=p in p out =0 |
| 物质守恒方程 | | V2 + : V3 + : V4 + : V5 + : | |
| 电荷守恒方程 | | | 电极和集流体界面上: 电极和隔膜界面上: |
| 能量守恒方程 | | | 入口: 出口: |
| Butler-Volmer 定律 | | ||
| 浓差过电位 | | ||
| Nernst方程 | |
| 参数 | 符号 | 数值 | 单位 |
|---|---|---|---|
| 电极的初始孔隙率 | | 0.929 | — |
| 电极的初始比表面积 | | 1.62×104 | m2 |
| 电极表观尺寸 | L×W×H | 60×75×5 | mm |
| 电极电子电导率 | | 3×103 | S· m-1 |
| 电极纤维直径 | d f | 1.76×10-5 | m |
| Carman-Kozeny常数 | K CK | 4.28 | — |
| 有效电极表观面积 | A | 45 | cm2 |
| 电极表观厚度 | d | 5 | mm |
| 电解液浓度 | | 1290 | kg·m-3 |
| 电解液黏度 | | 4.928×10-3 | Pa·s |
| 钒离子初始浓度 | | 1500 | mol·m-3 |
| 质子的初始浓度 | | 4500 | mol·m-3 |
| 质子扩散系数 | | 9.312×10-9 | m2·s-1 |
| V2+ 扩散系数 | | 2.4×10-10 | m2·s-1 |
| V3+ 扩散系数 | | 2.4×10-10 | m2·s-1 |
| V4+ 扩散系数 | | 3.9×10-10 | m2·s-1 |
| V5+ 扩散系数 | | 3.9×10-10 | m2·s-1 |
| 负极名义反应速率常数 | | 1.7×10-7 | m·s-1 |
| 正极名义反应速率常数 | | 6.8×10-7 | m·s-1 |
| 阳极反应传递系数 | | 0.45 | — |
| 阴极反应传递系数 | | 0.55 | — |
| V2+/V3+平衡电势 | | -0.255 | V |
| V4+/V5+平衡电势 | | 1.004 | V |
| 电解液的热导率 | | 0.67 | W·m-1·K-1 |
| 电极的热导率 | | 0.15 | W·m-1·K-1 |
| 电解液的热容 | | 4.19×106 | J·m-3·K-1 |
| 电极的热容 | | 3.33×105 | J·m-3·K-1 |
| 负极反应的熵变 | - | -100 | J·mol-1·K-1 |
| 操作温度 | T | 298.15 | K |
表2 物理及操作参数
Table 2 Physical and operational parameters
| 参数 | 符号 | 数值 | 单位 |
|---|---|---|---|
| 电极的初始孔隙率 | | 0.929 | — |
| 电极的初始比表面积 | | 1.62×104 | m2 |
| 电极表观尺寸 | L×W×H | 60×75×5 | mm |
| 电极电子电导率 | | 3×103 | S· m-1 |
| 电极纤维直径 | d f | 1.76×10-5 | m |
| Carman-Kozeny常数 | K CK | 4.28 | — |
| 有效电极表观面积 | A | 45 | cm2 |
| 电极表观厚度 | d | 5 | mm |
| 电解液浓度 | | 1290 | kg·m-3 |
| 电解液黏度 | | 4.928×10-3 | Pa·s |
| 钒离子初始浓度 | | 1500 | mol·m-3 |
| 质子的初始浓度 | | 4500 | mol·m-3 |
| 质子扩散系数 | | 9.312×10-9 | m2·s-1 |
| V2+ 扩散系数 | | 2.4×10-10 | m2·s-1 |
| V3+ 扩散系数 | | 2.4×10-10 | m2·s-1 |
| V4+ 扩散系数 | | 3.9×10-10 | m2·s-1 |
| V5+ 扩散系数 | | 3.9×10-10 | m2·s-1 |
| 负极名义反应速率常数 | | 1.7×10-7 | m·s-1 |
| 正极名义反应速率常数 | | 6.8×10-7 | m·s-1 |
| 阳极反应传递系数 | | 0.45 | — |
| 阴极反应传递系数 | | 0.55 | — |
| V2+/V3+平衡电势 | | -0.255 | V |
| V4+/V5+平衡电势 | | 1.004 | V |
| 电解液的热导率 | | 0.67 | W·m-1·K-1 |
| 电极的热导率 | | 0.15 | W·m-1·K-1 |
| 电解液的热容 | | 4.19×106 | J·m-3·K-1 |
| 电极的热容 | | 3.33×105 | J·m-3·K-1 |
| 负极反应的熵变 | - | -100 | J·mol-1·K-1 |
| 操作温度 | T | 298.15 | K |
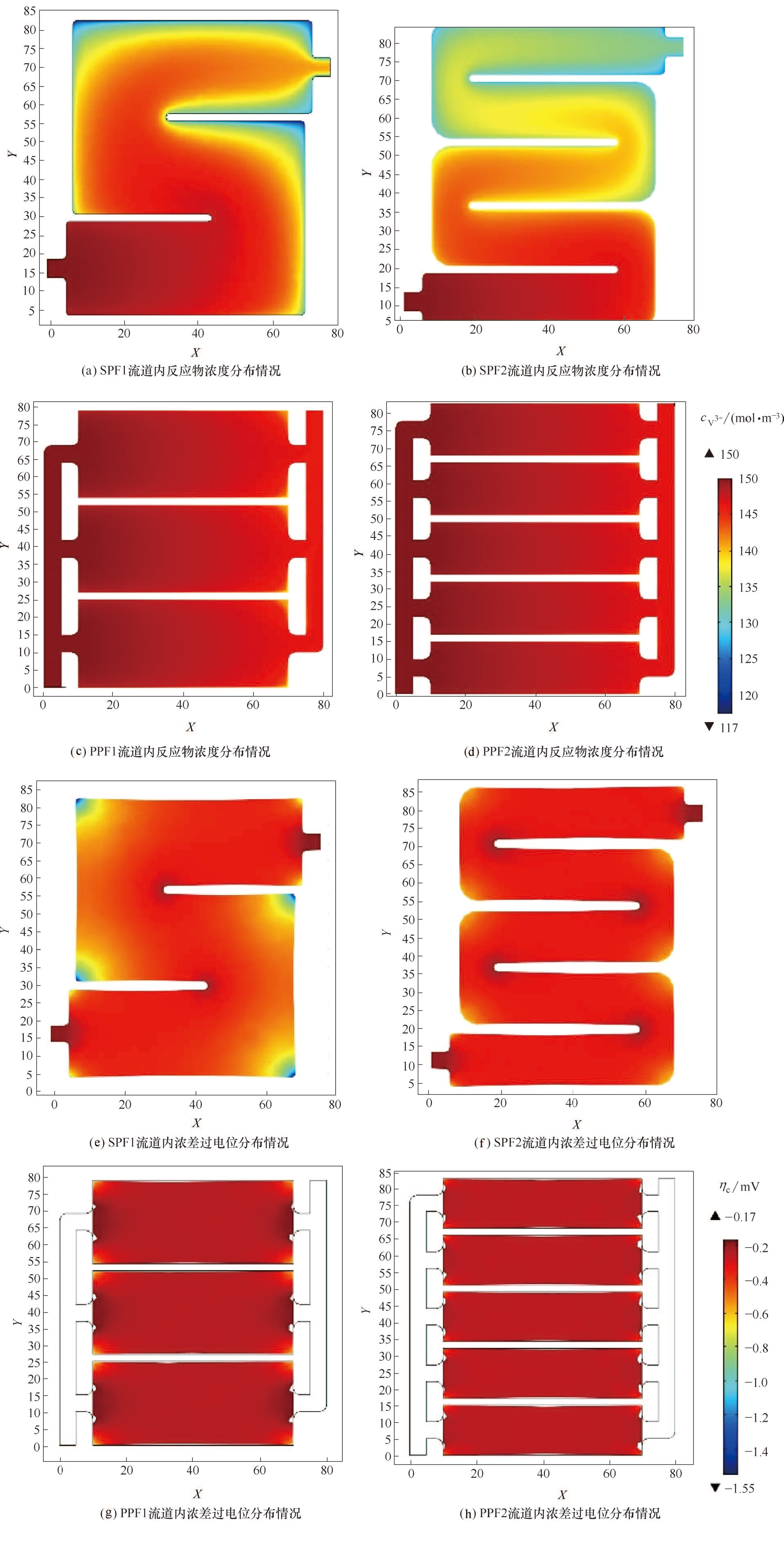
图7 不同流道内反应物V3+浓度[(a)~(d)]和浓差过电位[(e)~(h)]在XY平面(Z=2.5 mm)上的分布
Fig.7 Distribution of electrolyte concentration of V3+ [(a)~(d)] and concentration over-potential [(e)—(h)] on Z=2.5 mm XY plane in different flow channels
| 1 | Ma X K , Zhang H M , Xing F . A three-dimensional model for negative half cell of the vanadium redox flow battery [J]. Electrochimica Acta, 2011, 58(1): 238-246. |
| 2 | Tang A , Bao J , Skyllas-Kazacos M . Thermal modelling of battery configuration and self-discharge reactions in vanadium redox flow battery [J]. Journal of Power Sources, 2012, 216(1): 489-501. |
| 3 | Tang A , Bao J , Skyllas-Kazacos M . Dynamic modelling of the effects of ion diffusion and side reactions on the capacity loss for vanadium redox flow battery[J]. Journal of Power Sources, 2011, 196(24): 10737-10747. |
| 4 | Wandschneider F T , Rhm S , Fischer P , et al . A multi-stack simulation of shunt currents in vanadium redox flow batteries [J]. Journal of Power Sources, 2014, 261(1): 64-74. |
| 5 | Dixon D , Bsbu D J , Langner J , et al . Effect of oxygen plasma treatment on the electrochemical performance of the rayon and polyacrylonitrile based carbon felt for the vanadium redox flow battery application [J]. Journal of Power Sources, 2016, 332(1): 240-248. |
| 6 | He Z , Li M , Li Y , et al . Flexible electrospun carbon nanofiber embedded with TiO2 as excellent negative electrode for vanadium redox flow battery [J]. Electrochimica Acta, 2018, 281(2): 601-610. |
| 7 | Liu T , Li X , Zhang H , et al . Progress on the electrode materials towards vanadium flow batteries (VFBs) with improved power density[J]. Journal of Energy Chemistry, 2018, 27(5): 1292-1303. |
| 8 | Lu X , Zeng Y , Yu M , et al . Oxygen-deficient hematite nanorods as high-performance and novel negative electrodes for flexible asymmetric supercapacitors[J]. Adv. Mater., 2014, 26(19): 3148-3155. |
| 9 | Noack J , Roznyatovskaya N , Kunzendorf J , et al . The influence of electrochemical treatment on electrode reactions for vanadium redox-flow batteries[J]. Journal of Energy Chemistry, 2018, 27(5): 1341-1352. |
| 10 | Xiang Y , Daoud W A . Cr2O3-modified graphite felt as a novel positive electrode for vanadium redox flow battery [J]. Electrochimica Acta, 2018, 290(1): 176-184. |
| 11 | Zhao C , Li Y , He Z , et al . KHCO3 activated carbon microsphere as excellent electrocatalyst for VO2+/VO2 + redox couple for vanadium redox flow battery [J]. Journal of Energy Chemistry, 2019, 29(1): 103-110. |
| 12 | Tang A , Bao J , Skyllas-Kazacos M . Studies on pressure losses and flow rate optimization in vanadium redox flow battery [J]. Journal of Power Sources, 2014, 248(2): 154-162. |
| 13 | Xu Q , Zhao T S , Leung P K . Numerical investigations of flow field designs for vanadium redox flow batteries [J]. Applied Energy, 2013, 105(1): 47-56. |
| 14 | Yan Y , Skyllas-Kazacos M , Bao J . Effects of battery design, environmental temperature and electrolyte flowrate on thermal behaviour of a vanadium redox flow battery in different applications [J]. Journal of Energy Storage, 2017, 11(1): 104-118. |
| 15 | Yang X G , Ye Q , Cheng P , et al . Effects of the electric field on ion crossover in vanadium redox flow batteries [J]. Applied Energy, 2015, 145(3): 306-319. |
| 16 | You X , Ye Q , Cheng P . Scale-up of high power density redox flow batteries by introducing interdigitated flow fields [J]. International Communications in Heat and Mass Transfer, 2016, 75(1): 7-12. |
| 17 | Zheng Q , Zhang H , Xing F , et al . A three-dimensional model for thermal analysis in a vanadium flow battery [J]. Applied Energy, 2014, 113(19): 1675-1685. |
| 18 | Kumar S , Jayanti S . Effect of flow field on the performance of an all-vanadium redox flow battery [J]. Journal of Power Sources, 2016, 307(9): 782-787. |
| 19 | König S , Suriyah M R , Leibfried T . A plug flow reactor model of a vanadium redox flow battery considering the conductive current collectors [J]. Journal of Power Sources, 2017, 360(2): 221-231. |
| 20 | König S , Suriyah M R , Leibfried T . Innovative model-based flow rate optimization for vanadium redox flow batteries [J]. Journal of Power Sources, 2016, 333(2): 134-144. |
| 21 | Ke X , Prahl J M , Alexander J I D , et al . Mathematical modeling of electrolyte flow in a segment of flow channel over porous electrode layered system in vanadium flow battery with flow field design [J]. Electrochimica Acta, 2017, 223(2): 124-134. |
| 22 | Houser J , Pezeshki A , Clement J T , et al . Architecture for improved mass transport and system performance in redox flow batteries [J]. Journal of Power Sources, 2017, 351(2): 96-105. |
| 23 | Zheng Q , Xing F , Li X , et al . Flow field design and optimization based on the mass transport polarization regulation in a flow-through type vanadium flow battery [J]. Journal of Power Sources, 2016, 324(5): 402-411. |
| 24 | Bhattarai A , Wai N , Schweiss R , et al . Advanced porous electrodes with flow channels for vanadium redox flow battery [J]. Journal of Power Sources, 2017, 341(1): 83-90. |
| 25 | Al-Yasiri M , Park J . A novel cell design of vanadium redox flow batteries for enhancing energy and power performance [J]. Applied Energy, 2018, 222(6): 530-539. |
| 26 | Wei Z , Tseng K J , Wai N , et al . Adaptive estimation of state of charge and capacity with online identified battery model for vanadium redox flow battery [J]. Journal of Power Sources, 2016, 332(5): 389-398. |
| 27 | Oh K , Won S , Ju H . A comparative study of species migration and diffusion mechanisms in all-vanadium redox flow batteries [J]. Electrochimica Acta, 2015, 181(3): 238-247. |
| 28 | Zhang Y , Zhao J , Wang P , et al . A comprehensive equivalent circuit model of all-vanadium redox flow battery for power system analysis [J]. Journal of Power Sources, 2015, 290(1): 14-24. |
| 29 | Yin C , Gao Y , Guo S , et al . A coupled three dimensional model of vanadium redox flow battery for flow field designs [J]. Energy, 2014, 74(10): 886-895. |
| 30 | Chen L , He Y , Tao W Q , et al . Pore-scale study of multiphase reactive transport in fibrous electrodes of vanadium redox flow batteries [J]. Electrochimica Acta, 2017, 248(5): 425-439. |
| [1] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [2] | 于源, 陈薇薇, 付俊杰, 刘家祥, 焦志伟. 几何相似涡流空气分级机环形区流场变化规律研究及预测[J]. 化工学报, 2023, 74(6): 2363-2373. |
| [3] | 张正, 何永平, 孙海东, 张荣子, 孙正平, 陈金兰, 郑一璇, 杜晓, 郝晓刚. 蛇形流场电控离子交换装置用于选择性提锂[J]. 化工学报, 2023, 74(5): 2022-2033. |
| [4] | 吕阳光, 左培培, 杨正金, 徐铜文. 三嗪框架聚合物膜用于有机纳滤甲醇/正己烷分离[J]. 化工学报, 2023, 74(4): 1598-1606. |
| [5] | 李新亚, 邢雷, 蒋明虎, 赵立新. 倒锥注气强化井下油水分离水力旋流器性能研究[J]. 化工学报, 2023, 74(3): 1134-1144. |
| [6] | 李沐紫, 贾国伟, 赵砚珑, 张鑫, 李建荣. 金属有机框架材料对非二氧化碳温室气体捕捉研究进展[J]. 化工学报, 2023, 74(1): 365-379. |
| [7] | 范怡平, 卢春喜. 离心力场-移动床耦合气固分离装备的研究进展[J]. 化工学报, 2023, 74(1): 157-169. |
| [8] | 鲁文静, 李先锋. 液流电池多孔离子传导膜研究进展[J]. 化工学报, 2023, 74(1): 192-204. |
| [9] | 杜若晗, 逄博, 王宁, 崔福军, 郭明钢, 贺高红, 吴雪梅. 连续共价有机框架筛分复合膜及全钒电池性能[J]. 化工学报, 2022, 73(9): 4163-4172. |
| [10] | 董宜放, 于樱迎, 胡学功, 裴刚. 电场对竖直微槽润湿及毛细流动特性影响[J]. 化工学报, 2022, 73(7): 2952-2961. |
| [11] | 李彬, 宋文明, 杨坤龙, 姜爽, 张天永. 水系有机液流电池活性材料的分子工程研究进展[J]. 化工学报, 2022, 73(7): 2806-2818. |
| [12] | 毛恒, 王月, 王森, 刘伟民, 吕静, 陈甫雪, 赵之平. APTES改性ZIF-L/PEBA混合基质膜强化渗透汽化分离苯酚研究[J]. 化工学报, 2022, 73(3): 1389-1402. |
| [13] | 韩昌亮, 辛镜青, 于广滨, 刘俊秀, 许麒澳, 姚安卡, 尹鹏. 微通道内超临界氮气三维热流场实验与数值模拟[J]. 化工学报, 2022, 73(2): 653-662. |
| [14] | 叶诗洋, 程敏, 吉旭, 戴一阳, 党亚固, 毕可鑫, 赵志伟, 周利. 高性能COF材料的高通量筛选策略:己烷异构体分离[J]. 化工学报, 2022, 73(11): 5138-5149. |
| [15] | 张后虎, 吴晓莉, 陈冲冲, 陈静静, 王景涛. CD-MOF二维层状膜制备及混合溶剂精准分离研究[J]. 化工学报, 2022, 73(10): 4539-4550. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号