化工学报 ›› 2022, Vol. 73 ›› Issue (11): 5138-5149.DOI: 10.11949/0438-1157.20220988
叶诗洋1( ), 程敏1, 吉旭1, 戴一阳1, 党亚固1, 毕可鑫1, 赵志伟2, 周利1(
), 程敏1, 吉旭1, 戴一阳1, 党亚固1, 毕可鑫1, 赵志伟2, 周利1( )
)
收稿日期:2022-06-17
修回日期:2022-09-10
出版日期:2022-11-05
发布日期:2022-12-06
通讯作者:
周利
作者简介:叶诗洋(1997—),男,硕士研究生,ysyyes@163.com
基金资助:
Shiyang YE1( ), Min CHENG1, Xu JI1, Yiyang DAI1, Yagu DANG1, Kexin BI1, Zhiwei ZHAO2, Li ZHOU1(
), Min CHENG1, Xu JI1, Yiyang DAI1, Yagu DANG1, Kexin BI1, Zhiwei ZHAO2, Li ZHOU1( )
)
Received:2022-06-17
Revised:2022-09-10
Online:2022-11-05
Published:2022-12-06
Contact:
Li ZHOU
摘要:
己烷异构体中双支链异构体的分离可以提高汽油的辛烷值从而减少发动机的爆震现象。针对传统的蒸馏方法耗能高和新型吸附剂金属有机框架成本高、工作能力低、稳定性差的缺点,采用高通量计算筛选方法研究了688种共价有机框架(COFs)对己烷异构体的分离性能。首先计算了所有COF的几何结构描述符,通过限制孔径(PLD) 6.2~15 Å的范围筛选出209个可容纳所有己烷异构体的COF,再利用巨正则Monte Carlo (GCMC)方法模拟433 K下上述COF对己烷异构体的吸附解吸过程。对再生能力R>80%且吸附性能分值(APS)最高的COF进行排序,筛选出具有最高APS值的COF-DL229 2-fold,APS值为23.36 mol/kg,R为99.38%。分析了6个几何结构描述符与APS的相关性,发现对于COF来说较高的孔隙率(VF)、较高的孔隙体积(PV)、较低的密度(
中图分类号:
叶诗洋, 程敏, 吉旭, 戴一阳, 党亚固, 毕可鑫, 赵志伟, 周利. 高性能COF材料的高通量筛选策略:己烷异构体分离[J]. 化工学报, 2022, 73(11): 5138-5149.
Shiyang YE, Min CHENG, Xu JI, Yiyang DAI, Yagu DANG, Kexin BI, Zhiwei ZHAO, Li ZHOU. High-throughput computational screening strategy for high-performance COF materials: separation of hexane isomers[J]. CIESC Journal, 2022, 73(11): 5138-5149.
| COF ID | PLD/Å | LCD/Å | VF | ρ/(g/cm3) | SA/(m2/cm3) | PV/(cm3/g) | Sads | Cw/(mol/kg) | APS/(mol/kg) | R/% |
|---|---|---|---|---|---|---|---|---|---|---|
| 条件(a)吸附压力1 bar和解吸压力0.1 bar | ||||||||||
| 3D-Py-COF-2P | 13.47 | 12.29 | 0.90 | 0.28 | 2030.21 | 3.06 | 1.38 | 9.67 | 13.31 | 94.58 |
| JUC-550 3-fold | 10.44 | 9.58 | 0.87 | 0.33 | 2660.17 | 2.55 | 1.52 | 8.40 | 12.78 | 91.80 |
| FLT-COF-1 AB | 11.36 | 10.72 | 0.84 | 0.48 | 1965.35 | 1.58 | 2.57 | 4.95 | 12.71 | 94.30 |
| JUC-551 3-fold | 10.66 | 9.75 | 0.86 | 0.34 | 2769.82 | 2.45 | 1.51 | 7.90 | 11.95 | 88.68 |
| 3D-HNU5 | 14.32 | 12.10 | 0.97 | 0.32 | 1643.40 | 2.75 | 1.26 | 8.93 | 11.29 | 86.89 |
| 条件(b)吸附压力10 bar和解吸压力1 bar | ||||||||||
| COF-DL229 2-fold | 17.57 | 14.36 | 0.93 | 0.16 | 1303.54 | 5.88 | 1.10 | 19.75 | 21.82 | 92.83 |
| DL-COF-1-ctn | 16.21 | 14.26 | 0.93 | 0.19 | 1363.41 | 4.79 | 1.18 | 16.00 | 18.83 | 87.51 |
| DL-COF-2-ctn | 16.19 | 14.24 | 0.92 | 0.21 | 1380.14 | 4.29 | 1.17 | 14.23 | 16.72 | 87.47 |
| JUC-550 2-fold | 12.49 | 10.59 | 0.91 | 0.22 | 1813.73 | 4.00 | 1.16 | 11.90 | 13.83 | 77.54 |
| JUC-551 2-fold | 12.06 | 10.08 | 0.91 | 0.23 | 1883.71 | 3.79 | 1.19 | 9.25 | 10.99 | 62.95 |
| 条件(c)吸附压力10 bar和解吸压力0.1 bar | ||||||||||
| COF-DL229 2-fold | 17.57 | 14.36 | 0.93 | 0.16 | 1303.54 | 5.88 | 1.10 | 21.15 | 23.36 | 99.38 |
| DL-COF-1-ctn | 16.21 | 14.26 | 0.93 | 0.19 | 1363.41 | 4.79 | 1.18 | 18.12 | 21.32 | 99.08 |
| DL-COF-2-ctn | 16.19 | 14.24 | 0.92 | 0.21 | 1380.14 | 4.29 | 1.17 | 16.12 | 18.93 | 99.07 |
| FLT-COF-1 AB | 11.36 | 10.72 | 0.84 | 0.48 | 1965.35 | 1.58 | 2.54 | 7.07 | 17.94 | 95.95 |
| JUC-550 2-fold | 12.49 | 10.59 | 0.91 | 0.22 | 1813.73 | 4.00 | 1.16 | 15.15 | 17.61 | 98.73 |
表1 三组吸附-解吸压力下APS值排名前5位的COF材料
Table 1 Top 5 ranked COFs with best APS for the three sets of adsorption and desorption pressures
| COF ID | PLD/Å | LCD/Å | VF | ρ/(g/cm3) | SA/(m2/cm3) | PV/(cm3/g) | Sads | Cw/(mol/kg) | APS/(mol/kg) | R/% |
|---|---|---|---|---|---|---|---|---|---|---|
| 条件(a)吸附压力1 bar和解吸压力0.1 bar | ||||||||||
| 3D-Py-COF-2P | 13.47 | 12.29 | 0.90 | 0.28 | 2030.21 | 3.06 | 1.38 | 9.67 | 13.31 | 94.58 |
| JUC-550 3-fold | 10.44 | 9.58 | 0.87 | 0.33 | 2660.17 | 2.55 | 1.52 | 8.40 | 12.78 | 91.80 |
| FLT-COF-1 AB | 11.36 | 10.72 | 0.84 | 0.48 | 1965.35 | 1.58 | 2.57 | 4.95 | 12.71 | 94.30 |
| JUC-551 3-fold | 10.66 | 9.75 | 0.86 | 0.34 | 2769.82 | 2.45 | 1.51 | 7.90 | 11.95 | 88.68 |
| 3D-HNU5 | 14.32 | 12.10 | 0.97 | 0.32 | 1643.40 | 2.75 | 1.26 | 8.93 | 11.29 | 86.89 |
| 条件(b)吸附压力10 bar和解吸压力1 bar | ||||||||||
| COF-DL229 2-fold | 17.57 | 14.36 | 0.93 | 0.16 | 1303.54 | 5.88 | 1.10 | 19.75 | 21.82 | 92.83 |
| DL-COF-1-ctn | 16.21 | 14.26 | 0.93 | 0.19 | 1363.41 | 4.79 | 1.18 | 16.00 | 18.83 | 87.51 |
| DL-COF-2-ctn | 16.19 | 14.24 | 0.92 | 0.21 | 1380.14 | 4.29 | 1.17 | 14.23 | 16.72 | 87.47 |
| JUC-550 2-fold | 12.49 | 10.59 | 0.91 | 0.22 | 1813.73 | 4.00 | 1.16 | 11.90 | 13.83 | 77.54 |
| JUC-551 2-fold | 12.06 | 10.08 | 0.91 | 0.23 | 1883.71 | 3.79 | 1.19 | 9.25 | 10.99 | 62.95 |
| 条件(c)吸附压力10 bar和解吸压力0.1 bar | ||||||||||
| COF-DL229 2-fold | 17.57 | 14.36 | 0.93 | 0.16 | 1303.54 | 5.88 | 1.10 | 21.15 | 23.36 | 99.38 |
| DL-COF-1-ctn | 16.21 | 14.26 | 0.93 | 0.19 | 1363.41 | 4.79 | 1.18 | 18.12 | 21.32 | 99.08 |
| DL-COF-2-ctn | 16.19 | 14.24 | 0.92 | 0.21 | 1380.14 | 4.29 | 1.17 | 16.12 | 18.93 | 99.07 |
| FLT-COF-1 AB | 11.36 | 10.72 | 0.84 | 0.48 | 1965.35 | 1.58 | 2.54 | 7.07 | 17.94 | 95.95 |
| JUC-550 2-fold | 12.49 | 10.59 | 0.91 | 0.22 | 1813.73 | 4.00 | 1.16 | 15.15 | 17.61 | 98.73 |

图3 在433 K下nHex、2MP、3MP、22DMB、23DMB等摩尔五组分混合物在COF-DL229 2-fold上的吸附等温线
Fig.3 Adsorption isotherms of nHex, 2MP, 3MP, 22DMB, and 23DMB in an equimolar five-component mixture at 433 K in COF-DL229 2-fold
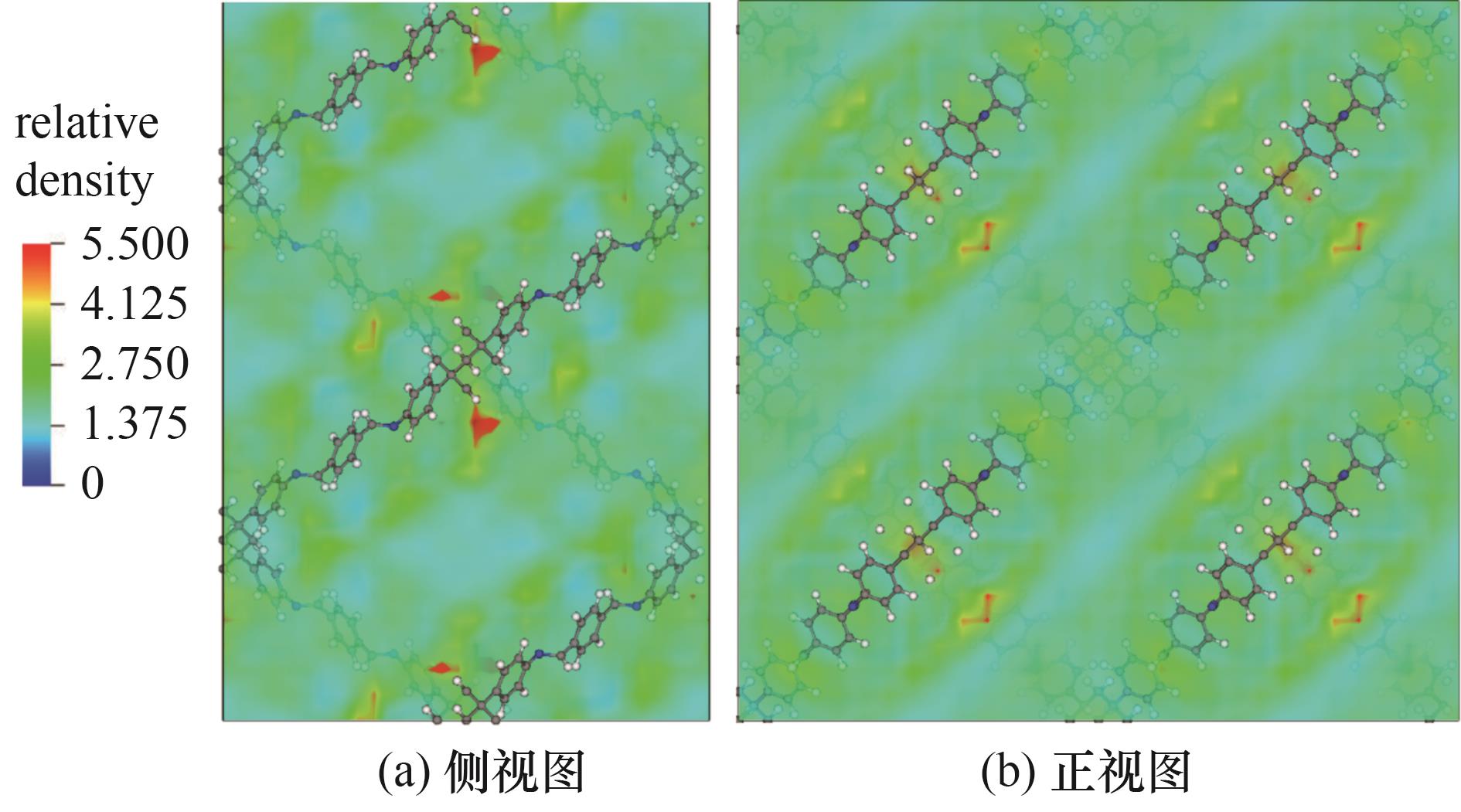
图4 在433 K、10 bar下己烷异构体等摩尔五组分混合物中nHex在COF-DL229 2-fold上的密度分布
Fig.4 Density distribution of nHex on COF-DL229 2-fold in an equimolar five-component mixture of hexane isomer at 433 K and 10 bar
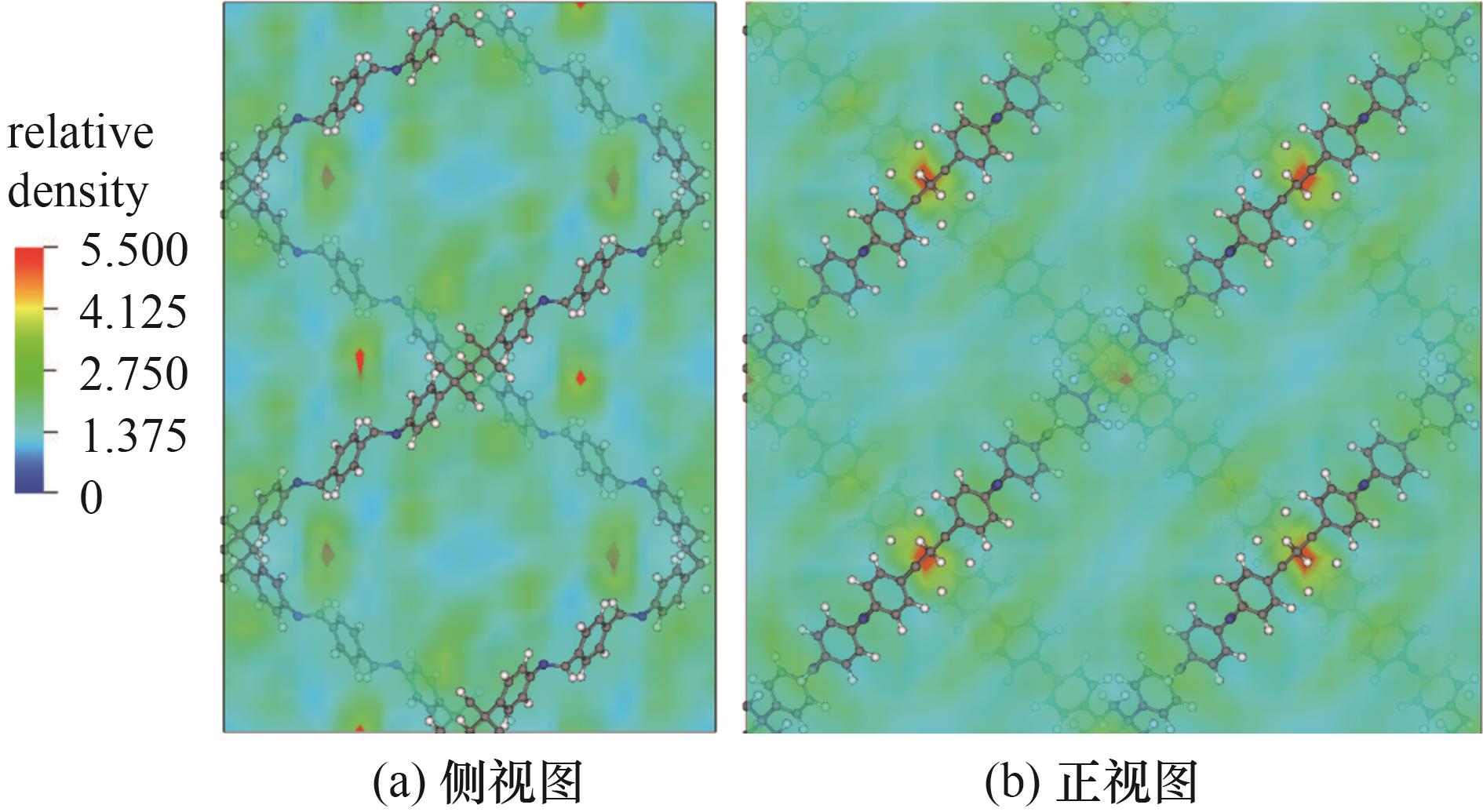
图5 在433 K、10 bar下己烷异构体等摩尔五组分混合物中22DMB在COF-DL229 2-fold上的密度分布
Fig.5 Density distribution of 22DMB on COF-DL229 2-fold in an equimolar five-component mixture of hexane isomer at 433 K and 10 bar
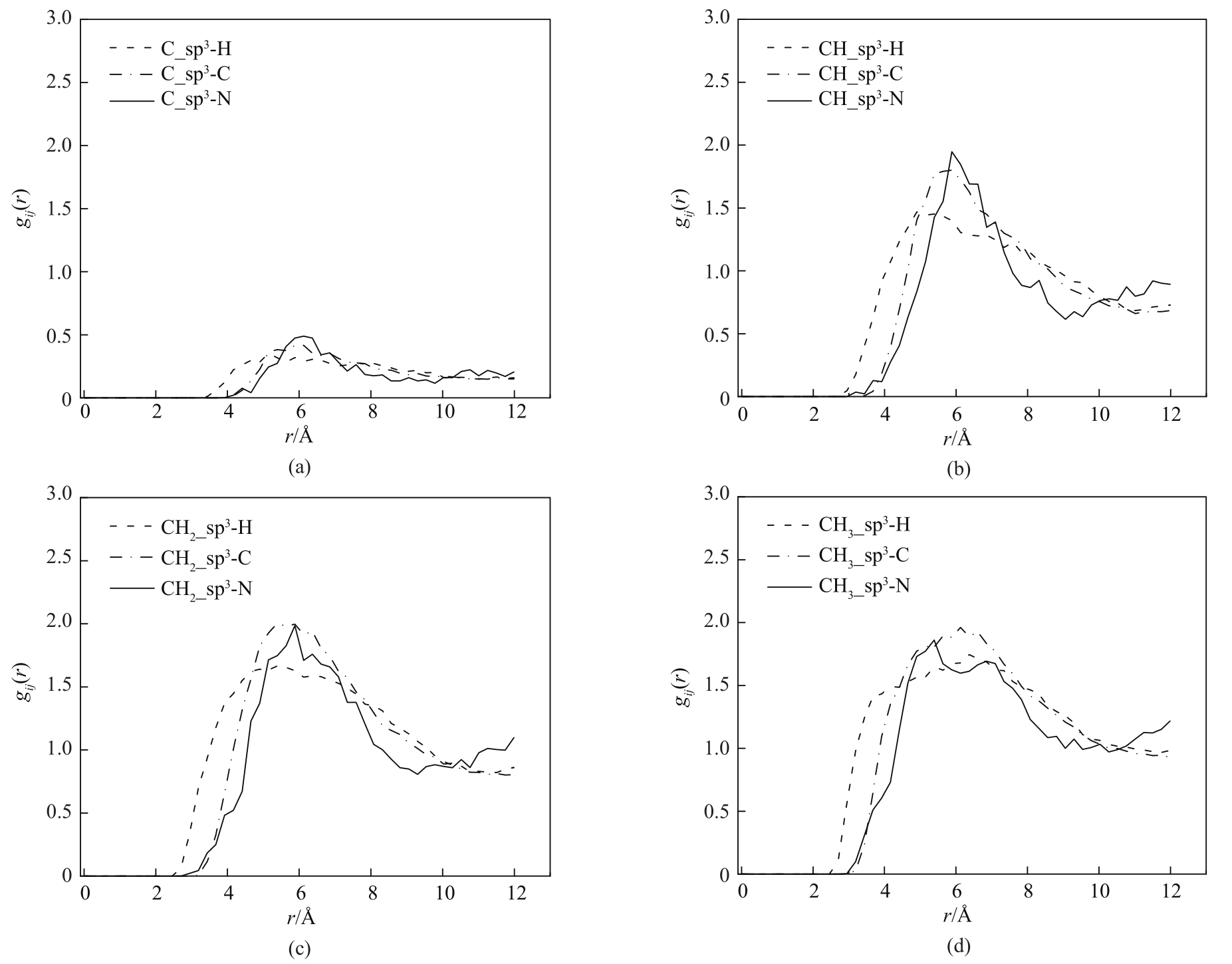
图6 433 K、0.1 bar下COF-DL229 2-fold不同骨架原子与己烷异构体相互作用原子对的径向分布函数
Fig.6 Radial distribution functions of interaction atom pairs between various framework atoms of COF-DL229 2-fold and hexane isomers at 433 K and 0.1 bar
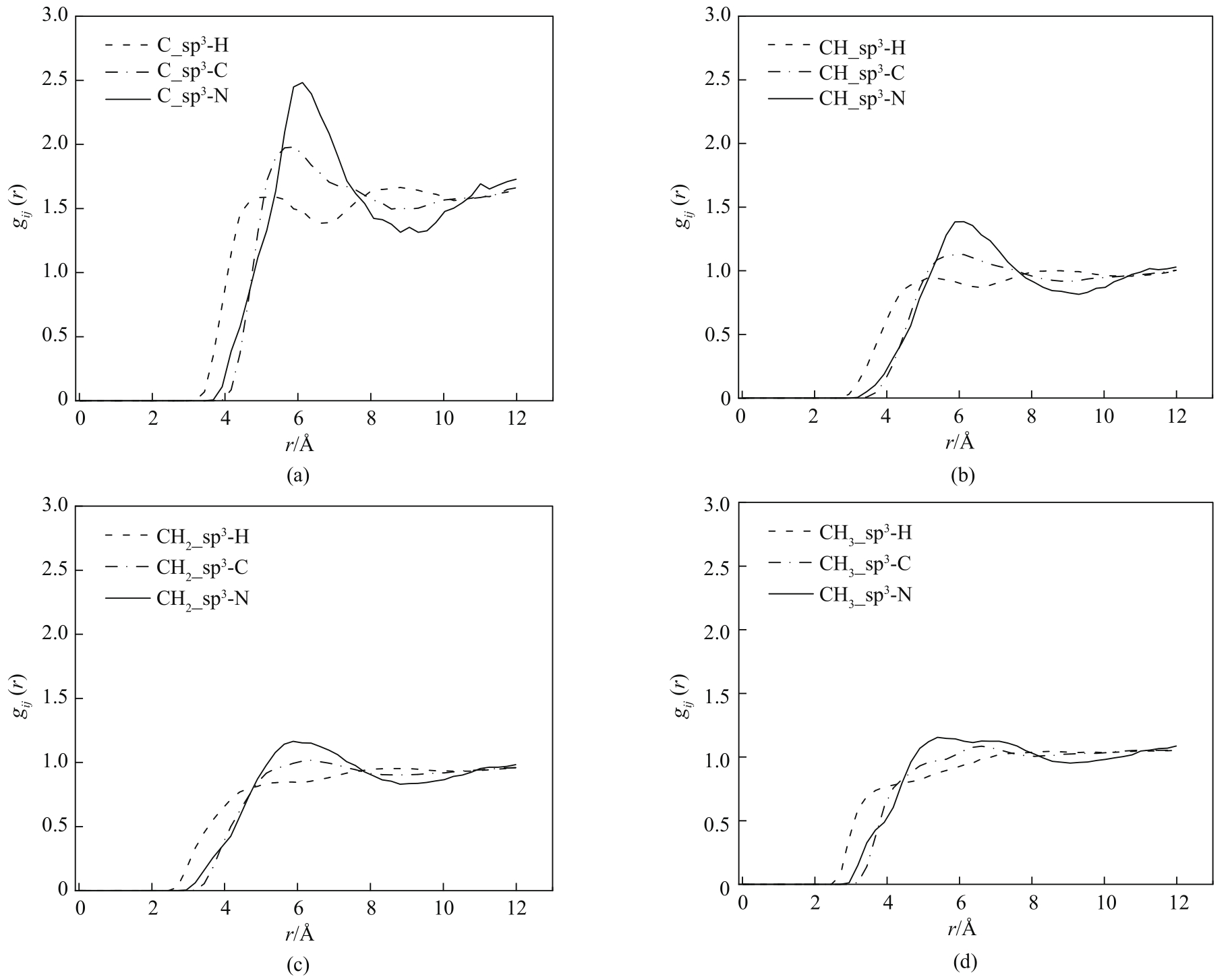
图7 433 K、10 bar下COF-DL229 2-fold不同骨架原子与己烷异构体相互作用原子对的径向分布函数
Fig.7 Radial distribution functions of interaction atom pairs between various framework atoms of COF-DL229 2-fold and hexane isomers at 433 K and 10 bar

图8 在433 K、吸附压力10 bar、解吸压力0.1 bar下各描述符与APS的关系
Fig.8 Relationship between descriptors at 433 K, 10 bar adsorption pressure and 0.1 bar desorption pressure
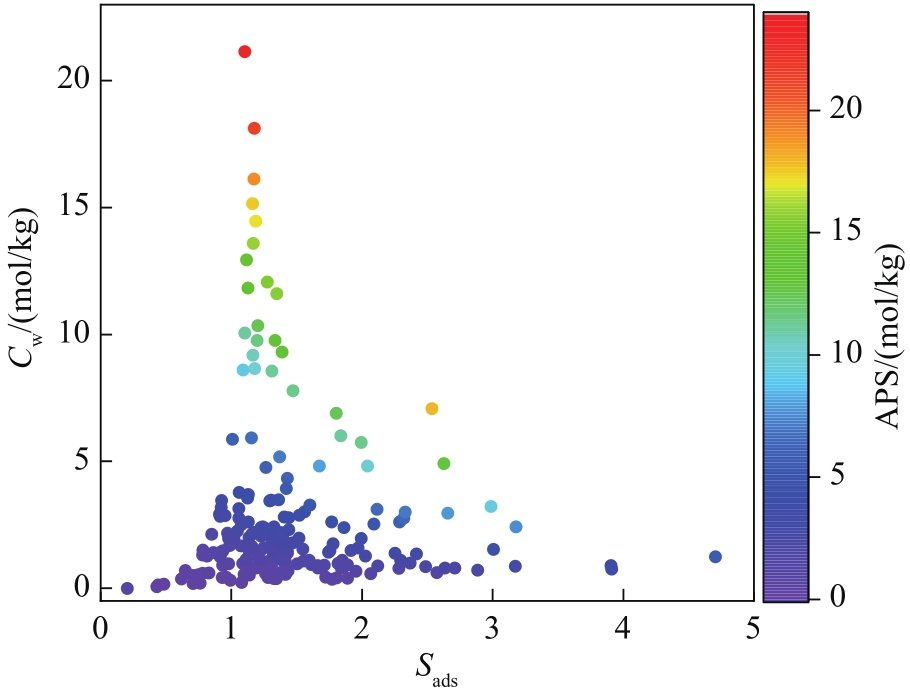
图9 Sads与Cw的关系(对各个点根据APS值进行上色,每个点代表一个COF结构)
Fig.9 Relationship between adsorption selectivity Sads and working capacity Cw (the points are color coded with respect to the APS value, each point represents one COF structure)
| 1 | 周晶晶. 中国能源依赖度指数构建及评价研究[D]. 徐州: 中国矿业大学, 2019. |
| Zhou J J. Research on the construction and evaluation for China’s energy dependence index[D]. Xuzhou: China University of Mining and Technology, 2019. | |
| 2 | 尧命发, 郑尊清, 沈捷, 等. 辛烷值对均质压燃发动机燃烧特性和性能的影响[J]. 燃烧科学与技术, 2004, 10(3): 244-249. |
| Yao M F, Zheng Z Q, Shen J, et al. Experimental study on the influence of fuel octane number on combustion characteristics and performance of HCCI engine[J]. Journal of Combustion Science and Technology, 2004, 10(3): 244-249. | |
| 3 | Peralta D, Chaplais G, Simon-Masseron A, et al. Separation of C6 paraffins using zeolitic imidazolate frameworks: comparison with zeolite 5A[J]. Industrial & Engineering Chemistry Research, 2012, 51(12): 4692-4702. |
| 4 | 郭辉, 王菊香, 景晓锋. UOP C5/C6低温异构化技术及工业应用[J]. 炼油技术与工程, 2018, 48(10): 9-13. |
| Guo H, Wang J X, Jing X F. UOP C5/C6 low-temperature isomerization process(Penex-DIH) and its commercial application[J]. Petroleum Refinery Engineering, 2018, 48(10): 9-13. | |
| 5 | 韩笑, 陈雨亭, 苏宝根, 等. 己烷异构体吸附分离材料研究进展[J]. 化工学报, 2021, 72(7): 3445-3465. |
| Han X, Chen Y T, Su B G, et al. Advances in adsorbents for hexane isomers separation[J]. CIESC Journal, 2021, 72(7): 3445-3465. | |
| 6 | Silva J A C, Rodrigues A E. Equilibrium and kinetics of n-hexane sorption in pellets of 5A zeolite[J]. AIChE Journal, 1997, 43(10): 2524-2534. |
| 7 | Arruebo M, Falconer J L, Noble R D. Separation of binary C5 and C6 hydrocarbon mixtures through MFI zeolite membranes[J]. Journal of Membrane Science, 2006, 269(1/2): 171-176. |
| 8 | Olson D H, Camblor M A, Villaescusa L A, et al. Light hydrocarbon sorption properties of pure silica Si-CHA and ITQ-3 and high silica ZSM-58[J]. Microporous and Mesoporous Materials, 2004, 67(1): 27-33. |
| 9 | Krishna R, Calero S, Smit B. Investigation of entropy effects during sorption of mixtures of alkanes in MFI zeolite[J]. Chemical Engineering Journal, 2002, 88(1/2/3): 81-94. |
| 10 | Bárcia P S, Silva J A C, Rodrigues A E. Multicomponent sorption of hexane isomers in zeolite BETA[J]. AIChE Journal, 2007, 53(8): 1970-1981. |
| 11 | Bárcia P S, Silva J A C, Rodrigues A E. Separation by fixed-bed adsorption of hexane isomers in zeolite BETA pellets[J]. Industrial & Engineering Chemistry Research, 2006, 45(12): 4316-4328. |
| 12 | Bárcia P S, Silva J A C, Rodrigues A E. Adsorption equilibrium and kinetics of branched hexane isomers in pellets of BETA zeolite[J]. Microporous and Mesoporous Materials, 2005, 79(1/2/3): 145-163. |
| 13 | Krishna R. Separating mixtures by exploiting molecular packing effects in microporous materials[J]. Physical Chemistry Chemical Physics: PCCP, 2015, 17(1): 39-59. |
| 14 | Chen B L, Liang C D, Yang J, et al. A microporous metal-organic framework for gas-chromatographic separation of alkanes[J]. Angewandte Chemie, 2006, 118(9): 1418-1421. |
| 15 | Chung Y G, Bai P, Haranczyk M, et al. Computational screening of nanoporous materials for hexane and heptane isomer separation[J]. Chemistry of Materials, 2017, 29(15): 6315-6328. |
| 16 | Dong X Q, Fan Q, Hao W Z, et al. Adsorption and separation of hexane isomers in metal-organic frameworks (MOFs): a computational study[J]. Computational and Theoretical Chemistry, 2021, 1197: 113164. |
| 17 | Herm Z R, Wiers B M, Mason J A, et al. Separation of hexane isomers in a metal-organic framework with triangular channels[J]. Science, 2013, 340(6135): 960-964. |
| 18 | Peng L, Zhu Q, Wu P L, et al. High-throughput computational screening of metal-organic frameworks with topological diversity for hexane isomer separations[J]. Physical Chemistry Chemical Physics: PCCP, 2019, 21(16): 8508-8516. |
| 19 | Solanki V A, Borah B. Ranking of metal-organic frameworks (MOFs) for separation of hexane isomers by selective adsorption[J]. Industrial & Engineering Chemistry Research, 2019, 58(43): 20047-20065. |
| 20 | Solanki V A, Borah B. Exploring the potentials of metal-organic frameworks as adsorbents and membranes for separation of hexane isomers[J]. The Journal of Physical Chemistry C, 2019, 123(29): 17808-17822. |
| 21 | Solanki V A, Borah B. Adsorption and diffusion of hexane isomers in a series of microporous metal-organic frameworks (MOF): a molecular simulation study[J]. AIP Conference Proceedings, 2020, 2220(1): 130014. |
| 22 | Solanki V A, Borah B. High-throughput computational screening of 12, 351 real metal-organic framework structures for separation of hexane isomers: a quest for a yet better adsorbent[J]. Journal of Physical Chemistry C, 2020, 124: 4582-4594. |
| 23 | Bury W, Walczak A M, Leszczyński M K, et al. Rational design of noncovalent diamondoid microporous materials for low-energy separation of C6-hydrocarbons[J]. Journal of the American Chemical Society, 2018, 140(44): 15031-15037.[ |
| 24 | Castro-Gutiérrez J, de Oliveira Jardim E, Canevesi R L S, et al. Molecular sieving of linear and branched C6 alkanes by tannin-derived carbons[J]. Carbon, 2021, 174: 413-422. |
| 25 | Yaghi O M, Li G M, Li H L. Selective binding and removal of guests in a microporous metal-organic framework[J]. Nature, 1995, 378(6558): 703-706. |
| 26 | Li H L, Eddaoudi M, O’Keeffe M, et al. Design and synthesis of an exceptionally stable and highly porous metal-organic framework[J]. Nature, 1999, 402(6759): 276-279. |
| 27 | Li H L, Eddaoudi M, Groy T, et al. Establishing microporosity in open metal-organic frameworks: gas sorption isotherms for Zn(BDC) (BDC=1, 4-benzenedicarboxylate)[J]. Journal of the American Chemical Society, 1998, 120(33): 8571-8572. |
| 28 | Burtch N C, Jasuja H, Walton K S. Water stability and adsorption in metal-organic frameworks[J]. Chemical Reviews, 2014, 114(20): 10575-10612. |
| 29 | Jasuja H, Burtch N C, Huang Y G, et al. Kinetic water stability of an isostructural family of zinc-based pillared metal-organic frameworks[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2013, 29(2): 633-642. |
| 30 | Aksu G O, Daglar H, Altintas C, et al. Computational selection of high-performing covalent organic frameworks for adsorption and membrane-based CO2/H2 separation[J]. The Journal of Physical Chemistry C, Nanomaterials and Interfaces, 2020, 124(41): 22577-22590. |
| 31 | Altundal O F, Altintas C, Keskin S. Can COFs replace MOFs in flue gas separation? High-throughput computational screening of COFs for CO2/N2 separation[J]. Journal of Materials Chemistry A, 2020, 8(29): 14609-14623. |
| 32 | Zeng Y F, Zou R Q, Zhao Y L. Covalent organic frameworks for CO2 capture[J]. Advanced Materials (Deerfield Beach, Fla.), 2016, 28(15): 2855-2873. |
| 33 | Tong M, Yang Q, Xiao Y, et al. Revealing the structure-property relationship of covalent organic frameworks for CO₂ capture from postcombustion gas: a multi-scale computational study[J]. Physical Chemistry Chemical Physics, 2014, 16(29): 15189-15198. |
| 34 | Tong M M, Yang Q Y, Zhong C L. Computational screening of covalent organic frameworks for CH4/H2, CO2/H2 and CO2/CH4 separations[J]. Microporous and Mesoporous Materials, 2015, 210: 142-148. |
| 35 | Adsorption Keskin S., diffusion, and separation of CH 4 /H2 mixtures in covalent organic frameworks: molecular simulations and theoretical predictions[J]. Journal of Physical Chemistry C, 2012, 116: 1772-1779. |
| 36 | Yan T, Lan Y, Tong M, et al. Screening and design of covalent organic framework membranes for CO2/CH4 separation[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(1): 1220-1227. |
| 37 | Tong M, Zhang Y, Yan T, et al. Computational insights on the role of nanochannel environment in the CO2/CH4 and H2/CH4 separation using restacked covalent organic framework membranes[J]. The Journal of Physical Chemistry C, 2019, 123(37): 22949-22958. |
| 38 | Zeng H W, Liu Y, Liu H L. Adsorption and diffusion of CO2 and CH4 in covalent organic frameworks: an MC/MD simulation study[J]. Molecular Simulation, 2018, 44(15): 1244-1251. |
| 39 | Wang Y, Wang G, Liu Y, et al. Identifying promising covalent-organic frameworks for decarburization and desulfurization from biogas via computational screening[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(26): 8858-8867. |
| 40 | Tong M M, Lan Y S, Yang Q Y, et al. Exploring the structure-property relationships of covalent organic frameworks for noble gas separations[J]. Chemical Engineering Science, 2017, 168: 456-464. |
| 41 | Huang J J, Han X, Yang S, et al. Microporous 3D covalent organic frameworks for liquid chromatographic separation of xylene isomers and ethylbenzene[J]. Journal of the American Chemical Society, 2019, 141(22): 8996-9003. |
| 42 | Ma H, Ren H, Meng S, et al. A 3D microporous covalent organic framework with exceedingly high C3H8/CH4 and C2 hydrocarbon/CH4 selectivity[J]. Chemical Communications, 2013, 49(84): 9773-9775. |
| 43 | Huang N, Chen X, Krishna R, et al. Two-dimensional covalent organic frameworks for carbon dioxide capture through channel-wall functionalization[J]. Angewandte Chemie (International Ed. in English), 2015, 54(10): 2986-2990. |
| 44 | Liu J, Wei Y, Li P, et al. Selective H2S/CO2 separation by metal-organic frameworks based on chemical-physical adsorption[J]. The Journal of Physical Chemistry C, 2017, 121(24): 13249-13255. |
| 45 | Vaesen S, Guillerm V, Yang Q Y, et al. A robust amino-functionalized titanium(Ⅳ) based MOF for improved separation of acid gases[J]. Chemical Communications (Cambridge, England), 2013, 49(86): 10082-10084. |
| 46 | Ongari D, Yakutovich A V, Talirz L, et al. Building a consistent and reproducible database for adsorption evaluation in covalent-organic frameworks[J]. ACS Central Science, 2019, 5(10): 1663-1675. |
| 47 | Willems T F, Rycroft C H, Kazi M, et al. Algorithms and tools for high-throughput geometry-based analysis of crystalline porous materials[J]. Microporous and Mesoporous Materials, 2012, 149(1): 134-141. |
| 48 | Dubbeldam D, Calero S, Ellis D E, et al. RASPA: molecular simulation software for adsorption and diffusion in flexible nanoporous materials[J]. Molecular Simulation, 2016, 42(2): 81-101. |
| 49 | Anderson R, Rodgers J, Argueta E, et al. Role of pore chemistry and topology in the CO2 capture capabilities of MOFs: from molecular simulation to machine learning[J]. Chemistry of Materials, 2018, 30(18): 6325-6337. |
| 50 | Dubbeldam D, Krishna R, Calero S, et al. Computer-assisted screening of ordered crystalline nanoporous adsorbents for separation of alkane isomers[J]. Angewandte Chemie International Edition, 2012, 51(47): 11867-11871. |
| 51 | Rappe A K, Casewit C J, Colwell K S, et al. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations[J]. Journal of the American Chemical Society, 1992, 114(25): 10024-10035. |
| 52 | Martin M G, Siepmann J I. Novel configurational-bias Monte Carlo method for branched molecules. Transferable potentials for phase equilibria. 2. United-atom description of branched alkanes[J]. Journal of Physical Chemistry B, 1999, 103: 4508-4517. |
| 53 | Martin M G, Siepmann J I. Transferable potentials for phase equilibria. 1. United-atom description of n-alkanes[J]. The Journal of Physical Chemistry B, 1998, 102(14): 2569-2577. |
| 54 | Henrique A, Rodrigues A E, Silva J A C. Separation of hexane isomers in ZIF-8 by fixed bed adsorption[J]. Industrial & Engineering Chemistry Research, 2019, 58(1): 378-394. |
| 55 | 杨磊, 吴宇静, 吴选军, 等. 面向C4烯烃混合物吸附分离的真实金属-有机骨架材料高通量筛选[J]. 化学学报, 2021, 79(4): 520-529. |
| Yang L, Wu Y J, Wu X J, et al. High-throughput screening of real metal-organic frameworks for adsorption separation of C4 olefins[J]. Acta Chimica Sinica, 2021, 79(4): 520-529. | |
| 56 | 王之婧, 王俊超, 赵行乐, 等. CO2在氨基改性MIL-101(Cr)中吸附的分子模拟[J]. 无机化学学报, 2018, 34(11): 1966-1974. |
| Wang Z J, Wang J C, Zhao X L, et al. Molecular simulation for CO2 adsorption in amine-functionalized MIL-101(Cr)[J]. Chinese Journal of Inorganic Chemistry, 2018, 34(11): 1966-1974. | |
| 57 | Wang C, Wang Y, Ge R L, et al. A 3D covalent organic framework with exceptionally high iodine capture capability[J]. Chemistry (Weinheim an Der Bergstrasse, Germany), 2018, 24(3): 585-589. |
| 58 | Thompson C M, Occhialini G, McCandless G T, et al. Computational and experimental studies on the effects of monomer planarity on covalent organic framework formation[J]. Journal of the American Chemical Society, 2017, 139(30): 10506-10513. |
| 59 | Rodgers J L, Nicewander W A. Thirteen ways to look at the correlation coefficient[J]. The American Statistician, 1988, 42(1): 59-66. |
| 60 | Ozturk T N, Keskin S, et al. Computational screening of porous coordination networks for adsorption and membrane-based gas separations[J]. Journal of Physical Chemistry C, 2014, 118(25):13988-13997. |
| 61 | Guan P, Qiu J, Zhao Y, et al. A novel crystalline azine-linked three-dimensional covalent organic framework for CO2 capture and conversion[J]. Chemical Communications, 2019, 55(83):12459-12462. |
| 62 | Breiman L, Friedman J, Olshen R, et al. Classification and regression trees[J]. Biometrics, 1984, 40(3): 358. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [5] | 赵佳佳, 田世祥, 李鹏, 谢洪高. SiO2-H2O纳米流体强化煤尘润湿性的微观机理研究[J]. 化工学报, 2023, 74(9): 3931-3945. |
| [6] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [7] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [8] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [9] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [10] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [11] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [12] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [13] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [14] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [15] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号