化工学报 ›› 2020, Vol. 71 ›› Issue (5): 2389-2400.DOI: 10.11949/0438-1157.20191319
收稿日期:2019-11-04
修回日期:2020-01-09
出版日期:2020-05-05
发布日期:2020-05-05
通讯作者:
刘咏
作者简介:彭娜(1995—),女,硕士研究生,基金资助:
Na PENG( ),Pengfei ZHAI,Jingtao WANG,Junxiao WANG,Yong LIU(
),Pengfei ZHAI,Jingtao WANG,Junxiao WANG,Yong LIU( )
)
Received:2019-11-04
Revised:2020-01-09
Online:2020-05-05
Published:2020-05-05
Contact:
Yong LIU
摘要:
锂硫电池具有较高的理论能量密度,被认为是最有发展潜力的下一代高能量密度储能器件之一。然而多硫化物穿过隔膜形成的穿梭效应导致电池容量衰减过快、使用寿命降低,严重阻碍了锂硫电池商业化。以层状氧化石墨烯为模板,采用氧化还原法合成了二氧化锰纳米片,通过低压抽滤获得二氧化锰改性隔膜。利用TEM、XRD、FTIR、SEM、AFM等对该二氧化锰纳米片及改性隔膜的微观结构、形貌等进行表征;采用恒电流充放电、循环伏安法、电化学阻抗法对二氧化锰改性隔膜电化学性能进行测试。研究结果表明,二氧化锰纳米片能均匀覆盖聚丙烯隔膜表面的微孔,通过物理阻隔和催化作用,有效抑制了多硫化物的穿梭,提高了锂硫电池的比容量和循环稳定性。
中图分类号:
彭娜, 翟鹏飞, 王景涛, 王俊晓, 刘咏. 二氧化锰纳米片改性隔膜在锂硫电池中的应用[J]. 化工学报, 2020, 71(5): 2389-2400.
Na PENG, Pengfei ZHAI, Jingtao WANG, Junxiao WANG, Yong LIU. Application of manganese dioxide nanosheets modified separator for lithium-sulfur batteries[J]. CIESC Journal, 2020, 71(5): 2389-2400.
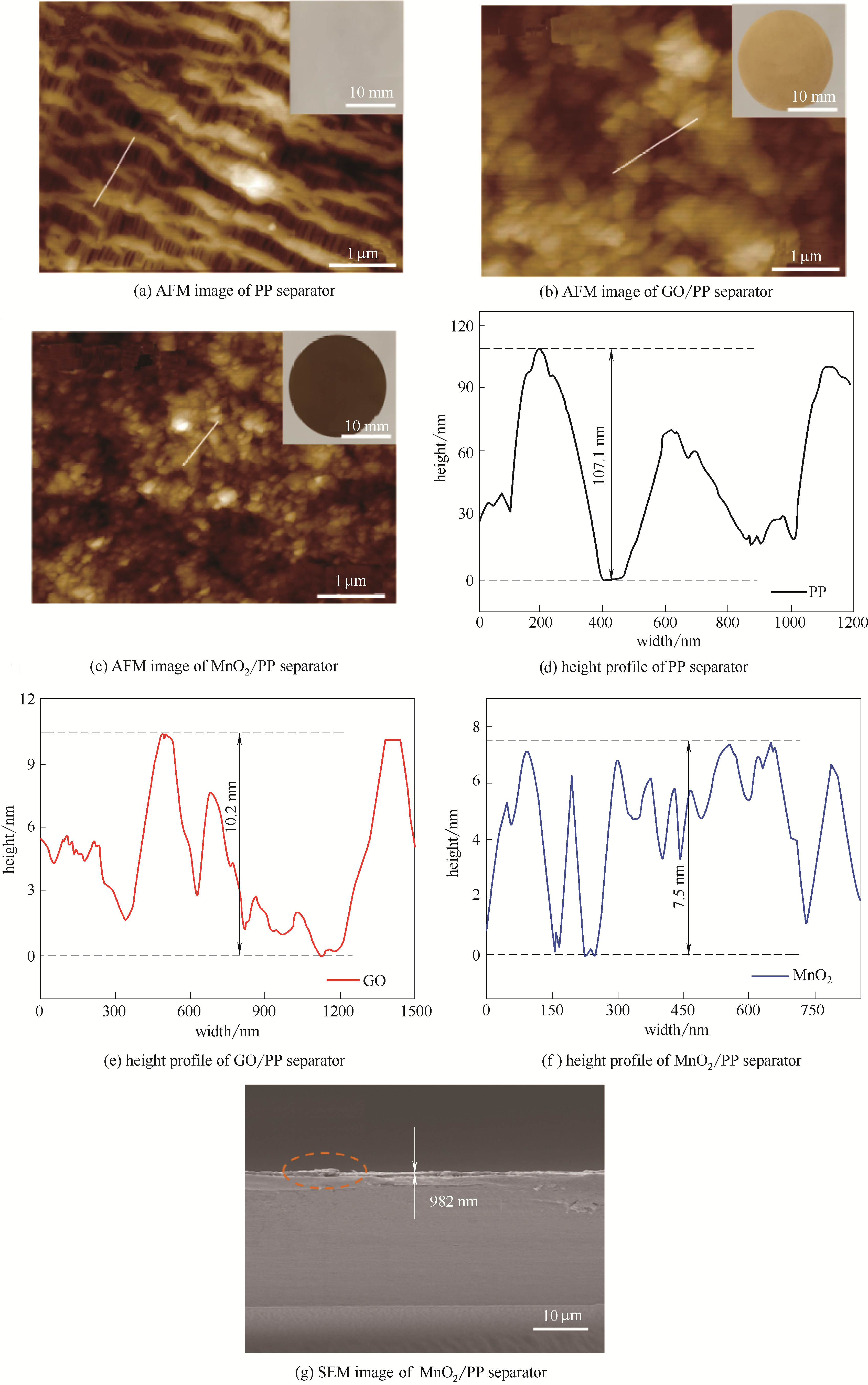
图5 PP、GO/PP、MnO2/PP隔膜的AFM图及高度分布和MnO2/PP隔膜的SEM图
Fig.5 AFM images and corresponding height profiles of PP, GO/PP and MnO2/PP separators, and SEM images of MnO2/PP separator
| 1 | Dunn B, Kamath H, Tarascon J M. Electrical energy storage for the grid: a battery of choices[J]. Science, 2011, 334(6058): 928-935. |
| 2 | Etacheri V, Marom R, Elazari R, et al. Challenges in the development of advanced Li-ion batteries: a review[J]. Energy & Environmental Science, 2011, 4(9): 3243-3262. |
| 3 | 刘帅, 姚路, 章琴, 等. 高性能锂硫电池研究进展[J]. 物理化学学报, 2017, 33(12): 2339-2358. |
| Liu S, Yao L, Zhang Q, et al. Advances in high-performance lithium-sulfur batteries[J]. Acta Physico-Chimica Sinica, 2017, 33(12): 2339-2358. | |
| 4 | Scrosati B, Garche J. Lithium batteries: status, prospects and future[J]. Journal of Power Sources, 2010, 195(9): 2419-2430. |
| 5 | Kim J S, Hwang T H, Kim B G, et al. A lithium‐sulfur battery with a high areal energy density[J]. Advanced Functional Materials, 2014, 24(34): 5359-5367. |
| 6 | Manthiram A, Fu Y, Chung S H, et al. Rechargeable lithium–sulfur batteries[J]. Chemical Reviews, 2014, 114(23): 11751-11787. |
| 7 | Liu D, Zhang C, Zhou G, et al. Catalytic effects in lithium–sulfur batteries: promoted sulfur transformation and reduced shuttle effect[J]. Advanced Science, 2018, 5(1): 1700270. |
| 8 | 许睿, 赵梦, 黄佳琦. 复合隔膜在锂硫电池中的应用评述[J]. 储能科学与技术, 2017, 6(3): 433-450. |
| Xu R, Zhao M, Huang J Q, Progress in composite separators for lithium sulfur batteries[J]. Energy Storage Science and Technology, 2017, 6(3): 433-450. | |
| 9 | Kolosnitsyn V S, Karaseva E V. Lithium-sulfur batteries: problems and solutions[J]. Russian Journal of Electrochemistry, 2008, 44(5): 506-509 |
| 10 | 黄佳琦, 孙滢智, 王云飞, 等. 锂硫电池先进功能隔膜的研究进展[J]. 化学学报, 2017, 75(2): 173-188. |
| Huang J Q, Sun Y Z, Wang Y F, et al. Review on advanced functional separators for lithium-sulfur batteries[J]. Acta Chimica Sinica, 2017, 75(2): 173-188. | |
| 11 | Chung S H, Manthiram A. Bifunctional separator with a light‐weight carbon‐coating for dynamically and statically stable lithium‐sulfur batteries[J]. Advanced Functional Materials, 2014, 24(33): 5299-5306. |
| 12 | Pei F, Lin L, Fu A, et al. A two-dimensional porous carbon-modified separator for high-energy-density Li-S batteries[J]. Joule, 2018, 2(2): 323-336. |
| 13 | Balach J, Jaumann T, Klose M, et al. Functional mesoporous carbon‐coated separator for long‐life, high‐energy lithium–sulfur batteries[J]. Advanced Functional Materials, 2015, 25(33): 5285-5291. |
| 14 | Manthiram A, Fu Y, Su Y S. Challenges and prospects of lithium-sulfur batteries[J]. Accounts of Chemical Research, 2012, 46(5): 1125-1134. |
| 15 | Jayaprakash N, Shen J, Moganty S S, et al. Porous hollow carbon@ sulfur composites for high power lithium-sulfur batteries[J]. Angewandte Chemie International Edition, 2011, 50(26): 5904-5908. |
| 16 | Yin Y X, Xin S, Guo Y G, et al. Lithium–sulfur batteries: electrochemistry, materials, and prospects[J]. Angewandte Chemie International Edition, 2013, 52(50): 13186-13200. |
| 17 | Xin S, Gu L, Zhao N H, et al. Smaller sulfur molecules promise better lithium–sulfur batteries[J]. Journal of the American Chemical Society, 2012, 134(45): 18510-18513. |
| 18 | Yim T, Han S H, Park N H, et al. Effective polysulfide rejection by dipole‐aligned BaTiO3 coated separator in lithium–sulfur batteries[J]. Advanced Functional Materials, 2016, 26(43): 7817-7823. |
| 19 | Zang Y, Pei F, Huang J, et al. Large‐area preparation of crack‐free crystalline microporous conductive membrane to upgrade high energy lithium-sulfur batteries[J]. Advanced Energy Materials, 2018, 8(31): 1802052. |
| 20 | Huang J Q, Zhuang T Z, Zhang Q, et al. Permselective graphene oxide membrane for highly stable and anti-self-discharge lithium-sulfur batteries[J]. ACS Nano, 2015, 9(3): 3002-3011. |
| 21 | Ma R, Bando Y, Zhang L, et al. Layered MnO2 nanobelts: hydrothermal synthesis and electrochemical measurements[J]. Advanced Materials, 2004, 16(11): 918-922. |
| 22 | Xu R, Wang X, Wang D, et al. Surface structure effects in nanocrystal MnO2 and Ag/MnO2 catalytic oxidation of CO[J]. Journal of Catalysis, 2006, 237(2): 426-430. |
| 23 | Liu Z, Xu K, Sun H, et al. One‐step synthesis of single‐layer MnO2 nanosheets with multi‐role sodium dodecyl sulfate for high‐performance pseudocapacitors[J]. Small, 2015, 11(18): 2182-2191 |
| 24 | Zhao G, Li J, Jiang L, et al. Synthesizing MnO2 nanosheets from graphene oxide templates for high performance pseudosupercapacitors[J]. Chemical Science, 2012, 3(2): 433-437. |
| 25 | Ananth M V, Pethkar S, Dakshinamurthi K. Distortion of MnO6 octahedra and electrochemical activity of Nstutite-based MnO2 polymorphs for alkaline electrolytes—an FTIR study[J]. Journal of Power Sources, 1998, 75(2): 278-282. |
| 26 | Zhang C, Wu H B, Yuan C, et al. Confining sulfur in double‐shelled hollow carbon spheres for lithium–sulfur batteries[J]. Angewandte Chemie International Edition, 2012, 51(38): 9592-9595. |
| 27 | Mao L, Park H, Soler-Crespo R A, et al. Stiffening of graphene oxide films by soft porous sheets[J]. Nature Communications, 2019, 10(1): 1-7. |
| 28 | Wang H, Zhang J, Hang X, et al. Half‐metallicity in single‐layered manganese dioxide nanosheets by defect engineering[J]. Angewandte Chemie International Edition, 2015, 54(4): 1195-1199. |
| 29 | Song X, Chen G, Wang S, et al. Self-assembled close-packed MnO2 nanoparticles anchored on a polyethylene separator for lithium–sulfur batteries[J]. ACS Applied Materials & Interfaces, 2018, 10(31): 26274-26282. |
| 30 | Cha E, Patel M D, Park J, et al. 2D MoS2 as an efficient protective layer for lithium metal anodes in high-performance Li–S batteries[J]. Nature Nanotechnology, 2018, 13(4): 337. |
| 31 | Liang X, Hart C, Pang Q, et al. A highly efficient polysulfide mediator for lithium–sulfur batteries[J]. Nature Communications, 2015, 6: 5682. |
| 32 | Miller F A, Wilkins C H. Infrared spectra and characteristic frequencies of inorganic ions[J]. Analytical Chemistry, 1952, 24(8): 1253-1294. |
| 33 | Jin B, Kim J U, Gu H B. Electrochemical properties of lithium-sulfur batteries[J]. Journal of Power Sources, 2003, 117(1/2): 148-152. |
| 34 | Kai K, Yoshida Y, Kageyama H, et al. Room-temperature synthesis of manganese oxide monosheets[J]. Journal of the American Chemical Society, 2008, 130(47): 15938-15943. |
| 35 | Seh Z W, Sun Y, Zhang Q, et al. Designing high-energy lithium-sulfur batteries[J]. Chemical Society Reviews, 2016, 45(20): 5605-5634. |
| 36 | Deng Z, Zhang Z, Lai Y, et al. Electrochemical impedance spectroscopy study of a lithium/sulfur battery: modeling and analysis of capacity fading[J]. Journal of the Electrochemical Society, 2013, 160(4): A553-A558. |
| 37 | Jeon B H, Yeon J H, Kim K M, et al. Preparation and electrochemical properties of lithium-sulfur polymer batteries[J]. Journal of Power Sources, 2002, 109(1): 89-97. |
| 38 | Ghazi Z A, He X, Khattak A M, et al. MoS2/celgard separator as efficient polysulfide barrier for long‐life lithium–sulfur batteries[J]. Advanced Materials, 2017, 29(21): 1606817. |
| 39 | Zhao T, Zhai P, Yang Z, et al. Self-supporting Ti3C2Tx foam/S cathodes with high sulfur loading for high-energy-density lithium-sulfur batteries[J]. Nanoscale, 2018, 10(48): 22954-22962. |
| [1] | 邵苛苛, 宋孟杰, 江正勇, 张旋, 张龙, 高润淼, 甄泽康. 水平方向上冰中受陷气泡形成和分布实验研究[J]. 化工学报, 2023, 74(S1): 161-164. |
| [2] | 吴延鹏, 李晓宇, 钟乔洋. 静电纺丝纳米纤维双疏膜油性细颗粒物过滤性能实验分析[J]. 化工学报, 2023, 74(S1): 259-264. |
| [3] | 刘远超, 关斌, 钟建斌, 徐一帆, 蒋旭浩, 李耑. 单层XSe2(X=Zr/Hf)的热电输运特性研究[J]. 化工学报, 2023, 74(9): 3968-3978. |
| [4] | 何松, 刘乔迈, 谢广烁, 王斯民, 肖娟. 高浓度水煤浆管道气膜减阻两相流模拟及代理辅助优化[J]. 化工学报, 2023, 74(9): 3766-3774. |
| [5] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [6] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [7] | 齐聪, 丁子, 余杰, 汤茂清, 梁林. 基于选择吸收纳米薄膜的太阳能温差发电特性研究[J]. 化工学报, 2023, 74(9): 3921-3930. |
| [8] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [9] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [10] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| [11] | 邢美波, 张中天, 景栋梁, 张洪发. 磁调控水基碳纳米管协同多孔材料强化相变储/释能特性[J]. 化工学报, 2023, 74(7): 3093-3102. |
| [12] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [13] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [14] | 张贲, 王松柏, 魏子亚, 郝婷婷, 马学虎, 温荣福. 超亲水多孔金属结构驱动的毛细液膜冷凝及传热强化[J]. 化工学报, 2023, 74(7): 2824-2835. |
| [15] | 韩奎奎, 谭湘龙, 李金芝, 杨婷, 张春, 张永汾, 刘洪全, 于中伟, 顾学红. 四通道中空纤维MFI分子筛膜用于二甲苯异构体分离[J]. 化工学报, 2023, 74(6): 2468-2476. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号