化工学报 ›› 2020, Vol. 71 ›› Issue (9): 3979-3994.DOI: 10.11949/0438-1157.20200516
收稿日期:2020-05-08
修回日期:2020-06-27
出版日期:2020-09-05
发布日期:2020-09-05
通讯作者:
李春
作者简介:秦磊(1987—),男,博士,基金资助:
Lei QIN1( ),Jie YU1,Xiaoyu NING1,Wentao SUN1,Chun LI1,2(
),Jie YU1,Xiaoyu NING1,Wentao SUN1,Chun LI1,2( )
)
Received:2020-05-08
Revised:2020-06-27
Online:2020-09-05
Published:2020-09-05
Contact:
Chun LI
摘要:
微生物细胞工厂生产化学品是解决能源和环境问题的有效方式之一。越来越多的化合物可通过合成生物系统实现在微生物中的合成,但菌株的生产能力和鲁棒性仍需进一步提高。提高细胞工厂的智能化,实现生物“智”造过程,将是解决菌株发酵生产能力不足和鲁棒性差的重要途径。本文从蛋白质设计的智能化、生物传感器的智能化、代谢调控的智能化、菌株进化的智能化以及发酵过程智能化等五个层面对生物“智”造的研究现状进行介绍。生物“智”造的发展将为提高工业生物过程的生产水平和过程节能减排做出重要贡献。
中图分类号:
秦磊, 俞杰, 宁小钰, 孙文涛, 李春. 合成生物系统构建与绿色生物“智”造[J]. 化工学报, 2020, 71(9): 3979-3994.
Lei QIN, Jie YU, Xiaoyu NING, Wentao SUN, Chun LI. Synthetic biological system construction and green intelligent biological manufacturing[J]. CIESC Journal, 2020, 71(9): 3979-3994.

图2 蛋白质变构调节的从头设计(a) pH诱导的α-螺旋同源三聚体的解离[26]; (b) 基于α-螺旋的蛋白质开关[30]Fig.2 De novo design of tunable conformational changes of proteins
(a) pH-induced dissociation of α-helix homotrimer[26]; (b) α-helix based protein switch[30]

图3 酶催化的智能化(a) 利用酶的杂泛性合成不同产物[35]; (b) 通过调节温度改变酶的性质[36]
Fig.3 Intelligence of enzymes(a) synthesis of various products by enzyme promiscuity[35]; (b) changing properties of enzymes by regulating temperature[36]

图4 转录调节元件作为生物传感器(a) 感应IPP的转录调节元件的理性设计[53]; (b) 低温诱导表达系统[59]; (c) 大肠杆菌中光诱导和光抑制表达系统[64];(d) 酿酒酵母中光诱导表达系统[65]
Fig.4 Transcription regulatory elements as biosensors(a) rational design of IPP-response element[53]; (b) cold-induced expression system[59]; (c) light-induced and light-repressed expression system inE. coli[64]; (d) light-induced expression system in S. cerevisiae[65]
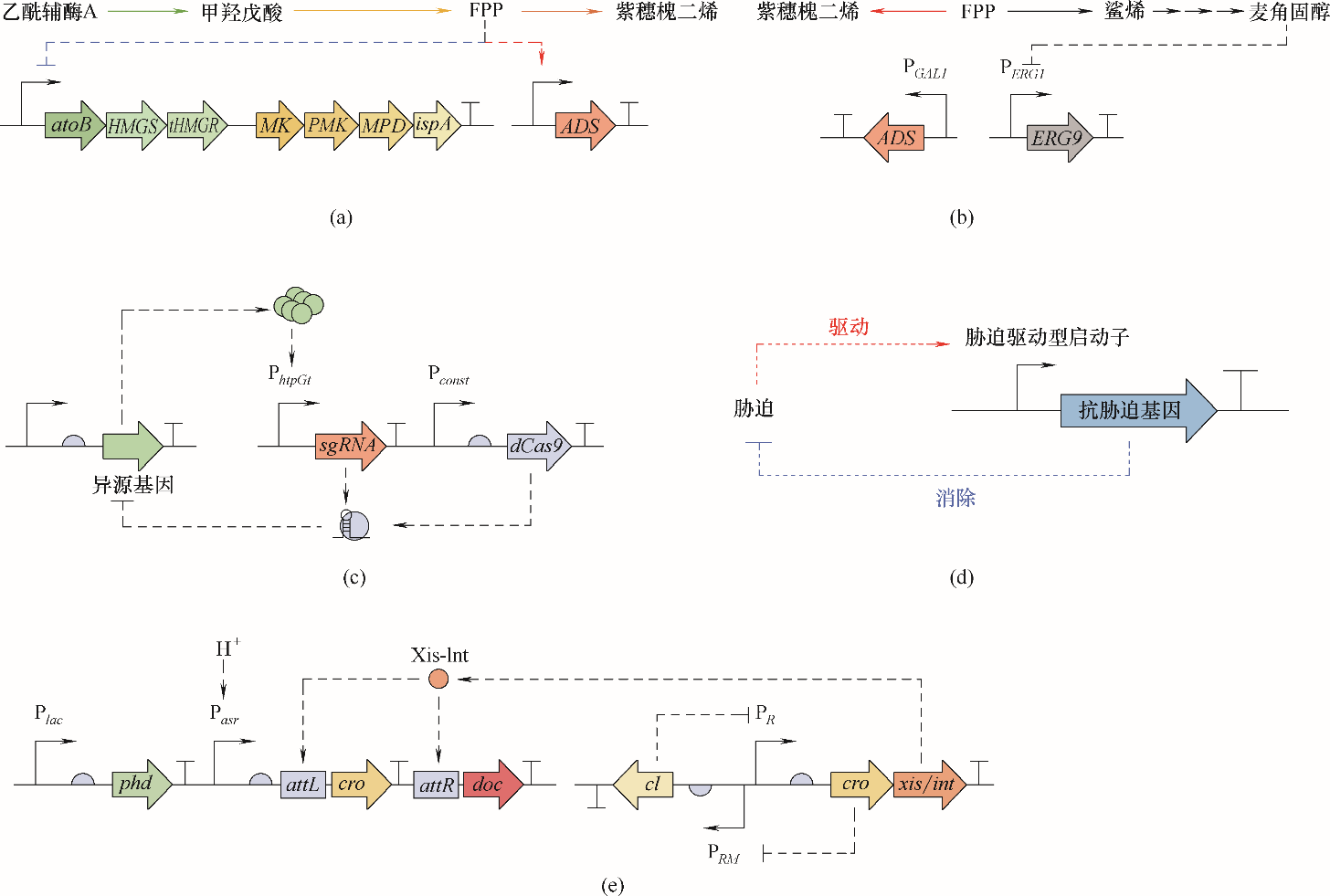
图5 自主动态调控策略(a) FPP反馈抑制与反馈诱导提高紫穗槐二烯产量[56]; (b) 麦角固醇反馈抑制降低竞争途径通量[91]; (c) 转录负担诱导的CRISPRi反馈调节[92];(d) 胁迫诱导的抗胁迫反馈调节[57]; (e) 二次酸感应细胞死亡系统[93]
Fig.5 Autonomous dynamic regulations(a) FPP feedback inhibition and induction increase amorphadiene production[56]; (b) ergosterol feedback inhibition decreases competitive pathway flux[91]; (c) burden induced CRISPRi feedback regulation[92]; (d) stress-driven feedback regulation of anti-stress system[57]; (e) two count pH sensitive kill switch[93]

图6 基于生物传感器的智能自主进化策略(a) FREP[53]; (b) AEMS[39]; (c) 利用pH感应的核糖体开关的自主进化[73]
Fig.6 Intelligent autonomous evolutions(a) FREP[53]; (b) AEMS[39]; (c) autonomous evolution based on pH-sensitive riboswitch[73]
| 1 | Dai Z, Nielsen J. Advancing metabolic engineering through systems biology of industrial microorganisms[J]. Current Opinion in Biotechnology, 2015, 36: 8-15. |
| 2 | Lee J W, Na D, Park J M, et al. Systems metabolic engineering of microorganisms for natural and non-natural chemicals[J]. Nature Chemical Biology, 2012, 8(6): 536. |
| 3 | Curran K A, Alper H S. Expanding the chemical palate of cells by combining systems biology and metabolic engineering[J]. Metabolic Engineering, 2012, 14(4): 289-297. |
| 4 | Zhang F, Rodriguez S, Keasling J D. Metabolic engineering of microbial pathways for advanced biofuels production[J]. Current Opinion in Biotechnology, 2011, 22(6): 775-783. |
| 5 | Mao X, Liu Z, Sun J, et al. Metabolic engineering for the microbial production of marine bioactive compounds[J]. Biotechnology Advances, 2017, 35(8): 1004-1021. |
| 6 | Pontrelli S, Chiu T Y, Lan E I, et al. Escherichia coli as a host for metabolic engineering[J]. Metabolic Engineering, 2018, 50: 16-46. |
| 7 | Matsumoto T, Tanaka T, Kondo A. Engineering metabolic pathways in Escherichia coli for constructing a “microbial chassis” for biochemical production[J]. Bioresource Technology, 2017, 245: 1362-1368. |
| 8 | Heider S A E, Wendisch V F. Engineering microbial cell factories: metabolic engineering of Corynebacterium glutamicum with a focus on non-natural products[J]. Biotechnology Journal, 2015, 10(8): 1170-1184. |
| 9 | Becker J, Wittmann C. Bio-based production of chemicals, materials and fuels—Corynebacterium glutamicum as versatile cell factory[J]. Current Opinion in Biotechnology, 2012, 23(4): 631-640. |
| 10 | Zhang Y, Nielsen J, Liu Z. Engineering yeast metabolism for production of terpenoids for use as perfume ingredients, pharmaceuticals and biofuels[J]. FEMS Yeast Research, 2017, 17: 8. |
| 11 | Bond C M, Tang Y. Engineering Saccharomyces cerevisiae for production of simvastatin[J]. Metabolic Engineering, 2019, 51: 1-8. |
| 12 | Yaguchi A, Spagnuolo M, Blenner M. Engineering yeast for utilization of alternative feedstocks[J]. Current Opinion in Biotechnology, 2018, 53: 122-129. |
| 13 | Ko J K, Lee S M. Advances in cellulosic conversion to fuels: engineering yeasts for cellulosic bioethanol and biodiesel production[J]. Current Opinion in Biotechnology, 2018, 50: 72-80. |
| 14 | Zhu M, Wang C, Sun W, et al. Boosting 11-oxo-β-amyrin and glycyrrhetinic acid synthesis in Saccharomyces cerevisiaevia pairing novel oxidation and reduction system from legume plants[J]. Metabolic Engineering, 2018, 45: 43-50. |
| 15 | Biggs B W, De Paepe B, Santos C N S, et al. Multivariate modular metabolic engineering for pathway and strain optimization[J]. Current Opinion in Biotechnology, 2014, 29: 156-162. |
| 16 | Caspeta L, Castillo T, Nielsen J. Modifying yeast tolerance to inhibitory conditions of ethanol production processes[J]. Frontiers in Bioengineering and Biotechnology, 2015, 3: 184. |
| 17 | Cunha J T, Romaní A, Costa C E, et al. Molecular and physiological basis of Saccharomyces cerevisiae tolerance to adverse lignocellulose-based process conditions[J]. Applied Microbiology and Biotechnology, 2019, 103: 159-175. |
| 18 | Xu K, Lv B, Huo Y X, et al. Toward the lowest energy consumption and emission in biofuel production: combination of ideal reactors and robust hosts[J]. Current Opinion in Biotechnology, 2017, 50: 19-24. |
| 19 | Deparis Q, Claes A, Foulquié-Moreno M R, et al. Engineering tolerance to industrially relevant stress factors in yeast cell factories[J]. FEMS Yeast Research, 2017, 17: 4. |
| 20 | Morano K A, Grant C M, Moye-Rowley W S. The response to heat shock and oxidative stress in Saccharomyces cerevisiae[J]. Genetics, 2012, 190(4): 1157-1195. |
| 21 | Xu K, Lee Y S, Li J, et al. Resistance mechanisms and reprogramming of microorganisms for efficient biorefinery under multiple environmental stresses[J]. Synthetic and Systems Biotechnology, 2019, 4(2): 92-98. |
| 22 | Xu K, Qin L, Bai W, et al. Multilevel defense system (MDS) relieves multiple stresses for economically boosting ethanol production of industrial Saccharomyces cerevisiae[J]. ACS Energy Letters, 2020, 5(2): 572-582. |
| 23 | Leman J K, Weitzner B D, Lewis S M, et al. Macromolecular modeling and design in Rosetta: recent methods and frameworks[J]. Nature Methods, 2020, 17: 665-680. |
| 24 | Weitzner B D, Kipnis Y, Daniel A G, et al. A computational method for design of connected catalytic networks in proteins[J]. Protein Science, 2019, 28(12): 2036-2041. |
| 25 | Chen Z, Boyken S E, Jia M, et al. Programmable design of orthogonal protein heterodimers[J]. Nature, 2019, 565: 106-111. |
| 26 | Boyken S E, Benhaim M A, Busch F, et al. De novo design of tunable, pH-driven conformational changes[J]. Science, 2019, 364: 658-664. |
| 27 | Zhang Y, Bartz R. Grigoryan G,et al. Computational design and experimental characterization of peptides intended for pH-dependent membrane insertion and pore formation[J]. ACS Chemical Biology, 2015, 10(4): 1082-1093. |
| 28 | Kisovec M, Rezelj S, Knap P, et al. Engineering a pH responsive pore forming protein[J]. Scientific Reports, 2017, 7: 42231. |
| 29 | Omersa N, Aden S, Kisovec M, et al. Design of protein logic gate system operating on lipid membranes[J]. ACS Synthetic Biology, 2020, 9: 316-328. |
| 30 | Langan R A, Boyken S E, Ng A H, et al. De novo design of bioactive protein switches[J]. Nature, 2019, 572: 205-210. |
| 31 | Ng A H, Nguyen T H, Gómez-Schiavon M, et al. Modular and tunable biological feedback control using a de novo protein switch[J]. Nature, 2019, 572: 265-269. |
| 32 | Chen Z, Kibler R D, Hunt A, et al. De novo design of protein logic gates[J]. Science, 2020, 368: 78-84. |
| 33 | Tinberg C E, Khare S D, Dou J, et al. Computational design of ligand-binding proteins with high affinity and selectivity[J]. Nature, 2013, 501(7466): 212-216. |
| 34 | Urlacher V B, Girhard M. Cytochrome P450 monooxygenases in biotechnology and synthetic biology[J]. Trends in Biotechnology, 2019, 37: 882. |
| 35 | Sun W, Xue H, Liu H, et al. Controlling chemo- and regioselectivity of a plant P450 in yeast cell toward rare licorice triterpenoid biosynthesis[J]. ACS Catalysis, 2020, 10: 4253-4260. |
| 36 | Inda M E, Vandenbranden M, Fernández A, et al. A lipid-mediated conformational switch modulates the thermosensing activity of DesK[J]. Proceedings of the National Academy of Sciences of the USA, 2014, 111(9): 3579-3584. |
| 37 | Wu M Y, Sung L Y, Li H, et al. Combining CRISPR and CRISPRi systems for metabolic engineering of E. coli and 1,4-BDO biosynthesis[J]. ACS Synthetic Biology, 2017, 6: 2350-2361. |
| 38 | Rodrigues A L, Becker J, de Souza Lima A O, et al. Systems metabolic engineering of Escherichia coli for gram scale production of the antitumor drug deoxyviolacein from glycerol[J]. Biotechnology and Bioengineering, 2014, 111: 2280-2289. |
| 39 | Wang S, Hou Y, Chen X, et al. Kick-starting evolution efficiency with an autonomous evolution mutation system[J]. Metabolic Engineering, 2019, 54: 127-136. |
| 40 | Xie W, Ye L, Lv X, et al. Sequential control of biosynthetic pathways for balanced utilization of metabolic intermediates in Saccharomyces cerevisiae[J]. Metabolic Engineering, 2015, 28: 8-18. |
| 41 | Paddon C J, Westfall P J, Pitera D J, et al. High-level semi-synthetic production of the potent antimalarial artemisinin[J]. Nature, 2013, 496: 528-532. |
| 42 | Šeputiene V, Motiejūnas D, Suziedelis K, et al. Molecular characterization of the acid-inducible asr gene of Escherichia coli and its role in acid stress response[J]. Journal of Bacteriology, 2003, 185(8): 2475-2484. |
| 43 | Ogasawara H, Hasegawa A, Kanda E, et al. Genomic SELEX search for target promoters under the control of the PhoQP-RstBA signal relay cascade[J]. Journal of Bacteriology, 2007, 189(13): 4791-4799. |
| 44 | Hoynes-O’Connor A, Shopera T, Hinman K, et al. Enabling complex genetic circuits to respond to extrinsic environmental signals[J]. Biotechnology and Bioengineering, 2017, 114: 1626-1631. |
| 45 | Haneburger I, Fritz G, Jurkschat N, et al. Deactivation of the E. coli pH stress sensor CadC by cadaverine[J]. Journal of Molecular Biology, 2012, 424: 15-27. |
| 46 | Moser F, Borujeni A E, Ghodasara A N, et al. Dynamic control of endogenous metabolism with combinatorial logic circuits[J]. Molecular Systems Biology, 2018, 14: 8605. |
| 47 | Xiong L, Zeng Y, Tang R Q, et al. Condition specific promoter activities in Saccharomyces cerevisiae[J]. Microbial Cell Factories, 2018, 17: 58. |
| 48 | Rajkumar A S, Liu G d, Bergenholm D, et al. Engineering of synthetic, stress-responsive yeast promoters[J]. Nucleic Acids Research, 2016, 44(17): 136. |
| 49 | Hanko E K R, Paiva A C, Jonczyk M, et al. A genome-wide approach for identification and characterisation of metabolite-inducible systems[J]. Nature Communications, 2020, 11: 1213. |
| 50 | Xu P, Wang W, Li L, et al. Design and kinetic analysis of a hybrid promoter-regulator system for malonyl-CoA sensing in Escherichia coli[J]. ACS Chemical Biology, 2014, 9(2): 451-458. |
| 51 | Xu P, Li L, Zhang F, et al. Improving fatty acids production by engineering dynamic pathway regulation and metabolic control[J]. Proceedings of the National Academy of Sciences of the USA, 2014, 111(31): 11299-11304. |
| 52 | Liang C, Zhang X, Wu J, et al. Dynamic control of toxic natural product biosynthesis by an artificial regulatory circuit[J]. Metabolic Engineering, 2020, 57: 239-246. |
| 53 | Chou H H, Keasling J D. Programming adaptive control to evolve increased metabolite production[J]. Nature Communications, 2013, 4: 2595. |
| 54 | Feng J, Jester B W, Tinberg C E, et al. A general strategy to construct small molecule biosensors in eukaryotes[J]. Elife, 2015, 4: e10606. |
| 55 | Jester B W, Tinberg C E, Rich M S, et al. Engineered biosensors from dimeric ligand-binding domains[J]. ACS Synthetic Biology, 2018, 7: 2457-2467. |
| 56 | Dahl R H, Zhang F, Alonso-Gutierrez J, et al. Engineering dynamic pathway regulation using stress-response promoters[J]. Nature Biotechnology, 2013, 31(11): 1039-1046. |
| 57 | Qin L, Dong S, Yu J, et al. Stress-driven dynamic regulation of multiple tolerance genes improves robustness and productive capacity of Saccharomyces cerevisiae in industrial lignocellulose fermentation[J]. Metabolic Engineering, 2020, 61:160-170. |
| 58 | Valdez-Cruz N A, Caspeta L, Pérez N O, et al. Production of recombinant proteins in E. coli by the heat inducible expression system based on the phage lambda pL and/or pR promoters[J]. Microbial Cell Factories, 2010, 9: 18. |
| 59 | Zheng Y, Meng F, Zhu Z, et al. A tight cold-inducible switch built by coupling thermosensitive transcriptional and proteolytic regulatory parts[J]. Nucleic Acids Research, 2019, 47(21): 137. |
| 60 | Guan C R, Cui W J, Cheng J T, et al. Construction and development of an auto-regulatory gene expression system in Bacillus subtilis[J]. Microbial Cell Factories, 2015, 14: 150. |
| 61 | Fuqua C, Parsek M R, Greenberg E P. Regulation of gene expression by cell-to-cell communication: acylhomoserine lactone quorum sensing[J]. Annual Review of Genetics, 2001, 35: 439-468. |
| 62 | Jia H, Sun X, Sun H, et al. Intelligent microbial heat-regulating engine (IMHeRE) for improved thermo-robustness and efficiency of bioconversion[J]. ACS Synthetic Biology, 2016, 5: 312-320. |
| 63 | Zoltowski B D, Motta-Mena L B, Gardner K H. Blue light-induced dimerization of a bacterial LOV-HTH DNA-binding protein[J]. Biochemistry, 2013, 52: 6653-6661. |
| 64 | Jayaraman P, Devarajan K, Chua T K, et al. Blue light-mediated transcriptional activation and repression of gene expression in bacteria[J]. Nucleic Acids Research, 2016, 44: 6994-7005. |
| 65 | Zhao E M, Zhang Y, Mehl J, et al. Optogenetic regulation of engineered cellular metabolism for microbial chemical production[J]. Nature, 2018, 555: 683. |
| 66 | Hochrein L, Machens F, Messerschmidt K, et al. PhiReX: a programmable and red light-regulated protein expression switch for yeast[J]. Nucleic Acids Research, 2017, 110: 21130-21135. |
| 67 | Schmidl S R, Sheth R U, Wu A, et al. Refactoring and optimization of light-switchable Escherichia coli two component systems[J]. ACS Synthetic Biology, 2014, 3: 820-831. |
| 68 | Olson E J, Hartsough L A, Landry B P, et al. Characterizing bacterial gene circuit dynamics with optically programmed gene expression signals[J]. Nature Methods, 2014, 11: 449-455. |
| 69 | Neupert J, Karcher D, Bock R. Design of simple synthetic RNA thermometers for temperature-controlled gene expression in Escherichia coli[J]. Nucleic Acids Research, 2008, 36: 124. |
| 70 | Giuliodori A M, Di Pietro F, Marzi S, et al. The cspA mRNA is a thermosensor that modulates translation of the cold-shock protein CspA[J]. Molecular Cell, 2010, 37(1): 21-33. |
| 71 | Nechooshtan G, Elgrably-Weiss M, Sheaffer A, et al. A pH-responsive riboregulatory[J]. Genes and Development, 2009, 23: 2650-2662. |
| 72 | Nechooshtan G, Elgrably-Weiss M, Altuvia S. Changes in transcriptional pausing modify the folding dynamics of the pH-responsive RNA element[J]. Nucleic Acids Research, 2014, 42: 622-630. |
| 73 | Pham H L, Wong A, Chua N, et al. Engineering a riboswitch-based genetic platform for the self-directed evolution of acid-tolerant phenotypes[J]. Nature Communications, 2017, 8: 411. |
| 74 | Lukyanov K A, Belousov V V. Genetically encoded fluorescent redox sensors[J]. Biochimica et Biophysica Acta, 2014, 1840(2): 745-756. |
| 75 | Bilan D S, Belousov V V. New tools for redox biology: from imaging to manipulation[J]. Free Radical Biology and Medicine, 2017, 109: 167-188. |
| 76 | 俞杰, 秦磊, 许可, 等. 细胞工厂氧化还原状态的荧光探针检测与调控[J].生物加工过程, 2020, 18(1): 60-69. |
| Yu J, Qin L, Xu K, et al. Detection and regulation of the redox state in cell factories by fluorescent probes[J]. Chinese Journal of Bioprocess Engineering, 2020, 18(1): 60-69. | |
| 77 | Bugaj L J, Choksi A T, Mesuda C K, et al. Optogenetic protein clustering and signaling activation in mammalian cells[J]. Nature Methods, 2013, 10: 249-252. |
| 78 | Taslimi A, Vrana J D, Chen D, et al. An optimized optogenetic clustering tool for probing protein interaction and function[J]. Nature Communications, 2014, 5: 4925. |
| 79 | Shin Y, Berry J, Pannucci N, et al. Spatiotemporal control of intracellular phase transitions using light-activated optoDroplets[J]. Cell, 2017, 168: 159-171. |
| 80 | Zhao E M, Suek N, Wilson M Z, et al. Light-based control of metabolic flux through assembly of synthetic organelles[J]. Nature Chemical Biology, 2019, 15: 589-597. |
| 81 | Dine E, Gil A A, Uribe G, et al. Protein phase separation provides long-term memory of transient spatial stimuli[J]. Cell Systems, 2018, 6: 655-663. |
| 82 | Gil A A, Laptenok S P, Iuliano J N, et al. Photoactivation of the BLUF protein PixD probed by the site-specific incorporation of fluorotyrosine residues[J]. Journal of the American Chemical Society, 2017, 139: 14638-14648. |
| 83 | Kim S K, Han G H, Seong W, et al. CRISPR interference-guided balancing of a biosynthetic mevalonate pathway increases terpenoid production[J]. Metabolic Engineering, 2016, 38: 228-240. |
| 84 | Cunningham-Bryant D, Sun J, Fernandez B, et al. CRISPR-Cas-mediated chemical control of transcriptional dynamics in yeast[J]. ChemBioChem, 2019, 20(12): 1519-1523. |
| 85 | Koopal B, Kruis A J, Claassens N J, et al. Incorporation of a synthetic amino acid into dCas9 improves control of gene silencing[J]. ACS Synthetic Biology, 2019, 8(2): 216-222. |
| 86 | Ni J, Wu Y T, Tao F, et al. A coenzyme-free biocatalyst for the value-added utilization of lignin-derived aromatics[J]. Journal of the American Chemical Society, 2018, 140: 16001-16005. |
| 87 | Bañares A B, Valdehuesa K N G, Ramos K R M, et al. A pH-responsive genetic sensor for the dynamic regulation of D-xylonic acid accumulation in Escherichia coli[J]. Applied Microbiology and Biotechnology, 2020, 104: 2097-2108. |
| 88 | Bañares A B, Valdehuesa K N G, Ramos K R M, et al. Discovering a novel D-xylonate-responsive promoter: the PyjhI-driven genetic switch towards better 1,2,4-butanetriol production[J]. Applied Microbiology and Biotechnology, 2019, 103: 8063-8074. |
| 89 | Anesiadis N, Kobayashi H, Cluett W R, et al. Analysis and design of a genetic circuit for dynamic metabolic engineering[J]. ACS Synthetic Biology, 2013, 2(8): 442-452. |
| 90 | Kim E M, Woo H M, Tian T, et al. Autonomous control of metabolic state by a quorum sensing (QS)-mediated regulator for bisabolene production in engineered E. coli[J]. Metabolic Engineering, 2017, 44: 325-336. |
| 91 | Yuan J, Ching C B. Dynamic control of ERG9 expression for improved amorpha-4,11-diene production in Saccharomyces cerevisiae[J]. Microbial Cell Factories, 2015, 14: 38. |
| 92 | Ceroni F, Boo A, Furini S, et al. Burden-driven feedback control of gene expression[J]. Nature Methods, 2018, 15(5): 387-393. |
| 93 | Stirling F, Naydich A, Bramante J, et al. Synthetic cassettes for pH-mediated sensing, counting and containment[J]. Cell Reports, 2020, 30(9): 3139. |
| 94 | Zhao N, Bai Y, Liu C G, et al. Flocculating Zymomonas mobilis is a promising host to be engineered for fuel ethanol production from lignocellulosic biomass[J]. Biotechnology Journal, 2014, 9(3): 362-371. |
| 95 | Govender P, Domingo J L, Bester M C, et al. Controlled expression of the dominant flocculation genes FLO1, FLO5, and FLO11 in Saccharomyces cerevisiae[J]. Applied and Environmental Microbiology, 2008, 74(19): 6041-6052. |
| 96 | Li Q, Zhao X Q, Chang A K, et al. Ethanol-induced yeast flocculation directed by the promoter of TPS1 encoding trehalose-6-phosphate synthase 1 for efficient ethanol production[J]. Metabolic Engineering, 2012, 14: 1-8. |
| 97 | Ling C, Qiao G Q, Shuai B W, et al. Engineering self-flocculating Halomonas campaniensis for wastewaterless open and continuous fermentation[J]. Biotechnology and Bioengineering, 2019, 116: 805-815. |
| 98 | Heler R, Wright A V, Vucelja M, et al. Mutations in Cas9 enhance the rate of acquisition of viral spacer sequences during the CRISPR-Cas immune response[J]. Molecular Cell, 2017, 65(1): 168-175. |
| 99 | Jiang W, Oikonomou P, Tavazoie S. Comprehensive genome-wide perturbations via CRISPR adaptation reveal complex genetics of antibiotic sensitivity[J]. Cell, 2020, 180: 1-16. |
| 100 | Xie Z X, Li B Z, Mitchell L A, et al. ‘Perfect’ designer chromosome V and behavior of a ring derivative[J]. Science, 2017, 355(6329): 4704. |
| 101 | Wu Y, Li B Z, Zhao M, et al. Bug mapping and fitness testing of chemically synthesized chromosome X[J]. Science, 2017, 355(6329): 4706. |
| 102 | Dymond J S, Richardson S M, Coombes C E, et al. Synthetic chromosome arms function in yeast and generate phenotypic diversity by design[J]. Nature, 2011, 477: 471-476. |
| 103 | Jia B, Wu Y, Li B Z, et al. Precise control of SCRaMbLE in synthetic haploid and diploid yeast[J]. Nature Communications, 2018, 9(1): 1933. |
| 104 | Hochrein L, Mitchell L A, Schulz K, et al. L-SCRaMbLE as a tool for light-controlled Cre-mediated recombination in yeast[J]. Nature Communications, 2018, 9(1): 1931. |
| 105 | Ma L, Li Y, Chen X, et al. SCRaMbLE generates evolved yeasts with increased alkali tolerance[J]. Microbial Cell Factories, 2019, 18(1): 52. |
| 106 | Shen M J, Wu Y, Yang K, et al. Heterozygous diploid and interspecies SCRaMbLEing[J]. Nature Communications, 2018, 9(1): 1934. |
| 107 | Luo Z, Wang L, Wang Y, et al. Identifying and characterizing SCRaMbLEd synthetic yeast using ReSCuES[J]. Nature Communications, 2018, 9(1): 1930. |
| 108 | Blount B A, Gowers G O F, Ho J C H, et al. Rapid host strain improvement by in vivo rearrangement of a synthetic yeast chromosome[J]. Nature Communications, 2018, 9(1): 1932. |
| 109 | Wu Y, Zhu R Y, Mitchell L A, et al. In vitro DNA SCRaMbLE[J]. Nature Communications, 2018, 9(1): 1935. |
| 110 | Wang J, Xie Z X, Ma Y, et al. Ring synthetic chromosome V SCRaMbLE[J]. Nature Communications, 2018, 9(1): 3783. |
| 111 | Gowers G O F, Chee S M, Bell D, et al. Improved betulinic acid biosynthesis using synthetic yeast chromosome recombination and semi-automated rapid LC-MS screening[J]. Nature Communications, 2020, 11(1): 868. |
| 112 | Liu W, Luo Z, Wang Y, et al. Rapid pathway prototyping and engineering using in vitro and in vivo synthetic genome SCRaMbLE-in methods[J]. Nature Communications, 2018, 9(1): 1936. |
| 113 | 田锡炜, 王冠, 张嗣良, 等. 工业生物过程智能控制原理和方法进展[J]. 生物工程学报, 2019, 35(10): 2014-2024. |
| Tian X W, Wang G, Zhang S L, et al. Progress in intelligent control of industrial bioprocess[J]. Chinese Journal of Biotechnology, 2019, 35(10): 2014-2024. | |
| 114 | Chen Y, Wang Z J, Chu J, et al. Significant decrease of broth viscosity and glucose consumption in erythromycin fermentation by dynamic regulation of ammonium sulfate and phosphate[J]. Bioresource Technology, 2013, 134: 173-179. |
| 115 | Zou X, Xia J Y, Chu J, et al. Real-time fluid dynamics investigation and physiological response for erythromycin fermentation scale-up from 50 L to 132 m3 fermenter[J]. Bioprocess and Biosystems Engineering, 2012, 35(5): 789-800. |
| 116 | 陈晓春. 啤酒发酵系统温度智能控制[J]. 食品工业, 2019, 40(11): 219-221. |
| Chen X C. Intelligent temperature control of beer fermentation system[J]. The Food Industry, 2019, 40(11): 219-221. | |
| 117 | Wang L, Yuan J, Wu C, et al. Practical algorithm for stochastic optimal control problem about microbial fermentation in batch culture[J]. Optimization Letters, 2019, 13: 527-541. |
| 118 | Grunberger A, Wiechert W, Kohlheyer D. Single-cell microfluidics: opportunity for bioprocess development[J]. Current Opinion in Biotechnology, 2014, 29: 15-23. |
| 119 | Chen J, Vestergaard M, Shen J, et al. Droplet-based microfluidics as a future tool for strain improvement in lactic acid bacteria[J]. FEMS Microbiology Letters, 2018, 365: 258. |
| 120 | Macosko E Z, Basu A, Satija R, et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets[J]. Cell, 2015, 161: 1202-1214. |
| 121 | Blattman S B, Jiang W, Oikonomou P, et al. Prokaryotic single-cell RNA sequencing by in situ combinatorial indexing[J]. Nature Microbiology, 2020, . |
| 122 | Si T, Chao R, Min Y, et al. Automated multiplex genome-scale engineering in yeast[J]. Nature Communications, 2017, 8: 15187. |
| [1] | 刘瑞琪, 周栖桐, 张悦, 贺莹, 高静, 马丽. 基于金纳米颗粒修饰二氧化硅纳米花的生物传感器构建及应用[J]. 化工学报, 2023, 74(3): 1247-1259. |
| [2] | 刘昕, 戈钧, 李春. 光驱动微生物杂合系统提高生物制造水平[J]. 化工学报, 2023, 74(1): 330-341. |
| [3] | 毕浩然, 张洋, 王凯, 徐晨晨, 霍奕影, 陈必强, 谭天伟. 微生物制造绿色化学品研究进展[J]. 化工学报, 2023, 74(1): 1-13. |
| [4] | 刘雪, 张莉娟, 赵广荣. 大肠杆菌偏利共培养系统合成大豆苷元[J]. 化工学报, 2022, 73(9): 4015-4024. |
| [5] | 王靖楠, 庞建, 秦磊, 郭超, 吕波, 李春, 王超. 丁烯基多杀菌素高产菌株的选育和改造策略[J]. 化工学报, 2022, 73(2): 566-576. |
| [6] | 孙怡, 张腾, 吕波, 李春. 胞内生物传感器提高微生物细胞工厂的精细调控[J]. 化工学报, 2022, 73(2): 521-534. |
| [7] | 王欣慧, 王颖, 姚明东, 肖文海. 维生素A生物合成的研究进展[J]. 化工学报, 2022, 73(10): 4311-4323. |
| [8] | 周武林, 高惠芳, 吴玉玲, 张显, 徐美娟, 杨套伟, 邵明龙, 饶志明. 重组酿酒酵母生物合成菜油甾醇[J]. 化工学报, 2021, 72(8): 4314-4324. |
| [9] | 陈婷婷, 韩恺忻, 陈翠雪, 凌雪萍, 沈亮, 卢英华. 铁还原菌Shewanella xiamenensis BC01的有机溶剂应激研究[J]. 化工学报, 2021, 72(7): 3747-3756. |
| [10] | 杨瑞雄, 郑鑫, 陆涛, 赵誉泽, 杨庆华, 卢英华, 何宁, 凌雪萍. 烯酰还原酶基因的替换对裂殖壶菌合成二十碳五烯酸的影响[J]. 化工学报, 2021, 72(7): 3768-3779. |
| [11] | 毛金竹, 肖淑玲, 杨智淳, 王孝宇, 张诗, 陈俊宏, 谢佶晟, 陈福德, 黄子诺, 冯天宇, 张瑷珲, 方柏山. 合成生物学在农残检测领域的应用[J]. 化工学报, 2021, 72(5): 2413-2425. |
| [12] | 王欣, 赵鹏, 李清扬, 田平芳. 半导体合成生物学的研究进展[J]. 化工学报, 2021, 72(5): 2426-2435. |
| [13] | 应雨轩, 林晓青, 吴昂键, 李晓东. 生活垃圾智慧焚烧的研究现状及展望[J]. 化工学报, 2021, 72(2): 886-900. |
| [14] | 苏楠, 吴亦楠, 陈韵亿, 金丽华, 张翀, Aikawa Shimpei, Hasunuma Tomohisa, Kondo Akihiko, 邢新会. ARTP诱变钝顶螺旋藻突变体比较组学研究[J]. 化工学报, 2021, 72(12): 6298-6310. |
| [15] | 张震, 曾雪城, 秦磊, 李春. 微生物细胞工厂的智能设计进展[J]. 化工学报, 2021, 72(12): 6093-6108. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号