化工学报 ›› 2021, Vol. 72 ›› Issue (4): 1895-1905.DOI: 10.11949/0438-1157.20200933
收稿日期:2020-07-13
修回日期:2020-09-10
出版日期:2021-04-05
发布日期:2021-04-05
通讯作者:
于旭东
作者简介:黄琴(1996—),女,硕士研究生,基金资助:
HUANG Qin1( ),YU Xudong1,2(
),YU Xudong1,2( ),LI Maolan1,ZHENG Hong1,ZENG Ying1,2
),LI Maolan1,ZHENG Hong1,ZENG Ying1,2
Received:2020-07-13
Revised:2020-09-10
Online:2021-04-05
Published:2021-04-05
Contact:
YU Xudong
摘要:
采用等温溶解平衡法和浊点法分别测定了308.2 K时三元体系KCl+PEG10000/20000+H2O的固液平衡组成和液液平衡组成,同时测定了固液平衡时体系的密度和折射率。根据实验数据,绘制了两个三元体系308.2 K时的完整相图、双液线对比图和结线-组成图。研究发现:三元体系KCl+PEG10000/20000+H2O 308.2 K时的完整相图均包含6个区域:不饱和液相区(L),2个一固一液区(L+S),双液相区(2L),两液一固区(2L+S),两固一液区(L+2S);其中,一液两固区(L+2S)中的两种固相分别为KCl和PEG10000/20000。在完整相图中,一固一液区面积最大,双液相区面积最小。对比308.2 K时聚乙二醇分子量为1000、4000、6000、10000和20000的KCl+PEG+H2O体系可知:分子量为1000、4000和6000时,体系中仅存在固液相平衡关系;分子量为10000和20000时,体系中同时存在固液和液液相平衡关系,且随着聚乙二醇分子量的增加,双液线向原点移动,双液相区(2L)和两液一固区(2L+S)增大,不饱和液相区(L)和一固一液区(L+S)均减小。采用Chen-NRTL-PDH热力学模型进行了液液相平衡计算,计算结果与实验数据吻合较好。
中图分类号:
黄琴, 于旭东, 李茂兰, 郑洪, 曾英. 308.2 K三元体系KCl+PEG10000/20000+H2O相平衡及热力学计算[J]. 化工学报, 2021, 72(4): 1895-1905.
HUANG Qin, YU Xudong, LI Maolan, ZHENG Hong, ZENG Ying. Experimental and thermodynamic simulation for ternary systems KCl+PEG10000/20000+ H2O at 308.2 K[J]. CIESC Journal, 2021, 72(4): 1895-1905.
| 三元体系 | a | b | c | R2 |
|---|---|---|---|---|
| KCl+PEG10000+H2O | -9.0880 | 22.0649 | -303.6913 | 0.9984 |
| KCl+PEG20000+H2O | -7.9581 | 21.2533 | -412.8902 | 0.9989 |
表1 回归参数值
Table 1 Parameters of regression
| 三元体系 | a | b | c | R2 |
|---|---|---|---|---|
| KCl+PEG10000+H2O | -9.0880 | 22.0649 | -303.6913 | 0.9984 |
| KCl+PEG20000+H2O | -7.9581 | 21.2533 | -412.8902 | 0.9989 |
| No. | Density(ρ)/ (g?cm-3) | Refractive index (nD) | Composition of equilibrated solution | Composition of wet solid phase | Equilibrated solid phase | ||
|---|---|---|---|---|---|---|---|
| wKCl | wPEG | wKCl | wPEG | ||||
| KCl+PEG10000+ H2O | |||||||
| 1,A | 1.1847 | 1.3692 | 0.2851 | 0.0000 | - | - | - |
| 2 | 1.1816 | 1.3722 | 0.2739 | 0.0197 | 0.6487 | 0.0095 | KCl |
| 3 | 1.1611 | 1.3904 | 0.2012 | 0.1997 | 0.6890 | 0.0778 | KCl |
| 4 | 1.1564 | 1.3941 | 0.1803 | 0.2459 | 0.6803 | 0.0959 | KCl |
| 5 | 1.1540 | 1.3995 | 0.1677 | 0.2913 | 0.6138 | 0.1352 | KCl |
| 6 | 1.1500 | 1.4050 | 0.1502 | 0.3399 | 0.6433 | 0.1427 | KCl |
| 7 | 1.1486 | 1.4105 | 0.1323 | 0.3905 | 0.5791 | 0.1894 | KCl |
| 8 | 1.1466 | 1.4164 | 0.1145 | 0.4428 | 0.5315 | 0.2343 | KCl |
| 9 | 1.1439 | 1.4221 | 0.0971 | 0.4966 | 0.4907 | 0.2801 | KCl |
| 10 | 1.1407 | 1.4280 | 0.0809 | 0.5515 | 0.3797 | 0.3722 | KCl |
| 11,B | 1.1060 | 1.4334 | 0.0000 | 0.7017 | - | - | - |
| KCl+PEG20000+ H2O | |||||||
| 12, C | 1.1847 | 1.3692 | 0.2851 | 0.0000 | - | - | - |
| 13 | 1.1581 | 1.3939 | 0.1811 | 0.2457 | 0.5370 | 0.1389 | KCl |
| 14 | 1.1538 | 1.3995 | 0.1595 | 0.2942 | 0.4868 | 0.1796 | KCl |
| 15 | 1.1500 | 1.4040 | 0.1439 | 0.3424 | 0.4829 | 0.2068 | KCl |
| 16 | 1.1417 | 1.4087 | 0.1190 | 0.3964 | 0.4494 | 0.2478 | KCl |
| 17 | 1.1404 | 1.4144 | 0.1032 | 0.4484 | 0.3937 | 0.3031 | KCl |
| 18 | 1.1272 | 1.4200 | 0.0794 | 0.5063 | 0.2966 | 0.3869 | KCl |
| 19 | 1.1235 | 1.4260 | 0.0517 | 0.5690 | 0.2295 | 0.4623 | KCl |
| 20, D | 1.1012 | 1.4280 | 0.0000 | 0.6742 | - | - | - |
表2 三元体系KCl+PEG10000/20000+H2O 308.2 K、94.77 kPa固液平衡实验数据
Table 2 Solid-liquid equilibrium data for ternary systems KCl+PEG10000/20000+H2O at T = 308.2 K and pressure p = 94.77 kPa
| No. | Density(ρ)/ (g?cm-3) | Refractive index (nD) | Composition of equilibrated solution | Composition of wet solid phase | Equilibrated solid phase | ||
|---|---|---|---|---|---|---|---|
| wKCl | wPEG | wKCl | wPEG | ||||
| KCl+PEG10000+ H2O | |||||||
| 1,A | 1.1847 | 1.3692 | 0.2851 | 0.0000 | - | - | - |
| 2 | 1.1816 | 1.3722 | 0.2739 | 0.0197 | 0.6487 | 0.0095 | KCl |
| 3 | 1.1611 | 1.3904 | 0.2012 | 0.1997 | 0.6890 | 0.0778 | KCl |
| 4 | 1.1564 | 1.3941 | 0.1803 | 0.2459 | 0.6803 | 0.0959 | KCl |
| 5 | 1.1540 | 1.3995 | 0.1677 | 0.2913 | 0.6138 | 0.1352 | KCl |
| 6 | 1.1500 | 1.4050 | 0.1502 | 0.3399 | 0.6433 | 0.1427 | KCl |
| 7 | 1.1486 | 1.4105 | 0.1323 | 0.3905 | 0.5791 | 0.1894 | KCl |
| 8 | 1.1466 | 1.4164 | 0.1145 | 0.4428 | 0.5315 | 0.2343 | KCl |
| 9 | 1.1439 | 1.4221 | 0.0971 | 0.4966 | 0.4907 | 0.2801 | KCl |
| 10 | 1.1407 | 1.4280 | 0.0809 | 0.5515 | 0.3797 | 0.3722 | KCl |
| 11,B | 1.1060 | 1.4334 | 0.0000 | 0.7017 | - | - | - |
| KCl+PEG20000+ H2O | |||||||
| 12, C | 1.1847 | 1.3692 | 0.2851 | 0.0000 | - | - | - |
| 13 | 1.1581 | 1.3939 | 0.1811 | 0.2457 | 0.5370 | 0.1389 | KCl |
| 14 | 1.1538 | 1.3995 | 0.1595 | 0.2942 | 0.4868 | 0.1796 | KCl |
| 15 | 1.1500 | 1.4040 | 0.1439 | 0.3424 | 0.4829 | 0.2068 | KCl |
| 16 | 1.1417 | 1.4087 | 0.1190 | 0.3964 | 0.4494 | 0.2478 | KCl |
| 17 | 1.1404 | 1.4144 | 0.1032 | 0.4484 | 0.3937 | 0.3031 | KCl |
| 18 | 1.1272 | 1.4200 | 0.0794 | 0.5063 | 0.2966 | 0.3869 | KCl |
| 19 | 1.1235 | 1.4260 | 0.0517 | 0.5690 | 0.2295 | 0.4623 | KCl |
| 20, D | 1.1012 | 1.4280 | 0.0000 | 0.6742 | - | - | - |
| No. | Composition of binodal curve | No. | Composition of binodal curve | No. | Composition of binodal curve | |||
|---|---|---|---|---|---|---|---|---|
| wKCl | wPEG | wKCl | wPEG | wKCl | wPEG | |||
| KCl+PEG10000+H2O | KCl+PEG20000+H2O | KCl+PEG20000+H2O | ||||||
| 1,E | 0.2637 | 0.0390 | 1,G | 0.2729 | 0.0059 | 20 | 0.2288 | 0.0637 |
| 2 | 0.2618 | 0.0398 | 2 | 0.2806 | 0.0057 | 21 | 0.2263 | 0.0706 |
| 3 | 0.2598 | 0.0418 | 3 | 0.2820 | 0.0056 | 22 | 0.2241 | 0.0765 |
| 4 | 0.2577 | 0.0444 | 4 | 0.2841 | 0.0054 | 23 | 0.2230 | 0.0809 |
| 5 | 0.2553 | 0.0479 | 5 | 0.2729 | 0.0059 | 24 | 0.2220 | 0.0852 |
| 6 | 0.2531 | 0.0523 | 6 | 0.2693 | 0.0083 | 25 | 0.2205 | 0.0909 |
| 7 | 0.2495 | 0.0611 | 7 | 0.2632 | 0.0091 | 26 | 0.2181 | 0.0965 |
| 8 | 0.2460 | 0.0700 | 8 | 0.2605 | 0.0100 | 27 | 0.2170 | 0.1052 |
| 9 | 0.2420 | 0.0803 | 9 | 0.2588 | 0.0110 | 28 | 0.2141 | 0.1149 |
| 10 | 0.2383 | 0.0898 | 10 | 0.2571 | 0.0119 | 29 | 0.2118 | 0.1225 |
| 11 | 0.2347 | 0.0990 | 11 | 0.2548 | 0.0160 | 30 | 0.2101 | 0.1309 |
| 12 | 0.2314 | 0.1080 | 12 | 0.2491 | 0.0250 | 31 | 0.2076 | 0.1402 |
| 13 | 0.2286 | 0.1150 | 13 | 0.2464 | 0.0275 | 32 | 0.2039 | 0.1521 |
| 14 | 0.2250 | 0.1234 | 14 | 0.2446 | 0.0308 | 33 | 0.2016 | 0.1632 |
| 15 | 0.2219 | 0.1317 | 15 | 0.2430 | 0.0329 | 34 | 0.1986 | 0.1790 |
| 16 | 0.2186 | 0.1405 | 16 | 0.2403 | 0.0399 | 35,H | 0.1951 | 0.1970 |
| 17 | 0.2168 | 0.1498 | 17 | 0.2376 | 0.0459 | |||
| 18 | 0.2141 | 0.1581 | 18 | 0.2343 | 0.0544 | |||
| 19,F | 0.2106 | 0.1648 | 19 | 0.2318 | 0.0581 | |||
表3 三元体系KCl+PEG10000/20000+H2O 308.2 K、94.77 kPa液液平衡实验数据
Table 3 Liquid-liquid equilibrium data of ternary systems KCl+PEG10000/20000+H2O at T = 308.2 K and pressure p = 94.77 kPa
| No. | Composition of binodal curve | No. | Composition of binodal curve | No. | Composition of binodal curve | |||
|---|---|---|---|---|---|---|---|---|
| wKCl | wPEG | wKCl | wPEG | wKCl | wPEG | |||
| KCl+PEG10000+H2O | KCl+PEG20000+H2O | KCl+PEG20000+H2O | ||||||
| 1,E | 0.2637 | 0.0390 | 1,G | 0.2729 | 0.0059 | 20 | 0.2288 | 0.0637 |
| 2 | 0.2618 | 0.0398 | 2 | 0.2806 | 0.0057 | 21 | 0.2263 | 0.0706 |
| 3 | 0.2598 | 0.0418 | 3 | 0.2820 | 0.0056 | 22 | 0.2241 | 0.0765 |
| 4 | 0.2577 | 0.0444 | 4 | 0.2841 | 0.0054 | 23 | 0.2230 | 0.0809 |
| 5 | 0.2553 | 0.0479 | 5 | 0.2729 | 0.0059 | 24 | 0.2220 | 0.0852 |
| 6 | 0.2531 | 0.0523 | 6 | 0.2693 | 0.0083 | 25 | 0.2205 | 0.0909 |
| 7 | 0.2495 | 0.0611 | 7 | 0.2632 | 0.0091 | 26 | 0.2181 | 0.0965 |
| 8 | 0.2460 | 0.0700 | 8 | 0.2605 | 0.0100 | 27 | 0.2170 | 0.1052 |
| 9 | 0.2420 | 0.0803 | 9 | 0.2588 | 0.0110 | 28 | 0.2141 | 0.1149 |
| 10 | 0.2383 | 0.0898 | 10 | 0.2571 | 0.0119 | 29 | 0.2118 | 0.1225 |
| 11 | 0.2347 | 0.0990 | 11 | 0.2548 | 0.0160 | 30 | 0.2101 | 0.1309 |
| 12 | 0.2314 | 0.1080 | 12 | 0.2491 | 0.0250 | 31 | 0.2076 | 0.1402 |
| 13 | 0.2286 | 0.1150 | 13 | 0.2464 | 0.0275 | 32 | 0.2039 | 0.1521 |
| 14 | 0.2250 | 0.1234 | 14 | 0.2446 | 0.0308 | 33 | 0.2016 | 0.1632 |
| 15 | 0.2219 | 0.1317 | 15 | 0.2430 | 0.0329 | 34 | 0.1986 | 0.1790 |
| 16 | 0.2186 | 0.1405 | 16 | 0.2403 | 0.0399 | 35,H | 0.1951 | 0.1970 |
| 17 | 0.2168 | 0.1498 | 17 | 0.2376 | 0.0459 | |||
| 18 | 0.2141 | 0.1581 | 18 | 0.2343 | 0.0544 | |||
| 19,F | 0.2106 | 0.1648 | 19 | 0.2318 | 0.0581 | |||
| No. | Kpc | TLL | STL | Composition of original solution | Composition of top phase | Composition of bottom phase | |||
|---|---|---|---|---|---|---|---|---|---|
| wKCl | wPEG | wKCl | wPEG | wKCl | wPEG | ||||
| KCl+PEG10000+H2O | |||||||||
| 1 | 0.85 | 0.1009 | -2.4945 | 0.2386 | 0.0900 | 0.2195 | 0.1407 | 0.2570 | 0.0470 |
| 2 | 0.84 | 0.1130 | -2.4703 | 0.2393 | 0.0903 | 0.2171 | 0.1473 | 0.2595 | 0.0426 |
| 3 | 0.82 | 0.1273 | -2.4229 | 0.2409 | 0.0909 | 0.2146 | 0.1544 | 0.2632 | 0.0367 |
| 4 | 0.81 | 0.1336 | -2.4022 | 0.2405 | 0.0908 | 0.2135 | 0.1576 | 0.2648 | 0.0343 |
| KCl+PEG20000+H2O | |||||||||
| 1 | 0.80 | 0.1525 | -2.7691 | 0.2400 | 0.0470 | 0.2026 | 0.1611 | 0.2544 | 0.0177 |
| 2 | 0.75 | 0.1877 | -2.6375 | 0.2443 | 0.0490 | 0.1972 | 0.1854 | 0.2637 | 0.0099 |
| 3 | 0.72 | 0.2067 | -2.5874 | 0.2487 | 0.0510 | 0.1939 | 0.2000 | 0.2684 | 0.0072 |
| 4 | 0.71 | 0.2146 | -2.5858 | 0.2509 | 0.0520 | 0.1924 | 0.2067 | 0.2698 | 0.0066 |
| 5 | 0.68 | 0.2354 | -2.5022 | 0.2531 | 0.0530 | 0.1888 | 0.2227 | 0.2761 | 0.0042 |
表4 三元体系KCl+PEG10000/20000+H2O 308.2 K、94.77 kPa结线实验数据
Table 4 Experimental data of tie lines of ternary systems KCl+PEG10000/20000+H2O at T = 308.2 K and pressure p = 94.77 kPa
| No. | Kpc | TLL | STL | Composition of original solution | Composition of top phase | Composition of bottom phase | |||
|---|---|---|---|---|---|---|---|---|---|
| wKCl | wPEG | wKCl | wPEG | wKCl | wPEG | ||||
| KCl+PEG10000+H2O | |||||||||
| 1 | 0.85 | 0.1009 | -2.4945 | 0.2386 | 0.0900 | 0.2195 | 0.1407 | 0.2570 | 0.0470 |
| 2 | 0.84 | 0.1130 | -2.4703 | 0.2393 | 0.0903 | 0.2171 | 0.1473 | 0.2595 | 0.0426 |
| 3 | 0.82 | 0.1273 | -2.4229 | 0.2409 | 0.0909 | 0.2146 | 0.1544 | 0.2632 | 0.0367 |
| 4 | 0.81 | 0.1336 | -2.4022 | 0.2405 | 0.0908 | 0.2135 | 0.1576 | 0.2648 | 0.0343 |
| KCl+PEG20000+H2O | |||||||||
| 1 | 0.80 | 0.1525 | -2.7691 | 0.2400 | 0.0470 | 0.2026 | 0.1611 | 0.2544 | 0.0177 |
| 2 | 0.75 | 0.1877 | -2.6375 | 0.2443 | 0.0490 | 0.1972 | 0.1854 | 0.2637 | 0.0099 |
| 3 | 0.72 | 0.2067 | -2.5874 | 0.2487 | 0.0510 | 0.1939 | 0.2000 | 0.2684 | 0.0072 |
| 4 | 0.71 | 0.2146 | -2.5858 | 0.2509 | 0.0520 | 0.1924 | 0.2067 | 0.2698 | 0.0066 |
| 5 | 0.68 | 0.2354 | -2.5022 | 0.2531 | 0.0530 | 0.1888 | 0.2227 | 0.2761 | 0.0042 |

图3 三元体系KCl+PEG1000/4000/6000/10000/20000+H2O 308.2 K完整相图对比
Fig.3 Diagrams comparison of ternary systems KCl+PEG1000/4000/6000/10000/20000+H2O at 308.2K
| Solvent | Molecular weight (M)① | Molar volume (V)/ (m3·mol-1) | Dielectric constant (D) | Segment number (r) |
|---|---|---|---|---|
| PEG10000 | 10251.00 | 8.25×10-3 | 2.20 | 227 |
| PEG20000 | 21245.00 | 1.65×10-2 | 2.20 | 482 |
| H2O | 18.02 | 18.13×10-6 | 74.83 | 1 |
表5 PEG10000/20000以及H2O在308.2 K时的物理性质参数[41-42]
Table 5 Physical property parameters of PEG10000/20000 and H2O at 308.2 K[41-42]
| Solvent | Molecular weight (M)① | Molar volume (V)/ (m3·mol-1) | Dielectric constant (D) | Segment number (r) |
|---|---|---|---|---|
| PEG10000 | 10251.00 | 8.25×10-3 | 2.20 | 227 |
| PEG20000 | 21245.00 | 1.65×10-2 | 2.20 | 482 |
| H2O | 18.02 | 18.13×10-6 | 74.83 | 1 |
| PEG10000/20000 | τ12 | τ21 | τ13 | τ31 | τ23 | τ32 | σ |
|---|---|---|---|---|---|---|---|
| PEG10000 | -95.8304 | 0.4592 | 5.1452 | -5.85×10-5 | 1.2935 | -103.4504 | 3.27×10-6 |
| PEG20000 | -158.4585 | -0.2582 | 8.1035 | 0.4067 | 3.0293 | -165.1540 | 8.04×10-7 |
表6 三元体系KCl+PEG10000/20000+H2O在 308.2 K时的热力学参数
Table 6 Thermodynamic parameters of ternary systems KCl+PEG10000/20000+H2O at 308.2 K
| PEG10000/20000 | τ12 | τ21 | τ13 | τ31 | τ23 | τ32 | σ |
|---|---|---|---|---|---|---|---|
| PEG10000 | -95.8304 | 0.4592 | 5.1452 | -5.85×10-5 | 1.2935 | -103.4504 | 3.27×10-6 |
| PEG20000 | -158.4585 | -0.2582 | 8.1035 | 0.4067 | 3.0293 | -165.1540 | 8.04×10-7 |
| No. | Composition of top phase | Composition of bottom phase | ||||
|---|---|---|---|---|---|---|
| wKCl | wPEG | ww | wKCl | wPEG | ww | |
| KCl+PEG10000+H2O | ||||||
| 1 | 0.2204 | 0.1418 | 0.6378 | 0.2560 | 0.0471 | 0.6969 |
| 2 | 0.2184 | 0.1487 | 0.6329 | 0.2581 | 0.0426 | 0.6993 |
| 3 | 0.2164 | 0.1559 | 0.6256 | 0.2624 | 0.0339 | 0.7037 |
| 4 | 0.2156 | 0.1588 | 0.6277 | 0.2613 | 0.0364 | 0.7023 |
| KCl+PEG20000+H2O | ||||||
| 1 | 0.2029 | 0.1613 | 0.6358 | 0.2538 | 0.0176 | 0.7286 |
| 2 | 0.1979 | 0.1855 | 0.6166 | 0.2628 | 0.0099 | 0.7273 |
| 3 | 0.2001 | 0.1949 | 0.6050 | 0.2673 | 0.0072 | 0.7255 |
| 4 | 0.2068 | 0.1934 | 0.5998 | 0.2687 | 0.0065 | 0.7248 |
| 5 | 0.2227 | 0.1905 | 0.5868 | 0.2746 | 0.0041 | 0.7213 |
表7 三元体系KCl+PEG10000/20000+H2O在 308.2 K时的热力学计算数据
Table 7 Calculated data of ternary systems KCl+PEG10000/20000+H2O at 308.2 K
| No. | Composition of top phase | Composition of bottom phase | ||||
|---|---|---|---|---|---|---|
| wKCl | wPEG | ww | wKCl | wPEG | ww | |
| KCl+PEG10000+H2O | ||||||
| 1 | 0.2204 | 0.1418 | 0.6378 | 0.2560 | 0.0471 | 0.6969 |
| 2 | 0.2184 | 0.1487 | 0.6329 | 0.2581 | 0.0426 | 0.6993 |
| 3 | 0.2164 | 0.1559 | 0.6256 | 0.2624 | 0.0339 | 0.7037 |
| 4 | 0.2156 | 0.1588 | 0.6277 | 0.2613 | 0.0364 | 0.7023 |
| KCl+PEG20000+H2O | ||||||
| 1 | 0.2029 | 0.1613 | 0.6358 | 0.2538 | 0.0176 | 0.7286 |
| 2 | 0.1979 | 0.1855 | 0.6166 | 0.2628 | 0.0099 | 0.7273 |
| 3 | 0.2001 | 0.1949 | 0.6050 | 0.2673 | 0.0072 | 0.7255 |
| 4 | 0.2068 | 0.1934 | 0.5998 | 0.2687 | 0.0065 | 0.7248 |
| 5 | 0.2227 | 0.1905 | 0.5868 | 0.2746 | 0.0041 | 0.7213 |
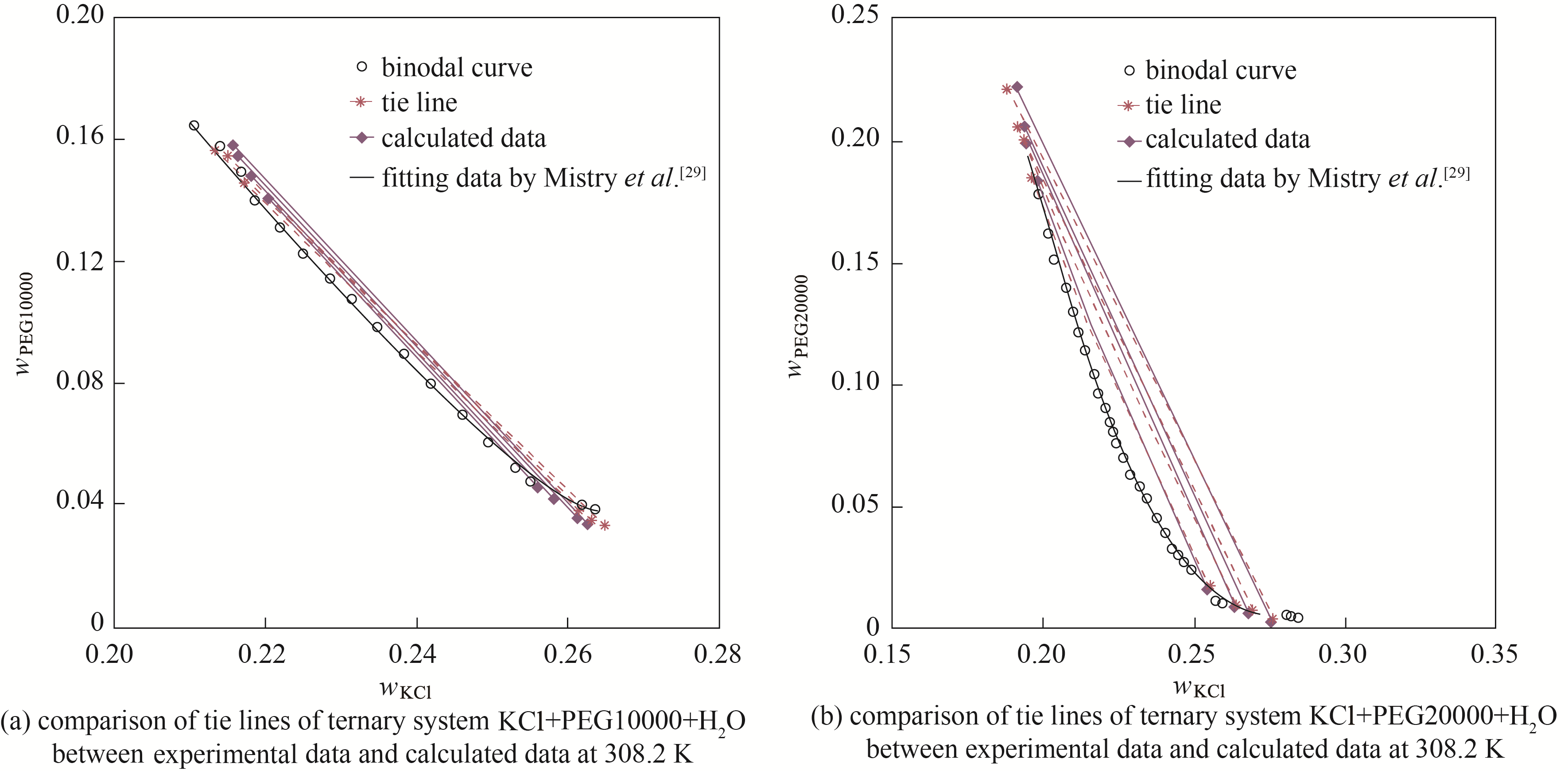
图4 三元体系KCl+PEG10000/20000+H2O 308.2 K实验结线与计算结线对比图
Fig.4 Comparison of tie lines of ternary systems KCl+PEG10000/20000+H2O between experimental data and calculated data at 308.2 K
| 1 | 时历杰, 王敏. 一里坪硫酸镁亚型盐湖钾镁混盐转化-浮选中物相行为[J]. 化工学报, 2019, 70(5): 1832-1841. |
| Shi L J, Wang M. Phase behavior of K-Mg mixed salt during transformation-flotation from Yiliping magnesium sulfate-type salt lake[J]. CIESC Journal, 2019, 70(5): 1832-1841. | |
| 2 | 侯献华, 樊馥, 郑绵平, 等. 青海盐湖钾盐资源开发利用及产业发展[J]. 科技导报, 2017, 35(12): 67-71. |
| Hou X H, Fan F, Zheng M P, et al. Development and utilization of potash resources of saline lakes in Qinghai province[J]. Science & Technology Review, 2017, 35(12): 67-71. | |
| 3 | Guo L J, Wang Y X, Tu L Y, et al. Thermodynamics and phase equilibrium of the system CsCl-MgCl2-H2O at 298.15 K[J]. Journal of Chemical & Engineering Data, 2017, 62(4): 1397-1402. |
| 4 | Yu X D, Zeng Y, Mu P T, et al. Solid-liquid equilibria in the quinary system LiCl-KCl-RbCl-MgCl2-H2O at T = 323 K[J]. Fluid Phase Equilibria, 2015, 387: 88-94. |
| 5 | Guo L J, Li H X. Solubility measurement of the solid solution containing the RbCl-CsCl-H2O system from T = 298.15 to 348.15 K[J]. Journal of Chemical & Engineering Data, 2020, 65(4): 1663-1668. |
| 6 | Li D D, Gao D D, Zeng D W, et al. Understanding the solid solution-aqueous solution equilibria in the KCl + RbCl + H2O system from experiments, atomistic simulation and thermodynamic modeling[J]. Journal of Solution Chemistry, 2017, 46(9/10): 1871-1902. |
| 7 | Zhang J, Gao S Y, Xia S P, et al. Study of thermodynamic properties of quaternary mixture RbCl + Rb2SO4 + CH3OH + H2O by EMF measurement at 298.15 K[J]. Fluid Phase Equilibria, 2004, 226: 307-312. |
| 8 | Królikowska M, Romańska K, Paduszyński K, et al. The study on the influence of glycols on the vapor pressure, density and dynamic viscosity of lithium bromide aqueous solution[J]. Fluid Phase Equilibria, 2020, 519: 112640. |
| 9 | Zhou X H, Zheng W J, Xu D H, et al. Solubility measurement and thermodynamics modelling for potassium dihydrogen phosphate in a water-ethanol system from 293.2 to 323.2 K[J]. Fluid Phase Equilibria, 2020, 512: 112533. |
| 10 | Justel F J, Villca G, Jimenez Y P. Measurement and modelling of water activity, density, sound velocity, refractive index and viscosity of the Na2MoO4 + poly(ethylene glycol) + H2O system in the temperature range from 313.15 to 333.15 K[J]. Fluid Phase Equilibria, 2020, 518: 112628. |
| 11 | Cao X Y, Chang Y H, Li S N, et al. Phase behavior of tetramethylurea + MCl (M = Na, K, Rb, Cs) + H2O systems at 288.2, 298.2 and 308.2 K[J]. The Journal of Chemical Thermodynamics, 2020, 144: 106058. |
| 12 | Lu Y M, Yang Y Z, Zhao X D, et al. Bovine serum albumin partitioning in polyethylene glycol (PEG)/potassium citrate aqueous two-phase systems[J]. Food and Bioproducts Processing, 2010, 88(1): 40-46. |
| 13 | Wysoczanska K, Macedo E A. Influence of the molecular weight of PEG on the polymer/salt phase diagrams of aqueous two-phase systems[J]. Journal of Chemical & Engineering Data, 2016, 61(12): 4229-4235. |
| 14 | Alvarenga B G, Virtuoso L S, Lemes N H T, et al. Measurement and correlation of the phase equilibrium of aqueous two-phase systems composed of polyethylene(glycol) 1500 or 4000 + sodium sulfite + water at different temperatures[J]. Journal of Chemical & Engineering Data, 2014, 59(2): 382-390. |
| 15 | de Oliveira A C, Sosa F H B, Costa M C D, et al. Study of liquid-liquid equilibria in aqueous two-phase systems formed by poly (ethylene glycol) (PEG) and sodium thiosulfate pentahydrate (Na2S2O3·5H2O) at different temperatures[J]. Fluid Phase Equilibria, 2018, 476: 118-125. |
| 16 | Pirdashti M, Bozorgzadeh A, Ketabi M, et al. Phase equilibria of aqueous mixtures of PEG with formate salt: effects of pH, type of cation, polymer molecular weight and temperature[J]. Fluid Phase Equilibria, 2019, 485: 158-167. |
| 17 | Ferreira L A, Teixeira J A. Salt effect on the (polyethylene glycol 8000 + sodium sulfate) aqueous two-phase system: relative hydrophobicity of the equilibrium phases[J]. The Journal of Chemical Thermodynamics, 2011, 43(8): 1299-1304. |
| 18 | Carvalho R, Penido J A, Lobo F A, et al. Thermodynamic investigation of the aqueous two-phase systems formed by PEG 400 + water + either sodium carbonate or potassium carbonate at different temperatures: experimental and correlational approaches[J]. Journal of Chemical & Engineering Data, 2019, 64(2): 448-458. |
| 19 | Huang Q, Wang L, Li M L, et al. Measurements and simulation of the polyethylene glycol 1000–water–KCl ternary system at 288.2, 298.2, and 308.2 K[J]. Journal of Chemical Engineering of Japan, 2019, 52(4): 325-332. |
| 20 | 于旭东, 黄琴, 王林, 等. KCl-PEG4000-H2O三元体系288、298、308 K相平衡测定及计算[J]. 化工学报, 2019, 70(3): 830-839. |
| Yu X D, Huang Q, Wang L, et al. Measurements and simulation for ternary system KCl-PEG4000-H2O at 288, 298 and 308 K[J]. CIESC Journal, 2019, 70(3): 830-839. | |
| 21 | Yu X D, Huang Q, Zheng M P, et al. Solid–liquid equilibria of KCl in polyethylene glycol 6000–H2O mixed solvent at 288.2, 298.2, and 308.2 K: experiment and correlation[J]. Journal of Chemical Engineering of Japan, 2020, 53(6): 229-236. |
| 22 | Yu X D, Wang L, Li M L, et al. Phase equilibria of CsCl-polyethylene glycol (PEG)-H2O at 298.15 K: effect of different polymer molar masses (PEG1000/4000/6000)[J]. The Journal of Chemical Thermodynamics, 2019, 135: 45-54. |
| 23 | Li M L, Wang L, Zheng H, et al. Solid: liquid equilibrium in ternary system RbCl + polyethylene glycol PEG1000 + H2O at 288.15, 298.15, and 308.15 K[J]. Russian Journal of Physical Chemistry A, 2019, 93(13): 2586-2592. |
| 24 | Li M L, Yu X D, Huang Q, et al. Phase equilibria measurements and correlation of aqueous solvent of PEG4000 with rubidium chloride at (288.15, 298.15, and 308.15) K[J]. The Journal of Chemical Thermodynamics, 2020, 149: 106151. |
| 25 | Chen C C. A segment-based local composition model for the Gibbs energy of polymer solutions[J]. Fluid Phase Equilibria, 1993, 83: 301-312. |
| 26 | 中国科学院青海盐湖研究所分析室. 卤水和盐的分析方法[M]. 2版. 北京: 科学出版社, 1988: 69-72. |
| Institute of Qinghai Salt-Lake of Chinese Academy of Sciences. Analytical Methods of Brines and Salts[M]. 2nd ed. Beijing: Science Press, 1988: 69-72. | |
| 27 | Cheluget E L, Gelinas S, Vera J H, et al. Liquid-liquid equilibrium of aqueous mixtures of poly(propylene glycol) with sodium chloride[J]. Journal of Chemical & Engineering Data, 1994, 39(1): 127-130. |
| 28 | Villalobos I, Barrueto Y, Garnica K, et al. Measurement and correlation of phase equilibrium of the aqueous two-phase system formed by Fe2(SO4)3 + PEG 4000 + H2O at different temperatures[J]. Journal of Molecular Liquids, 2017, 237: 372-379. |
| 29 | Mistry S L, Kaul A, Merchuk J C, et al. Mathematical modelling and computer simulation of aqueous two-phase continuous protein extraction[J]. Journal of Chromatography A, 1996, 741(2): 151-163. |
| 30 | Wang Y, Xu X H, Yan Y S, et al. Phase behavior for the [Bmim]BF4 aqueous two-phase systems containing ammonium sulfate/sodium carbonate salts at different temperatures: experimental and correlation[J]. Thermochimica Acta, 2010, 501(1/2): 112-118. |
| 31 | Han J, Pan R, Xie X Q, et al. Liquid-liquid equilibria of ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate + sodium and ammonium citrate aqueous two-phase systems at (298.15, 308.15, and 323.15) K[J]. Journal of Chemical & Engineering Data, 2010, 55(9): 3749-3754. |
| 32 | Othmer D F, Tobias P E. Liquid -liquid extraction data -toluene and acetaldehyde systems[J]. Industrial & Engineering Chemistry, 1942, 34(6): 690-692. |
| 33 | González-Tello P, Camacho F, Blázquez G, et al. Liquid-liquid equilibrium in the system poly(ethylene glycol) + MgSO4 + H2O at 298 K[J]. Journal of Chemical & Engineering Data, 1996, 41(6): 1333-1336. |
| 34 | Hand D B. Dineric distribution[J]. The Journal of Physical Chemistry, 1930, 34(9): 1961-2000. |
| 35 | Bachman I. Tie lines in ternary liquid systems[J]. Industrial & Engineering Chemistry Analytical Edition, 1940, 12(1): 38-39. |
| 36 | Zhang W L, Hu Y T, Wang Y, et al. Liquid-liquid equilibrium of aqueous two-phase systems containing poly(ethylene glycol) of different molecular weights and several ammonium salts at 298.15 K[J]. Thermochimica Acta, 2013, 560: 47-54. |
| 37 | Tubío G, Pellegrini L, Nerli B B, et al. Liquid-liquid equilibria of aqueous two-phase systems containing poly(ethylene glycols) of different molecular weight and sodium citrate[J]. Journal of Chemical & Engineering Data, 2006, 51(1): 209-212. |
| 38 | Edrisi S, Bakhshi H, Rahimnejad M. Aqueous two-phase systems for cephalexin monohydrate partitioning using poly ethylene glycol and sodium tartrate dihydrate: experimental and thermodynamic modeling[J]. Korean Journal of Chemical Engineering, 2019, 36(5): 780-788. |
| 39 | Zafarani-Moattar M T, Sadeghi R. Measurement and correlation of liquid-liquid equilibria of the aqueous two-phase system polyvinylpyrrolidone-sodium dihydrogen phosphate[J]. Fluid Phase Equilibria, 2002, 203(1/2): 177-191. |
| 40 | Simonson J M, Pitzer K S. Thermodynamics of multicomponent, miscible ionic systems: the system lithium nitrate-potassium nitrate-water[J]. The Journal of Physical Chemistry, 1986, 90(13): 3009-3013. |
| 41 | van Krevelen D W, Te Nijenhuis K. Properties of Polymers[M].Amsterdam: Elsevier, 2009: 287-318. |
| 42 | Haynes W M. CRC Handbook of Chemistry and Physics[M]. 97th ed. New York: CRC Press, 2017: 1-9. |
| [1] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [2] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [3] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [4] | 刘杰, 吴立盛, 李锦锦, 罗正鸿, 周寅宁. 含乙烯基胺酯键聚醚类可逆交联聚合物的制备及性能研究[J]. 化工学报, 2023, 74(7): 3051-3057. |
| [5] | 龙臻, 王谨航, 任俊杰, 何勇, 周雪冰, 梁德青. 离子液体协同PVCap抑制天然气水合物生成实验研究[J]. 化工学报, 2023, 74(6): 2639-2646. |
| [6] | 杨琴, 秦传鉴, 李明梓, 杨文晶, 赵卫杰, 刘虎. 用于柔性传感的双形状记忆MXene基水凝胶的制备及性能研究[J]. 化工学报, 2023, 74(6): 2699-2707. |
| [7] | 陈韶云, 徐东, 陈龙, 张禹, 张远方, 尤庆亮, 胡成龙, 陈建. 单层聚苯胺微球阵列结构的制备及其吸附性能[J]. 化工学报, 2023, 74(5): 2228-2238. |
| [8] | 姚晓宇, 沈俊, 李健, 李振兴, 康慧芳, 唐博, 董学强, 公茂琼. 流体气液临界参数测量方法研究进展[J]. 化工学报, 2023, 74(5): 1847-1861. |
| [9] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [10] | 张建华, 陈萌萌, 孙雅雯, 彭永臻. 部分短程硝化同步除磷耦合Anammox实现生活污水高效脱氮除磷[J]. 化工学报, 2023, 74(5): 2147-2156. |
| [11] | 龙臻, 王谨航, 何勇, 梁德青. 离子液体与动力学抑制剂作用下混合气体水合物生成特性研究[J]. 化工学报, 2023, 74(4): 1703-1711. |
| [12] | 吴学红, 栾林林, 陈亚南, 赵敏, 吕财, 刘勇. 可降解柔性相变薄膜的制备及其热性能[J]. 化工学报, 2023, 74(4): 1818-1826. |
| [13] | 吕阳光, 左培培, 杨正金, 徐铜文. 三嗪框架聚合物膜用于有机纳滤甲醇/正己烷分离[J]. 化工学报, 2023, 74(4): 1598-1606. |
| [14] | 罗来明, 张劲, 郭志斌, 王海宁, 卢善富, 相艳. 1~5 kW高温聚合物电解质膜燃料电池堆的理论模拟与组装测试[J]. 化工学报, 2023, 74(4): 1724-1734. |
| [15] | 刘海芹, 李博文, 凌喆, 刘亮, 俞娟, 范一民, 勇强. 羟基-炔点击化学改性半乳甘露聚糖薄膜的制备及性能研究[J]. 化工学报, 2023, 74(3): 1370-1378. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号