化工学报 ›› 2021, Vol. 72 ›› Issue (7): 3869-3879.DOI: 10.11949/0438-1157.20201825
肖弦1( ),徐文昊1,沈亮1,2(
),徐文昊1,沈亮1,2( ),王远鹏1,2,卢英华1,2
),王远鹏1,2,卢英华1,2
收稿日期:2020-12-15
修回日期:2021-04-04
出版日期:2021-07-05
发布日期:2021-07-05
通讯作者:
沈亮
作者简介:肖弦(1997—),女,硕士研究生,基金资助:
XIAO Xian1( ),XU Wenhao1,SHEN Liang1,2(
),XU Wenhao1,SHEN Liang1,2( ),WANG Yuanpeng1,2,LU Yinghua1,2
),WANG Yuanpeng1,2,LU Yinghua1,2
Received:2020-12-15
Revised:2021-04-04
Online:2021-07-05
Published:2021-07-05
Contact:
SHEN Liang
摘要:
石墨烯是导电性良好的二维材料,但易重新堆叠而导致导电率和电容量下降。氧化石墨烯(GO)的生物相容性和细菌的胶体特性可使二者在水溶液中聚集为三维石墨烯基材料。将剩余活性污泥与GO悬浮液共培养形成活性污泥石墨烯水凝胶(SGH),剩余活性污泥中的细菌可将GO还原为导电的rGO。SGH经冻干可得到具有良好亲水性和导电性的O、N自掺杂多孔材料,即活性污泥石墨烯气凝胶(SGA)。在氩气中高温退火可进一步提高材料的电化学性能。经700℃、2 h退火后的改性SGA(ANSGA)具有174 F/g的比电容值(2 A/g),以及优异的倍率性能、离子传输性能和循环稳定性,具有进一步加工制备电极材料的应用潜力,为石墨烯基材料绿色制备和剩余活性污泥资源化利用提供方向。
中图分类号:
肖弦, 徐文昊, 沈亮, 王远鹏, 卢英华. 氧化石墨烯与剩余活性污泥聚合制备多孔碳材料及其电化学性能[J]. 化工学报, 2021, 72(7): 3869-3879.
XIAO Xian, XU Wenhao, SHEN Liang, WANG Yuanpeng, LU Yinghua. Preparation and electrochemical properties of new porous carbon materials by synthesizing graphene oxide and waste activated sludge[J]. CIESC Journal, 2021, 72(7): 3869-3879.
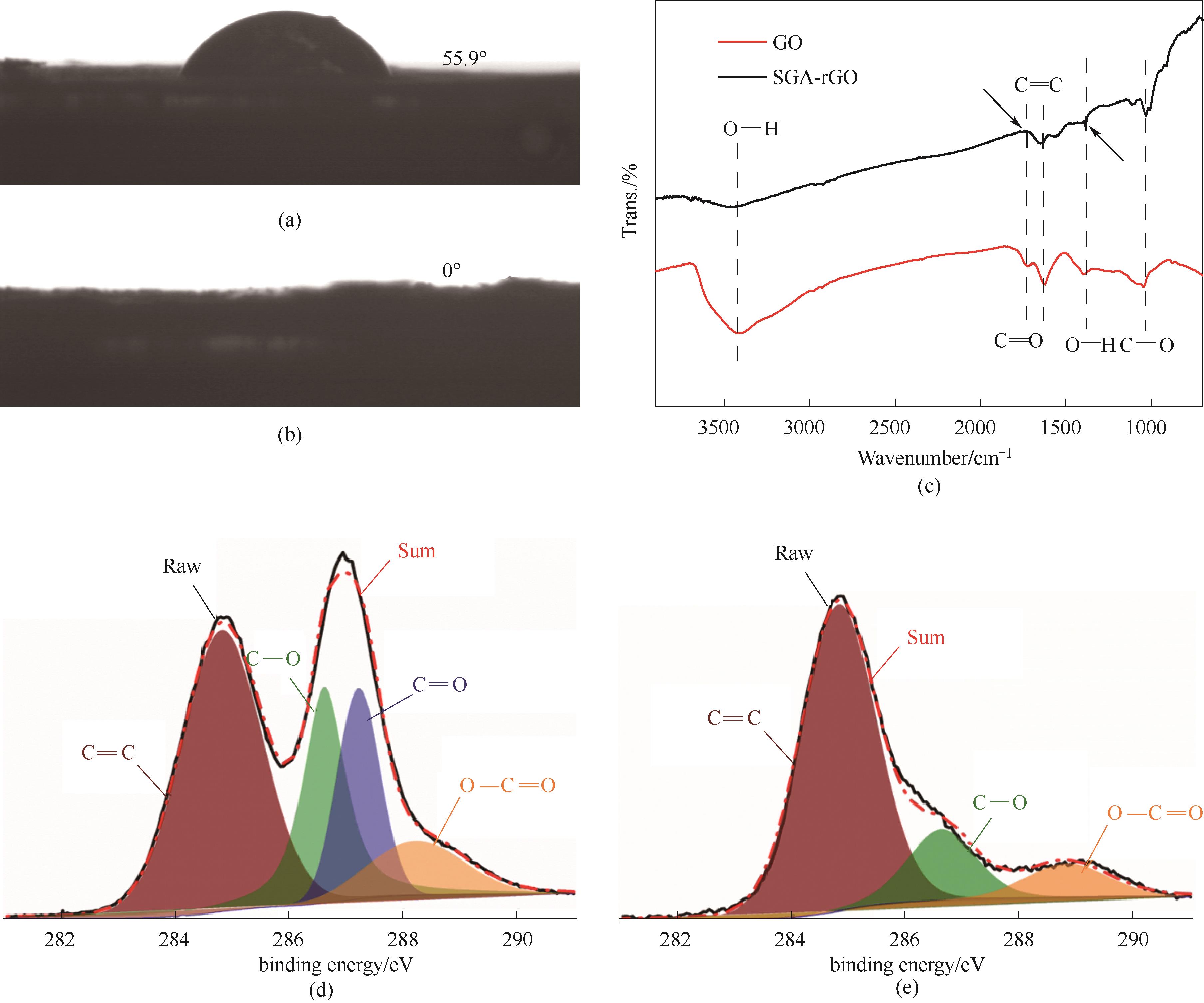
图4 0 s时SGA的接触角(a);9 s时SGA的接触角(b);GO和SGA-rGO的FTIR光谱图(c);GO(d)和SGA-rGO(e)的XPS C 1s光谱图
Fig.4 Contact angle of SGA at 0 s(a); Contact angle of SGA at 9 s (b); FTIR spectra (c) and XPS C 1s spectra[(d),(e)] of GO and SGA-rGO
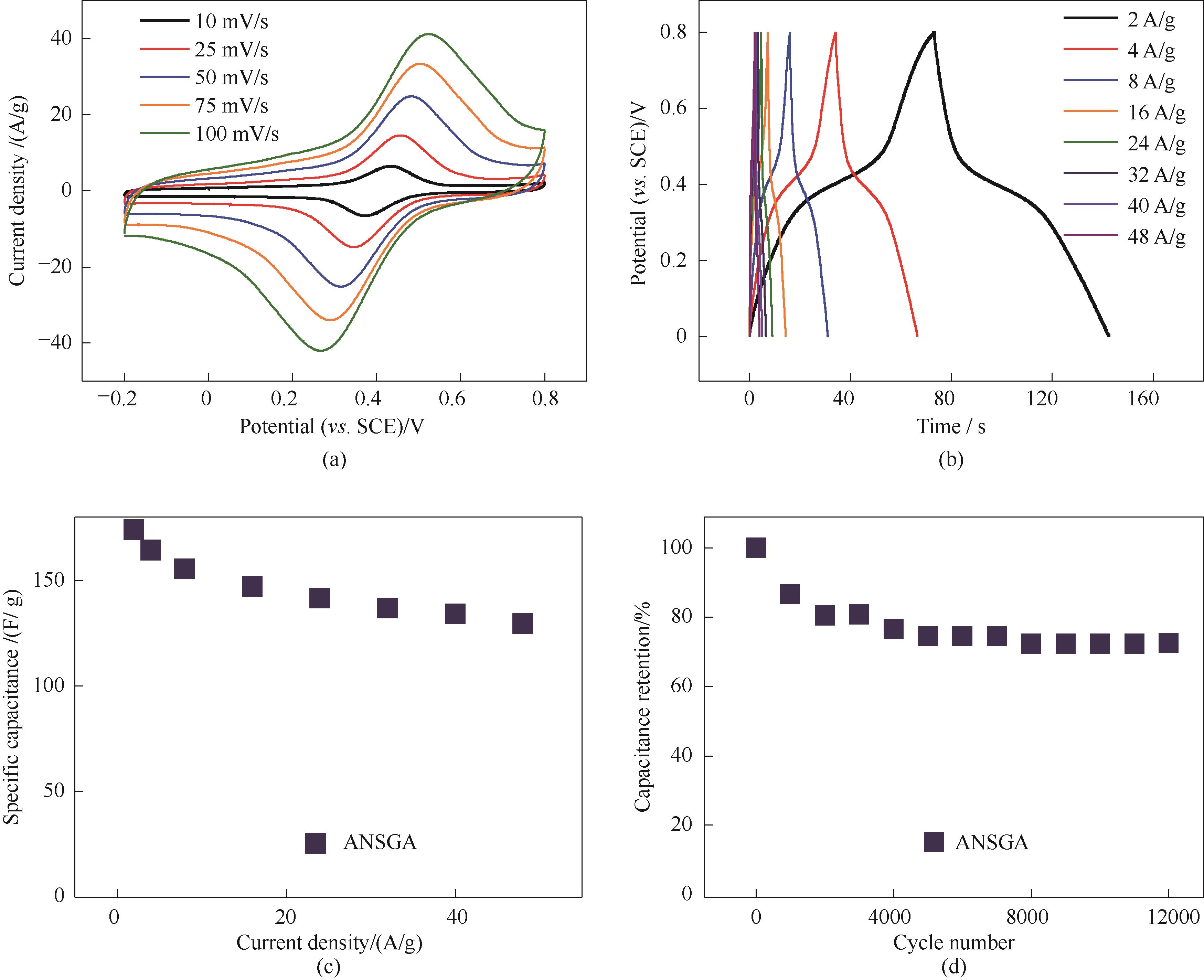
图7 基于退火后的SGH的电化学性能:循环伏安测试(a);不同电流密度下恒流充放电测试(b);不同电流密度下的比电容(c);21 A/g下的循环稳定性测试(d)
Fig.7 Electrochemical performance of ANSGA: CV curves (a), GCD curves (b), specific capacitance at different current densities (c), and cyclic stability test at a current density of 21 A/g (d)
| 主要材料 | 比电容/(F/g) | 电容保留率/% | 掺氮量/%(质量) | 文献 |
|---|---|---|---|---|
| GO/ammonia water | 233.3(0.5 A/g) | 64.3(20 A/g) | 4.4 | [ |
| GO/NH3 | 175(2 A/g) | 53.8(6 A/g) | 3.97 | [ |
| GO/uric acid | 196(2 A/g) | 79.1(3 A/g) | 5.63 | [ |
| GO/MnCo2O4/DMF | 95.1(2 A/g) | 65.2(4 A/g) | 4.14 | [ |
| GO/CuO/NH4NO3 | 197(5 A/g) | 57.9(5 A/g) | 4.54 | [ |
| GO/Fe3O4/Mn2O3/N2H4 | 90.58(0.5 A/g) | 80.7(2.5 A/g) | — | [ |
| GO/FeCl3/E.coli | 224(2 A/g) | 51.3(5 A/g) | 5.58 | [ |
| ANSGA(GO/activated sludge) | 174(2 A/g) | 74.7(48 A/g) | 5.61 | 本文 |
表1 石墨烯基碳材料的比电容、电容保留率及掺氮量的对比
Table 1 Comparison of specific capapcitance, capacitance retention and N-doped content of graphene-based carbon material
| 主要材料 | 比电容/(F/g) | 电容保留率/% | 掺氮量/%(质量) | 文献 |
|---|---|---|---|---|
| GO/ammonia water | 233.3(0.5 A/g) | 64.3(20 A/g) | 4.4 | [ |
| GO/NH3 | 175(2 A/g) | 53.8(6 A/g) | 3.97 | [ |
| GO/uric acid | 196(2 A/g) | 79.1(3 A/g) | 5.63 | [ |
| GO/MnCo2O4/DMF | 95.1(2 A/g) | 65.2(4 A/g) | 4.14 | [ |
| GO/CuO/NH4NO3 | 197(5 A/g) | 57.9(5 A/g) | 4.54 | [ |
| GO/Fe3O4/Mn2O3/N2H4 | 90.58(0.5 A/g) | 80.7(2.5 A/g) | — | [ |
| GO/FeCl3/E.coli | 224(2 A/g) | 51.3(5 A/g) | 5.58 | [ |
| ANSGA(GO/activated sludge) | 174(2 A/g) | 74.7(48 A/g) | 5.61 | 本文 |
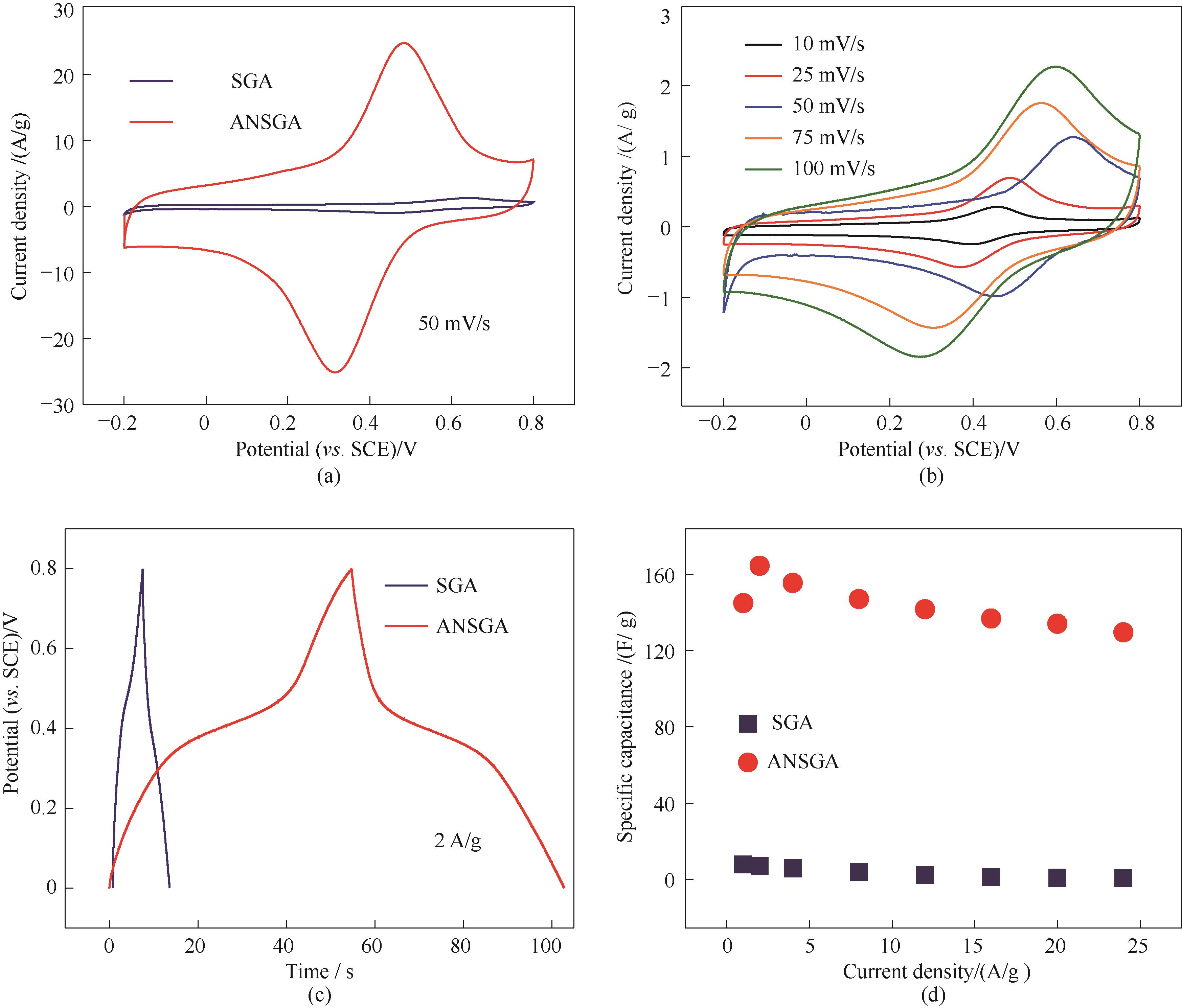
图8 基于SGA及ANSGA的电化学性能:50 mV/s下循环伏安曲线(a); SGA的循环伏安测试(b); 2 A/g下恒流充放电曲线(c);不同电流密度下的比电容(d)
Fig.8 Electrochemical performance of SGA and ANSGA: CV curves at a scan rate of 50 mV/s (a), CV curves of SGA (b), GCD curves at a current density of 2 A/g (c), and specific capacitance at different current densities (d)
| 1 | Yuan S J, Dai X H. Sewage sludge-based functional nanomaterials: development and applications[J]. Environmental Science: Nano, 2017, 4(1): 17-26. |
| 2 | Feng H B, Zheng M T, Dong H W, et al. Three-dimensional honeycomb-like hierarchically structured carbon for high-performance supercapacitors derived from high-ash-content sewage sludge[J]. Journal of Materials Chemistry A, 2015, 3(29): 15225-15234. |
| 3 | Lee C, Wei X, Kysar J W, et al. Measurement of the elastic properties and intrinsic strength of monolayer graphene[J]. Science, 2008, 321(5887): 385-388. |
| 4 | Zhang Y, Tan Y W, Stormer H L, et al. Experimental observation of the quantum Hall effect and Berry's phase in graphene[J]. Nature, 2005, 438(7065): 201-204. |
| 5 | Chae H K, Siberio-Pérez D Y, Kim J, et al. A route to high surface area, porosity and inclusion of large molecules in crystals[J]. Nature, 2004, 427(6974): 523-527. |
| 6 | Ma Y F, Chen Y S. Three-dimensional graphene networks: synthesis, properties and applications[J]. National Science Review, 2015, 2(1): 40-53. |
| 7 | Wang Y, Wei Z D, Nie Y, et al. Generation of three dimensional pore-controlled nitrogen-doped graphene hydrogels for high-performance supercapacitors by employing formamide as the modulator[J]. Journal of Materials Chemistry A, 2017, 5(4): 1442-1445. |
| 8 | Ruiz O N, Fernando K A, Wang B, et al. Graphene oxide: a nonspecific enhancer of cellular growth[J]. ACS Nano, 2011, 5(10): 8100-8107. |
| 9 | Chen J, Deng F, Hu Y Y, et al. Antibacterial activity of graphene-modified anode on Shewanella oneidensis MR-1 biofilm in microbial fuel cell[J]. Journal of Power Sources, 2015, 290: 80-86. |
| 10 | Yoshida N, Miyata Y, Mugita A, et al. Electricity recovery from municipal sewage wastewater using a hydrogel complex composed of microbially reduced graphene oxide and sludge[J]. Materials, 2016, 9(9): E742. |
| 11 | Xu W H, Jin Z H, Pang X, et al. Interaction between biocompatible graphene oxide and live shewanella in the self-assembled hydrogel: the role of physicochemical properties[J]. ACS Applied Bio Materials, 2020, 3(7): 4263-4272. |
| 12 | 周群英, 王士芬. 环境工程微生物学[M]. 3版. 北京: 高等教育出版社, 2018. |
| Zhou Q Y, Wang S F. Microbiology of Environmental Engineering[M]. 3rd ed. Beijing: Higher Education Press, 2018. | |
| 13 | Shen L, Hu H Y, Ji H F, et al. Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from excess activated sludge as a promising substitute of pure culture[J]. Bioresource Technology, 2015, 189: 236-242. |
| 14 | Shen L, Yuan X, Shen W H, et al. Positive impact of biofilm on reducing the permeation of ampicillin through membrane for membrane bioreactor[J]. Chemosphere, 2014, 97: 34-39. |
| 15 | Shen L, Jin Z H, Wang D, et al. Enhance wastewater biological treatment through the bacteria induced graphene oxide hydrogel[J]. Chemosphere, 2018, 190: 201-210. |
| 16 | Shen L, Jin Z H, Xu W H, et al. Enhanced treatment of anionic and cationic dyes in wastewater through live bacteria encapsulation using graphene hydrogel[J]. Industrial & Engineering Chemistry Research, 2019, 58(19): 7817-7824. |
| 17 | Salas E C, Sun Z, Lüttge A, et al. Reduction of graphene oxide via bacterial respiration[J]. ACS Nano, 2010, 4(8): 4852-4856. |
| 18 | Dreyer D R, Park S, Bielawski C W, et al. The chemistry of graphene oxide[J]. Chemical Society Reviews, 2010, 39(1): 228-240. |
| 19 | Wang D, Jin Z H, Pang X, et al. Fabrication and functionalization of biological graphene aerogel by reusing microorganism in activated sludge and ionic dyes[J]. Chemical Engineering Journal, 2020, 392: 124823. |
| 20 | Wei Y, Hu X Y, Jiang Q R, et al. Influence of graphene oxide with different oxidation levels on the properties of epoxy composites [J]. Composites Science and Technology, 2018, 161: 74-84. |
| 21 | Sui Z Y, Meng Q H, Li J T, et al. High surface area porous carbons produced by steam activation of graphene aerogels[J]. Journal of Materials Chemistry A, 2014, 2(25): 9891-9898. |
| 22 | Chen J, Zhang Y, Zhang M, et al. Water-enhanced oxidation of graphite to graphene oxide with controlled species of oxygenated groups[J]. Chemical Science, 2016, 7(3): 1874-1881. |
| 23 | Zhao J, Li Y J, Wang G L, et al. Enabling high-volumetric-energy-density supercapacitors: designing open, low-tortuosity heteroatom-doped porous carbon-tube bundle electrodes[J]. Journal of Materials Chemistry A, 2017, 5(44): 23085-23093. |
| 24 | Wu J F, Zhang Q N, Wang J J, et al. A self-assembly route to porous polyaniline/reduced graphene oxide composite materials with molecular-level uniformity for high-performance supercapacitors[J]. Energy & Environmental Science, 2018, 11(5): 1280-1286. |
| 25 | Smith K M, Fowler G D, Pullket S, et al. Sewage sludge-based adsorbents: a review of their production, properties and use in water treatment applications[J]. Water Research, 2009, 43(10): 2569-2594. |
| 26 | Zhou Q Y, Jiang X, Li X, et al. Preparation of high-yield N-doped biochar from nitrogen-containing phosphate and its effective adsorption for toluene[J]. RSC Advances, 2018, 8(53): 30171-30179. |
| 27 | 魏风, 毕宏晖, 焦帅, 等. 超级电容器用相互连接的类石墨烯纳米片[J]. 物理化学学报, 2020, 36(2): 142-149. |
| Wei F, Bi H H, Jiao S, et al. Interconnected graphene-like nanosheets for supercapacitors[J]. Acta Physico-Chimica Sinica, 2020, 36(2): 142-149. | |
| 28 | Wei F, Zhang H F, He X J, et al. Synthesis of porous carbons from coal tar pitch for high-performance supercapacitors[J]. New Carbon Materials, 2019, 34(2): 132-139. |
| 29 | Tajik S, Dubal D P, Gomez-Romero P, et al. Nanostructured mixed transition metal oxides for high performance asymmetric supercapacitors: facile synthetic strategy[J]. International Journal of Hydrogen Energy, 2017, 42(17): 12384-12395. |
| 30 | Gao F, Qin S H, Zang Y H, et al. Highly efficient formation of Mn3O4-graphene oxide hybrid aerogels for use as the cathode material of high performance lithium ion batteries[J]. New Carbon Materials, 2020, 35(2): 121-130. |
| 31 | Du X S, Zhou C F, Liu H Y, et al. Facile chemical synthesis of nitrogen-doped graphene sheets and their electrochemical capacitance[J]. Journal of Power Sources, 2013, 241: 460-466. |
| 32 | Li D L, Yu C Z, Wang M S, et al. Synthesis of nitrogen doped graphene from graphene oxide within an ammonia flame for high performance supercapacitors[J]. RSC Advances, 2014, 4(98): 55394-55399. |
| 33 | Wang X Q, Ding Y J, Chen F, et al. Hierarchical porous N-doped graphene monoliths for flexible solid-state supercapacitors with excellent cycle stability[J]. ACS Applied Energy Materials, 2018, 1(9): 5024-5032. |
| 34 | Pettong T, Iamprasertkun P, Krittayavathananon A, et al. High-performance asymmetric supercapacitors of MnCo2O4 nanofibers and N-doped reduced graphene oxide aerogel[J]. ACS Applied Materials & Interfaces, 2016, 8(49): 34045-34053. |
| 35 | Li Y J, Ye K, Cheng K, et al. Anchoring CuO nanoparticles on nitrogen-doped reduced graphene oxide nanosheets as electrode material for supercapacitors[J]. Journal of Electroanalytical Chemistry, 2014, 727: 154-162. |
| 36 | Chong B, Azman N, Mohd Abdah M, et al. Supercapacitive performance of N-doped graphene/Mn3O4/Fe3O4 as an electrode material[J]. Applied Sciences, 2019, 9(6): 1040. |
| 37 | Sun H M, Cao L Y, Lu L H. Bacteria promoted hierarchical carbon materials for high-performance supercapacitor[J]. Energy & Environmental Science, 2012, 5(3): 6206-6213. |
| 38 | Wang Z C, Wang Y, Shu X, et al. Hierarchical three-dimensional MnO2/carbon@TiO2 nanotube arrays for high-performance supercapacitors[J]. RSC Advances, 2016, 6(68): 63642-63651. |
| [1] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [2] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [3] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [4] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [5] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [6] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [7] | 张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| [8] | 郭旭, 张永政, 夏厚兵, 杨娜, 朱真珍, 齐晶瑶. 碳基材料电氧化去除水体污染物的研究进展[J]. 化工学报, 2023, 74(5): 1862-1874. |
| [9] | 顾浩, 张福建, 刘珍, 周文轩, 张鹏, 张忠强. 力电耦合作用下多孔石墨烯膜时间维度的脱盐性能及机理研究[J]. 化工学报, 2023, 74(5): 2067-2074. |
| [10] | 张正, 何永平, 孙海东, 张荣子, 孙正平, 陈金兰, 郑一璇, 杜晓, 郝晓刚. 蛇形流场电控离子交换装置用于选择性提锂[J]. 化工学报, 2023, 74(5): 2022-2033. |
| [11] | 李瑞康, 何盈盈, 卢维鹏, 王园园, 丁皓东, 骆勇名. 电化学强化钴基阴极活化过一硫酸盐的研究[J]. 化工学报, 2023, 74(5): 2207-2216. |
| [12] | 王承泽, 顾凯丽, 张晋华, 石建轩, 刘艺娓, 李锦祥. 硫化协同老化零价铁增效去除水中Cr(Ⅵ)的作用机制[J]. 化工学报, 2023, 74(5): 2197-2206. |
| [13] | 刘瑞琪, 周栖桐, 张悦, 贺莹, 高静, 马丽. 基于金纳米颗粒修饰二氧化硅纳米花的生物传感器构建及应用[J]. 化工学报, 2023, 74(3): 1247-1259. |
| [14] | 杜江龙, 杨雯棋, 黄凯, 练成, 刘洪来. 复合相变材料/空冷复合式锂离子电池模块散热性能[J]. 化工学报, 2023, 74(2): 674-689. |
| [15] | 程伟江, 汪何琦, 高翔, 李娜, 马赛男. 锂离子电池硅基负极电解液成膜添加剂的研究进展[J]. 化工学报, 2023, 74(2): 571-584. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号