化工学报 ›› 2021, Vol. 72 ›› Issue (11): 5738-5750.DOI: 10.11949/0438-1157.20210668
收稿日期:2021-05-17
修回日期:2021-07-30
出版日期:2021-11-05
发布日期:2021-11-12
通讯作者:
李文翠
作者简介:王博阳(1996—),男,硕士研究生,基金资助:
Boyang WANG( ),Jili XIA,Xiaoling DONG,Hang GUO,Wencui LI(
),Jili XIA,Xiaoling DONG,Hang GUO,Wencui LI( )
)
Received:2021-05-17
Revised:2021-07-30
Online:2021-11-05
Published:2021-11-12
Contact:
Wencui LI
摘要:
煤具有碳含量高、芳香结构发达、成本低廉等优点,是制备钠离子电池硬炭负极材料的优质前驱体。然而煤种类繁多且含有无机杂质,不同种煤热解成炭后材料的石墨化度、碳层间距和表面化学组成各异,导致煤基硬炭负极的电化学性能优化难以展开。选择四种不同变质程度的煤,采用酸洗脱灰、高温炭化的方法制备了系列煤基硬炭,研究了变质程度、炭化温度对煤基硬炭微晶结构和表面杂原子组成的影响,并考察了其相应的储钠行为。其中,褐煤1400℃炭化得到的硬炭性能最佳,在0.02 A·g-1电流密度下表现出338.8 mA·h·g-1的比容量和81.1%的首次库仑效率。优异的电化学性能归因于褐煤硬炭较大的碳层间距和丰富的储钠缺陷位点,提供了高嵌入和吸附储钠容量。
中图分类号:
王博阳, 夏吉利, 董晓玲, 郭行, 李文翠. 不同变质程度煤衍生硬炭的储钠行为研究[J]. 化工学报, 2021, 72(11): 5738-5750.
Boyang WANG, Jili XIA, Xiaoling DONG, Hang GUO, Wencui LI. Study on sodium storage behavior of hard carbons derived from coal with different grades of metamorphism[J]. CIESC Journal, 2021, 72(11): 5738-5750.
| 煤种 | 原煤工业分析/%(质量) | 脱灰样工业分析/%(质量) | 脱灰样元素分析/%(质量) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | Mad | Ad | Vdaf | C | N | H | S | O | |
| 无烟煤(WYM) | 0.11 | 10.37 | 10.23 | 1.50 | 1.00 | 9.70 | 88.91 | 0.42 | 4.04 | 0.66 | 5.97 |
| 烟煤(YM) | 2.32 | 3.78 | 30.72 | 3.20 | 0.41 | 28.01 | 76.01 | 0.83 | 4.62 | 0.60 | 17.90 |
| 次烟煤(CYM) | 5.21 | 4.80 | 47.51 | 4.56 | 0.31 | 42.62 | 71.64 | 0.85 | 6.01 | 0.42 | 21.08 |
| 褐煤(HM) | 8.45 | 15.27 | 49.96 | 6.71 | 0.75 | 44.01 | 67.83 | 1.33 | 5.10 | 1.10 | 24.55 |
表1 不同煤种煤脱灰前后的工业分析与脱灰后的元素分析结果
Table 1 Industrial analysis before and after deashing of different coals and elemental analysis results of coals after deashing
| 煤种 | 原煤工业分析/%(质量) | 脱灰样工业分析/%(质量) | 脱灰样元素分析/%(质量) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | Mad | Ad | Vdaf | C | N | H | S | O | |
| 无烟煤(WYM) | 0.11 | 10.37 | 10.23 | 1.50 | 1.00 | 9.70 | 88.91 | 0.42 | 4.04 | 0.66 | 5.97 |
| 烟煤(YM) | 2.32 | 3.78 | 30.72 | 3.20 | 0.41 | 28.01 | 76.01 | 0.83 | 4.62 | 0.60 | 17.90 |
| 次烟煤(CYM) | 5.21 | 4.80 | 47.51 | 4.56 | 0.31 | 42.62 | 71.64 | 0.85 | 6.01 | 0.42 | 21.08 |
| 褐煤(HM) | 8.45 | 15.27 | 49.96 | 6.71 | 0.75 | 44.01 | 67.83 | 1.33 | 5.10 | 1.10 | 24.55 |
| 组分 | 原煤XRF分析/%(质量) | 脱灰样XRF分析/%(质量) | ||||||
|---|---|---|---|---|---|---|---|---|
| WYM | YM | CYM | HM | WYM | YM | CYM | HM | |
| SiO2 | 4.240 | 0.320 | 0.360 | 7.560 | 0.180 | 0.093 | 0.000 | 0.011 |
| Al2O3 | 3.790 | 0.300 | 0.410 | 2.440 | 0.680 | 0.130 | 0.000 | 0.096 |
| Fe2O3 | 0.410 | 1.210 | 0.785 | 0.779 | 0.040 | 0.166 | 0.561 | 0.221 |
| CaO | 0.520 | 2.690 | 2.150 | 0.973 | 0.060 | 0.050 | 0.110 | 0.010 |
| MgO | 0.000 | 0.360 | 0.000 | 0.240 | 0.000 | 0.000 | 0.000 | 0.000 |
| Cr2O3 | 0.024 | 0.033 | 0.000 | 0.033 | 0.004 | 0.000 | 0.000 | 0.000 |
| Na2O | 0.110 | 0.000 | 0.000 | 0.280 | 0.000 | 0.000 | 0.000 | 0.000 |
| CuO | 0.009 | 0.000 | 0.011 | 0.013 | 0.001 | 0.000 | 0.000 | 0.006 |
表2 原煤和脱灰煤的X射线荧光光谱分析
Table 2 X-Ray fluorescence spectrum analysis results for the raw coals and demineralized coals
| 组分 | 原煤XRF分析/%(质量) | 脱灰样XRF分析/%(质量) | ||||||
|---|---|---|---|---|---|---|---|---|
| WYM | YM | CYM | HM | WYM | YM | CYM | HM | |
| SiO2 | 4.240 | 0.320 | 0.360 | 7.560 | 0.180 | 0.093 | 0.000 | 0.011 |
| Al2O3 | 3.790 | 0.300 | 0.410 | 2.440 | 0.680 | 0.130 | 0.000 | 0.096 |
| Fe2O3 | 0.410 | 1.210 | 0.785 | 0.779 | 0.040 | 0.166 | 0.561 | 0.221 |
| CaO | 0.520 | 2.690 | 2.150 | 0.973 | 0.060 | 0.050 | 0.110 | 0.010 |
| MgO | 0.000 | 0.360 | 0.000 | 0.240 | 0.000 | 0.000 | 0.000 | 0.000 |
| Cr2O3 | 0.024 | 0.033 | 0.000 | 0.033 | 0.004 | 0.000 | 0.000 | 0.000 |
| Na2O | 0.110 | 0.000 | 0.000 | 0.280 | 0.000 | 0.000 | 0.000 | 0.000 |
| CuO | 0.009 | 0.000 | 0.011 | 0.013 | 0.001 | 0.000 | 0.000 | 0.006 |
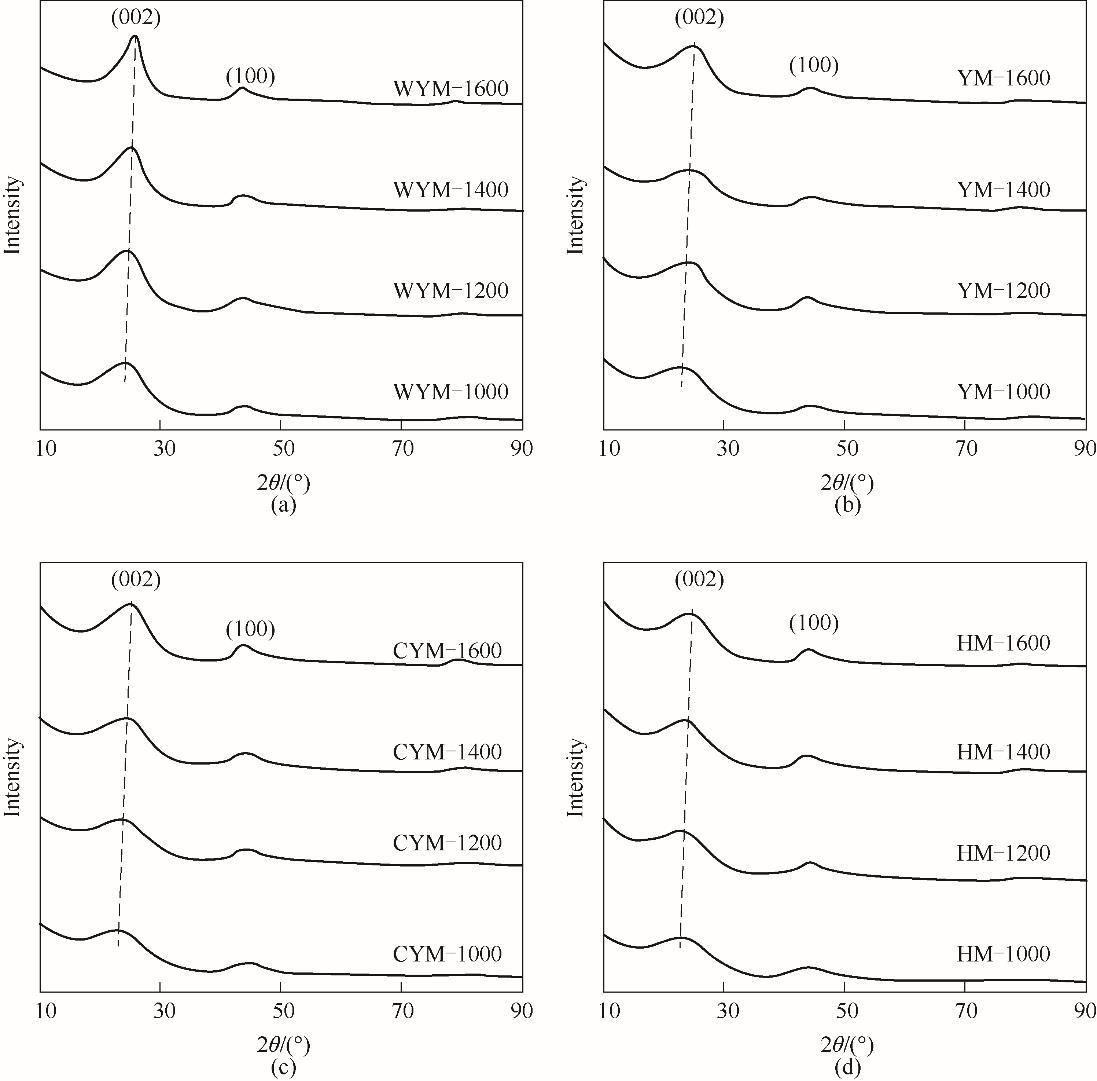
图3 不同炭化温度下制备的样品的XRD谱图(a) 无烟煤基硬炭;(b) 烟煤基硬炭;(c) 次烟煤基硬炭;(d) 褐煤基硬炭
Fig.3 XRD patterns of the samples prepared at different carbonization temperatures(a) WYM-based carbons; (b) YM-based carbons; (c) CYM-based carbons; (d) HM coal-based carbons
| Sample | d002/nm | Lc/nm | La/nm | AG/AD | SBET/(m2·g-1) |
|---|---|---|---|---|---|
| WYM-1000 | 0.364 | 2.43 | 1.86 | 0.36 | 3.66 |
| WYM-1200 | 0.362 | 2.59 | 1.94 | 0.40 | 8.06 |
| WYM-1400 | 0.358 | 3.10 | 2.03 | 0.48 | 3.00 |
| WYM-1600 | 0.349 | 4.03 | 2.43 | 0.51 | 4.32 |
| YM-1000 | 0.381 | 2.13 | 1.75 | 0.27 | 4.53 |
| YM-1200 | 0.374 | 2.32 | 1.83 | 0.29 | 3.95 |
| YM-1400 | 0.370 | 2.46 | 2.06 | 0.44 | 2.63 |
| YM-1600 | 0.364 | 2.60 | 2.13 | 0.49 | 1.96 |
| CYM-1000 | 0.380 | 2.13 | 1.77 | 0.26 | 5.53 |
| CYM-1200 | 0.377 | 2.25 | 1.80 | 0.30 | 9.94 |
| CYM-1400 | 0.372 | 2.32 | 2.09 | 0.38 | 2.28 |
| CYM-1600 | 0.365 | 2.56 | 2.18 | 0.44 | 2.20 |
| HM-1000 | 0.384 | 2.11 | 1.80 | 0.21 | 4.13 |
| HM-1200 | 0.381 | 2.20 | 1.82 | 0.24 | 4.24 |
| HM-1400 | 0.376 | 2.33 | 1.98 | 0.28 | 3.42 |
| HM-1600 | 0.368 | 2.46 | 2.07 | 0.40 | 4.42 |
表3 不同炭化温度下制备的煤基硬炭的结构参数
Table 3 Structural parameters of coal-based hard carbons prepared at different carbonization temperatures
| Sample | d002/nm | Lc/nm | La/nm | AG/AD | SBET/(m2·g-1) |
|---|---|---|---|---|---|
| WYM-1000 | 0.364 | 2.43 | 1.86 | 0.36 | 3.66 |
| WYM-1200 | 0.362 | 2.59 | 1.94 | 0.40 | 8.06 |
| WYM-1400 | 0.358 | 3.10 | 2.03 | 0.48 | 3.00 |
| WYM-1600 | 0.349 | 4.03 | 2.43 | 0.51 | 4.32 |
| YM-1000 | 0.381 | 2.13 | 1.75 | 0.27 | 4.53 |
| YM-1200 | 0.374 | 2.32 | 1.83 | 0.29 | 3.95 |
| YM-1400 | 0.370 | 2.46 | 2.06 | 0.44 | 2.63 |
| YM-1600 | 0.364 | 2.60 | 2.13 | 0.49 | 1.96 |
| CYM-1000 | 0.380 | 2.13 | 1.77 | 0.26 | 5.53 |
| CYM-1200 | 0.377 | 2.25 | 1.80 | 0.30 | 9.94 |
| CYM-1400 | 0.372 | 2.32 | 2.09 | 0.38 | 2.28 |
| CYM-1600 | 0.365 | 2.56 | 2.18 | 0.44 | 2.20 |
| HM-1000 | 0.384 | 2.11 | 1.80 | 0.21 | 4.13 |
| HM-1200 | 0.381 | 2.20 | 1.82 | 0.24 | 4.24 |
| HM-1400 | 0.376 | 2.33 | 1.98 | 0.28 | 3.42 |
| HM-1600 | 0.368 | 2.46 | 2.07 | 0.40 | 4.42 |
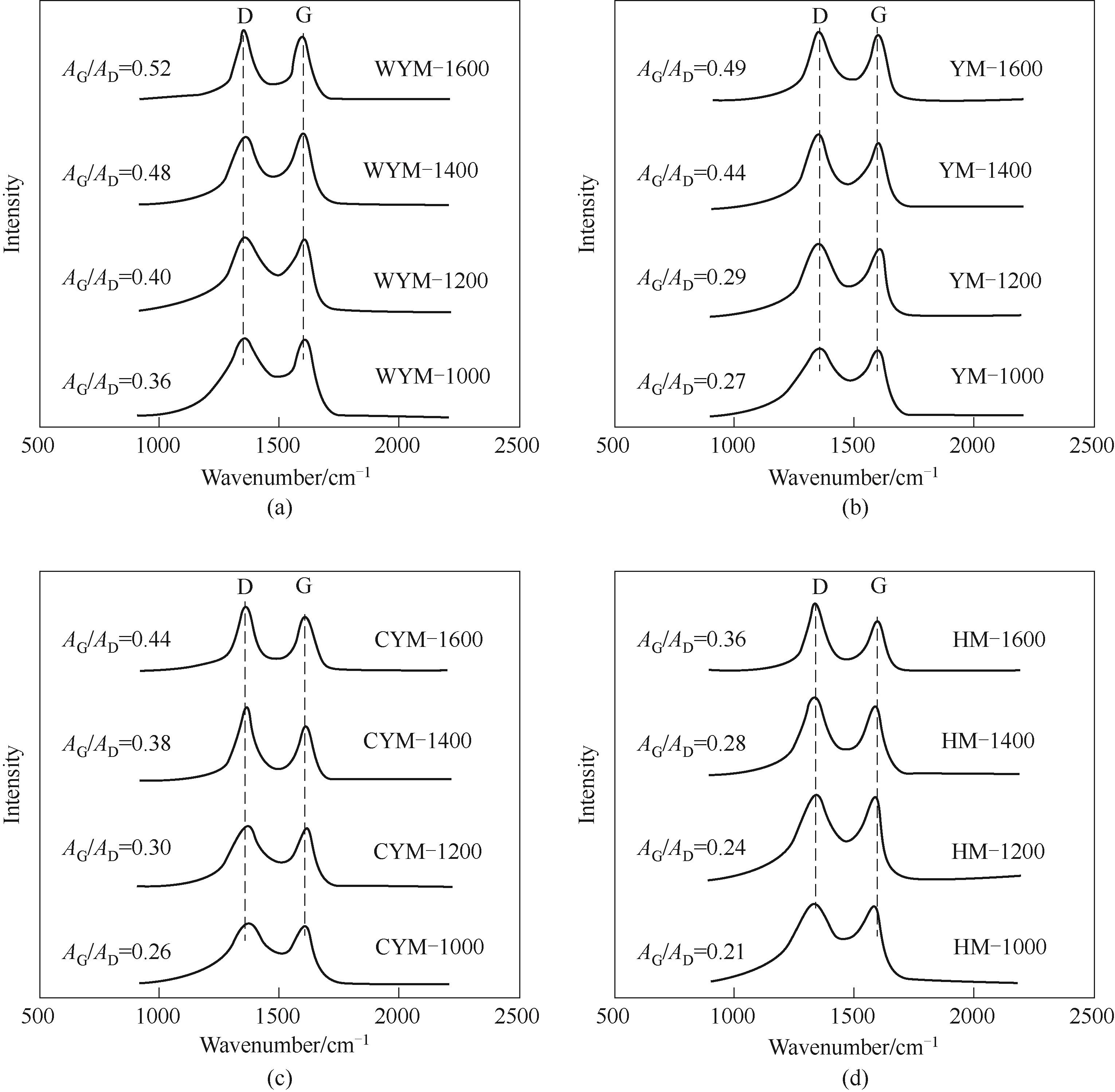
图4 不同炭化温度下得到的样品的拉曼光谱图(a) 无烟煤基硬炭; (b) 烟煤基硬炭; (c) 次烟煤基硬炭; (d) 褐煤基硬炭
Fig.4 Raman spectra of the samples prepared at different carbonization temperatures(a) WYM-based carbons; (b) YM-based carbons; (c) CYM-based carbons; (d) HM-based carbons
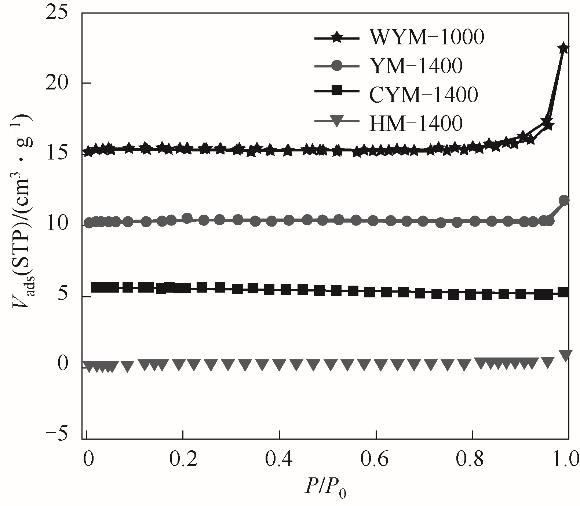
图5 样品WYM-1000、YM-1400、CYM-1400和HM-1400的氮吸附等温线(WYM-1000、YM-1400和CYM-1400的等温线分别垂直平移15、10和5 cm3·g-1)
Fig.5 Nitrogen sorption isotherms of WYM-1000, YM-1400, CYM-1400 and HM-1400(The isotherm of WYM-1000, YM-1400 and CYM-1400 are vertically offset by 15, 10 and 5 cm3·g-1, respectively)
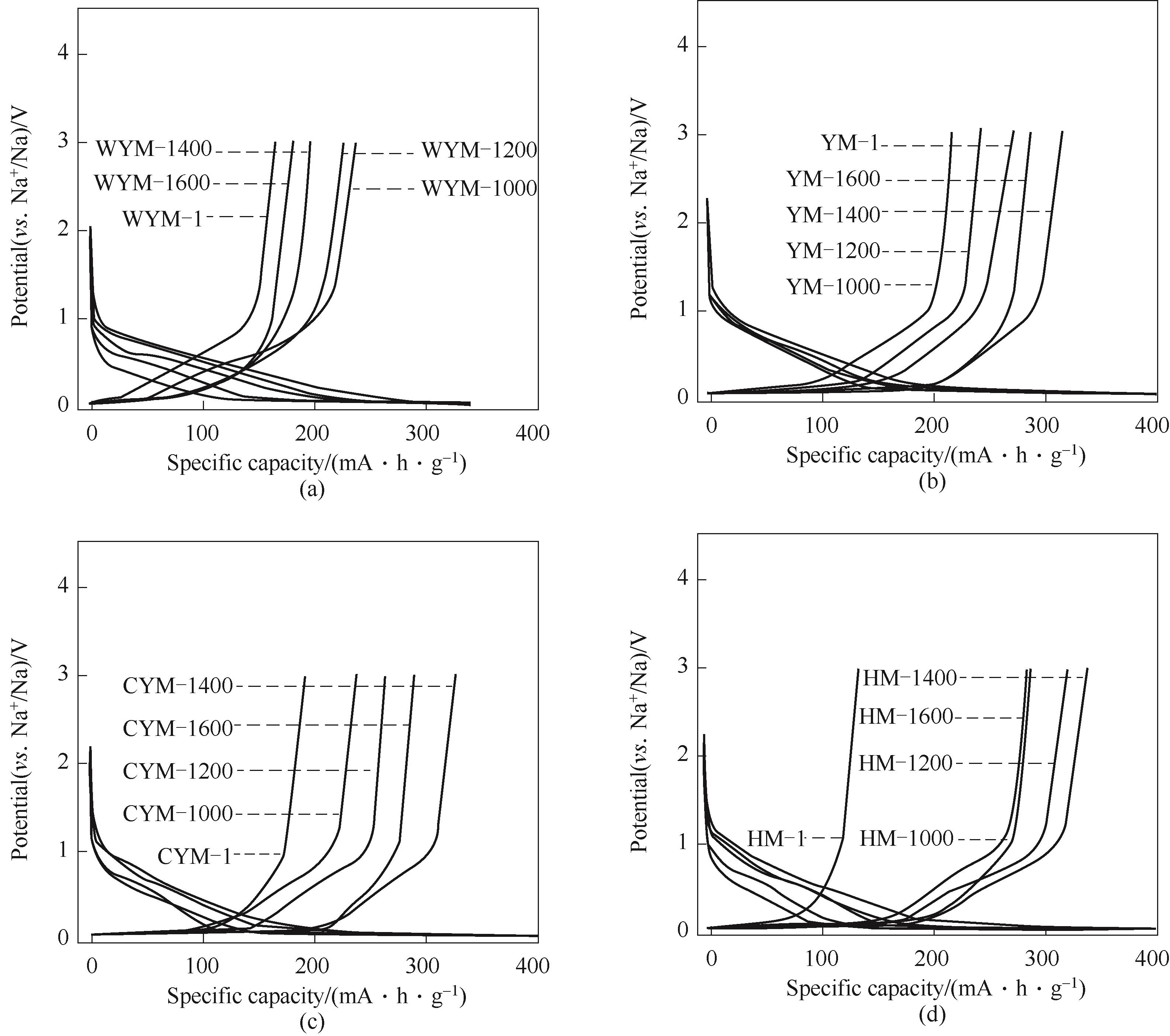
图7 各煤基硬炭作为钠电负极材料在0.02 A·g-1下的首次恒电流放电/充电曲线(a) 无烟煤基硬炭; (b) 烟煤基硬炭; (c) 次烟煤基硬炭; (d) 褐煤基硬炭
Fig.7 The first galvanostatic charge/discharge profiles at 0.02 A·g-1 of each coal-based carbons as anode materials of sodium-ion batteries(a) WYM-based carbons; (b) YM-based carbons; (c) CYM-based carbons; (d) HM coal-based carbons
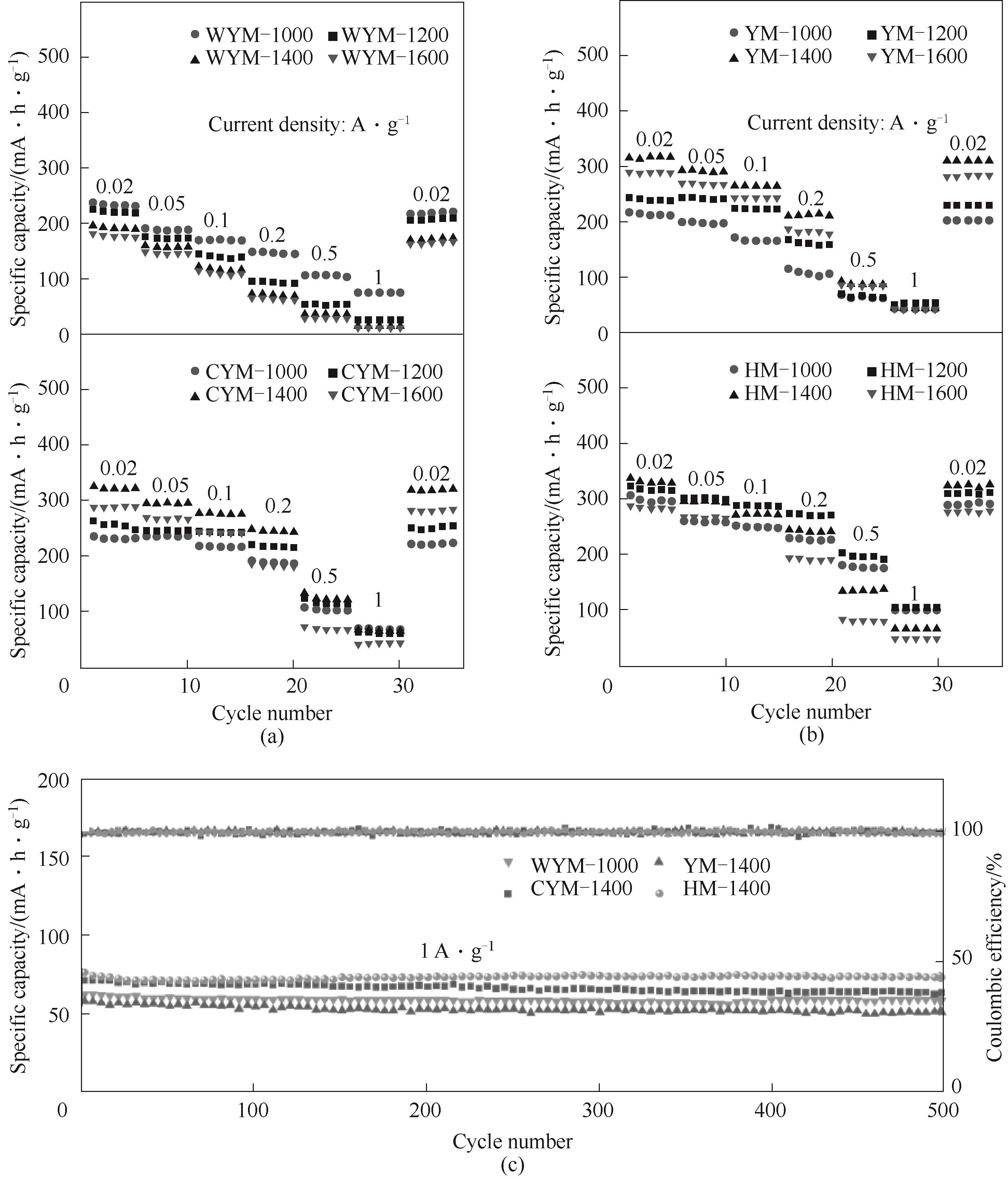
图8 无烟煤与次烟煤基硬炭(a)、烟煤与褐煤基硬炭(b)在0.02~1 A·g-1电流密度下的倍率性能;煤基硬炭在1 A·g-1电流密度下的循环性能(c)
Fig.8 Rate performance of the samples under the current density ranging from 0.02—1 A·g-1: (a) WYM and CYM-based hard carbons; (b) YM and HM-based hard carbons; (c) Cycle performance of the hard carbons at a current density of 1 A·g-1
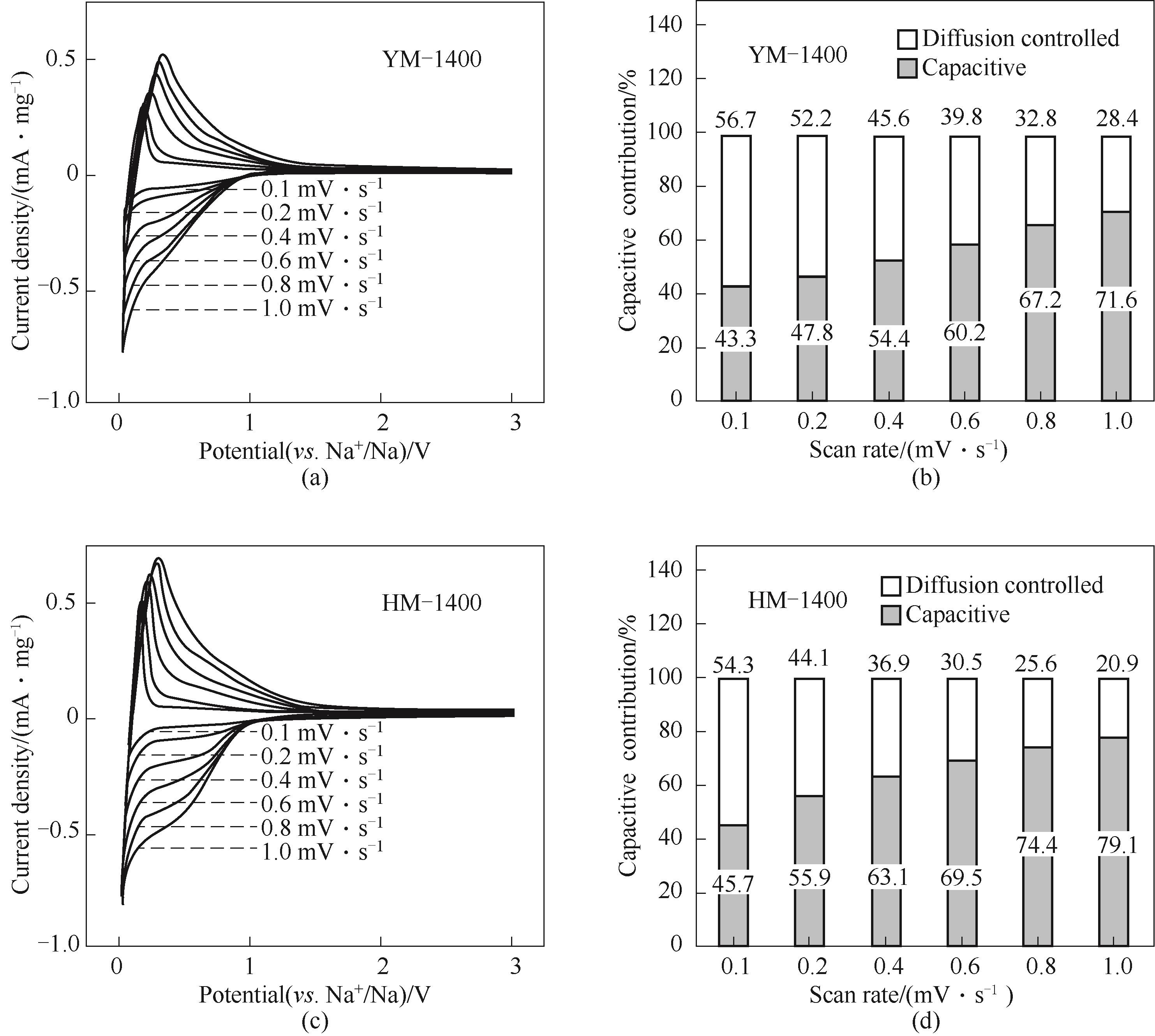
图9 YM-1400(a)和HM-1400(c)在0.1~1mV·s-1的不同扫速下的CV曲线;YM-1400(b)和HM-1400(d)在不同扫速下的归一化电容容量贡献占比
Fig.9 CV curves of YM-1400 (a) and HM-1400 (c) at different scan rates from 0.1—1 mV·s-1; Normalized contribution ratio of capacitive capacities of YM-1400 (b) and HM-1400 (d) at different scan rates
| 样品编号 | 元素分析/%(质量) | XPS分峰C 1s/% | XPS分峰O 1s/% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | C | H | O | sp2C | sp3C | C—O | —OH | C O O | |
| YM-1000 | 1.65 | 94.47 | 0.42 | 3.46 | 57.43 | 18.02 | 5.76 | 43.82 | 50.42 |
| YM-1200 | 1.06 | 96.23 | 0.21 | 2.5 | 64.04 | 18.20 | 5.17 | 45.60 | 49.23 |
| YM-1400 | 0.3 | 98.06 | 0.10 | 1.54 | 70.62 | 10.76 | 4.31 | 54.13 | 41.56 |
| YM-1600 | 0.08 | 99.09 | 0.06 | 0.77 | 72.01 | 11.91 | 1.08 | 58.71 | 40.21 |
| HM-1000 | 1.09 | 91.42 | 0.48 | 7.01 | 61.74 | 22.12 | 30.67 | 31.21 | 38.13 |
| HM-1200 | 0.45 | 95.69 | 0.22 | 3.64 | 66.05 | 17.57 | 14.38 | 40.06 | 45.57 |
| HM-1400 | 0.30 | 97.74 | 0.10 | 1.86 | 66.54 | 16.97 | 10.24 | 46.86 | 42.68 |
| HM-1600 | 0.08 | 98.63 | 0.08 | 1.21 | 68.37 | 13.96 | 0.43 | 64.85 | 34.73 |
表4 烟煤和褐煤基硬炭的元素分析与XPS分析结果
Table 4 Elemental and XPS analysis results of YM and HM coal-based hard carbons
| 样品编号 | 元素分析/%(质量) | XPS分峰C 1s/% | XPS分峰O 1s/% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | C | H | O | sp2C | sp3C | C—O | —OH | C O O | |
| YM-1000 | 1.65 | 94.47 | 0.42 | 3.46 | 57.43 | 18.02 | 5.76 | 43.82 | 50.42 |
| YM-1200 | 1.06 | 96.23 | 0.21 | 2.5 | 64.04 | 18.20 | 5.17 | 45.60 | 49.23 |
| YM-1400 | 0.3 | 98.06 | 0.10 | 1.54 | 70.62 | 10.76 | 4.31 | 54.13 | 41.56 |
| YM-1600 | 0.08 | 99.09 | 0.06 | 0.77 | 72.01 | 11.91 | 1.08 | 58.71 | 40.21 |
| HM-1000 | 1.09 | 91.42 | 0.48 | 7.01 | 61.74 | 22.12 | 30.67 | 31.21 | 38.13 |
| HM-1200 | 0.45 | 95.69 | 0.22 | 3.64 | 66.05 | 17.57 | 14.38 | 40.06 | 45.57 |
| HM-1400 | 0.30 | 97.74 | 0.10 | 1.86 | 66.54 | 16.97 | 10.24 | 46.86 | 42.68 |
| HM-1600 | 0.08 | 98.63 | 0.08 | 1.21 | 68.37 | 13.96 | 0.43 | 64.85 | 34.73 |
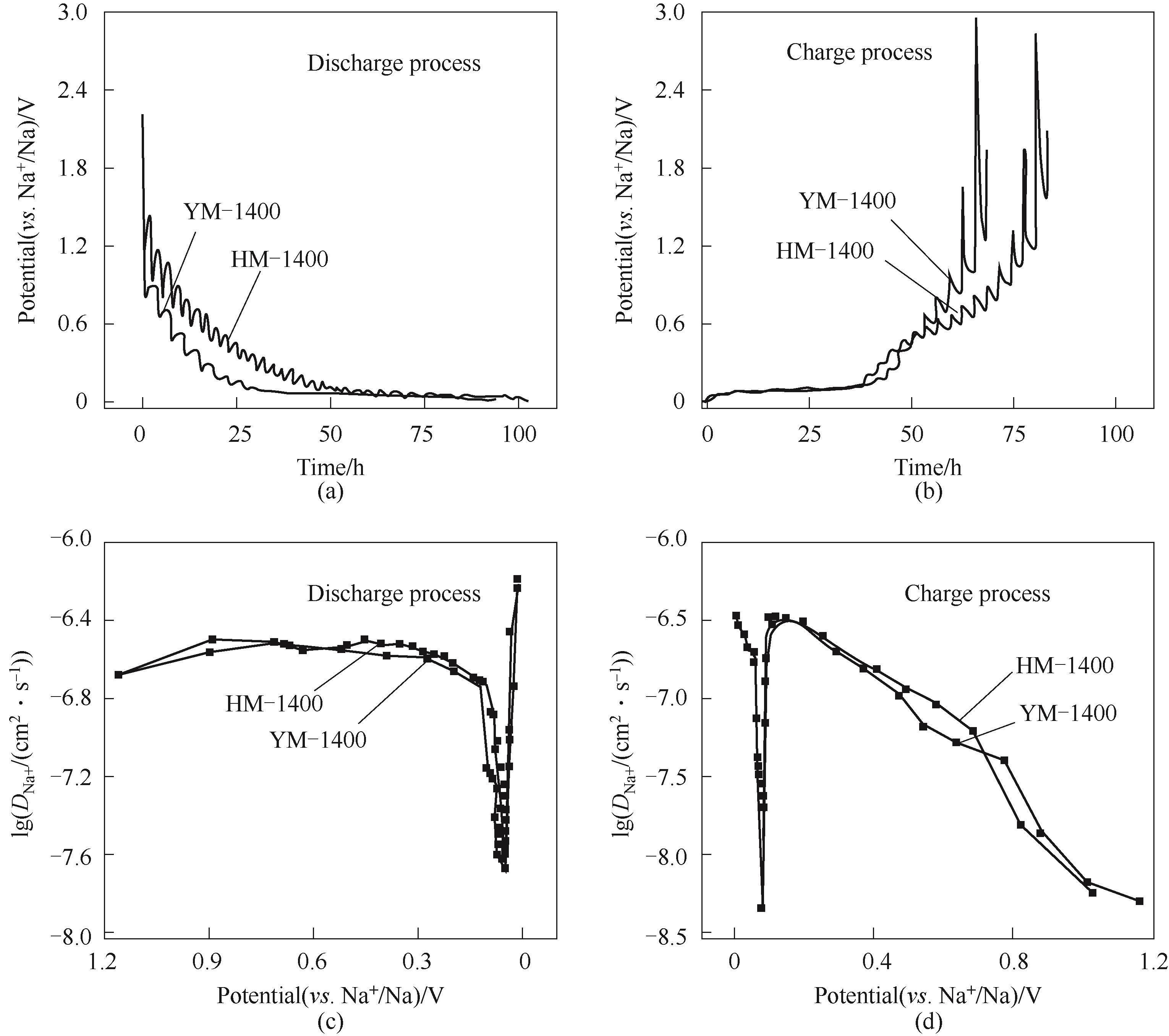
图11 YM-1400和HM-1400在充放电过程中的GITT曲线[(a), (b)]; 根据 YM-1400和HM-1400 在放电和充电过程中的GITT电势分布计算出的钠离子扩散系数[(c), (d)]
Fig.11 GITT measurements of YM-1400和HM-1400[(a), (b)]; Sodium-ion apparent diffusion coefficients calculated from the GITT potential profiles of the HM-1400 and YM-1400 electrodes during discharge and charge process[(c), (d)]
| 1 | Yabuuchi N, Kubota K, Dahbi M, et al. Research development on sodium-ion batteries[J]. Chemical Reviews, 2014, 114(23): 11636-11682. |
| 2 | Zhao C L, Wang Q D, Yao Z P, et al. Rational design of layered oxide materials for sodium-ion batteries[J]. Science, 2020, 370(6517): 708-711. |
| 3 | Pu X J, Wang H M, Zhao D, et al. Recent progress in rechargeable sodium-ion batteries: toward high-power applications[J]. Small, 2019, 15(32): 1805427. |
| 4 | He Y Z, Xu P, Zhang B, et al. Ultrasmall MnO nanoparticles supported on nitrogen-doped carbon nanotubes as efficient anode materials for sodium ion batteries[J]. ACS Applied Materials & Interfaces, 2017, 9(44): 38401-38408. |
| 5 | Li Z, Zhang C Z, Han F, et al. Improving the cycle stability of FeCl3-graphite intercalation compounds by polar Fe2O3 trapping in lithium-ion batteries[J]. Nano Research, 2019, 12(8): 1836-1844. |
| 6 | Li Z, Zhang C Z, Han F, et al. Towards high-volumetric performance of Na/Li-ion batteries: a better anode material with molybdenum pentachloride-graphite intercalation compounds (MoCl5-GICs)[J]. Journal of Materials Chemistry A, 2020, 8(5): 2430-2438. |
| 7 | Deng W W, Qian J F, Cao Y L, et al. Graphene-wrapped Na2C12H6O4 nanoflowers as high performance anodes for sodium-ion batteries[J]. Small, 2016, 12(5): 583-587. |
| 8 | Fu S D, Ni J F, Xu Y, et al. Hydrogenation driven conductive Na2Ti3O7 nanoarrays as robust binder-free anodes for sodium-ion batteries[J]. Nano Letters, 2016, 16(7): 4544-4551. |
| 9 | Sun N, Guan Z R X, Liu Y W, et al. Extended “adsorption-insertion” model: a new insight into the sodium storage mechanism of hard carbons[J]. Advanced Energy Materials, 2019, 9(32): 1901351. |
| 10 | Qu W H, Guo Y B, Shen W Z, et al. Using asphaltene supermolecules derived from coal for the preparation of efficient carbon electrodes for supercapacitors[J]. The Journal of Physical Chemistry C, 2016, 120(28): 15105-15113. |
| 11 | Qi Y R, Lu Y X, Liu L L, et al. Retarding graphitization of soft carbon precursor: from fusion-state to solid-state carbonization[J]. Energy Storage Materials, 2020, 26: 577-584. |
| 12 | 陆倩, 徐园园, 木沙江, 等. 不粘煤基活性炭作超级电容器电极材料: 硼、氮掺杂对其电化学性能的影响[J]. 新型炭材料, 2017, 32(5): 442-450. |
| Lu Q, Xu Y Y, Mu S J, et al. The effect of nitrogen and/or boron doping on the electrochemical performance of non-caking coal-derived activated carbons for use as supercapacitor electrodes[J]. New Carbon Materials, 2017, 32(5): 442-450. | |
| 13 | Li Y M, Hu Y S, Qi X G, et al. Advanced sodium-ion batteries using superior low cost pyrolyzed anthracite anode: towards practical applications[J]. Energy Storage Materials, 2016, 5: 191-197. |
| 14 | Lu H Y, Sun S F, Xiao L F, et al. High-capacity hard carbon pyrolyzed from subbituminous coal as anode for sodium-ion batteries[J]. ACS Applied Energy Materials, 2019, 2(1): 729-735. |
| 15 | Liu X F, Song D Z, He X Q, et al. Insight into the macromolecular structural differences between hard coal and deformed soft coal[J]. Fuel, 2019, 245: 188-197. |
| 16 | Xie X, Zhao Y, Qiu P H, et al. Investigation of the relationship between infrared structure and pyrolysis reactivity of coals with different ranks[J]. Fuel, 2018, 216: 521-530. |
| 17 | Steel K M, Patrick J W. The production of ultra clean coal by sequential leaching with HF followed by HNO3[J]. Fuel, 2003, 82(15/16/17): 1917-1920. |
| 18 | Mathews J P, Chaffee A L. The molecular representations of coal—a review[J]. Fuel, 2012, 96: 1-14. |
| 19 | Wang B Y, Xia J L, Dong X L, et al. Highly purified carbon derived from deashed anthracite for sodium-ion storage with enhanced capacity and rate performance[J]. Energy & Fuels, 2020, 34(12): 16831-16837. |
| 20 | Li Q, Wang Z H, He Y, et al. Pyrolysis characteristics and evolution of char structure during pulverized coal pyrolysis in drop tube furnace: influence of temperature[J]. Energy & Fuels, 2017, 31(5): 4799-4807. |
| 21 | Wang D C, Jin L J, Wei B Y, et al. Oxidative catalytic cracking and reforming of coal pyrolysis volatiles over NiO[J]. Energy & Fuels, 2020, 34(6): 6928-6937. |
| 22 | He X Q, Liu X F, Nie B S, et al. FTIR and Raman spectroscopy characterization of functional groups in various rank coals[J]. Fuel, 2017, 206: 555-563. |
| 23 | Zhang S X, Li C R, Huang R, et al. Thermogravimetric study of the kinetics and characteristics of the pyrolysis of pulverized coal[J]. Materials Research Express, 2020, 7(8): 085604. |
| 24 | Wang C Y, Xing Y W, Xia Y C, et al. Investigation of interactions between oxygen-containing groups and water molecules on coal surfaces using density functional theory[J]. Fuel, 2021, 287: 119556. |
| 25 | Miura K. Mild conversion of coal for producing valuable chemicals[J]. Fuel Processing Technology, 2000, 62(2/3): 119-135. |
| 26 | Lu Y X, Zhao C L, Qi X G, et al. Pre-oxidation-tuned microstructures of carbon anodes derived from pitch for enhancing Na storage performance[J]. Advanced Energy Materials, 2018, 8(27): 1800108. |
| 27 | Sun D, Luo B, Wang H Y, et al. Engineering the trap effect of residual oxygen atoms and defects in hard carbon anode towards high initial Coulombic efficiency[J]. Nano Energy, 2019, 64: 103937. |
| 28 | Sadezky A, Muckenhuber H, Grothe H, et al. Raman microspectroscopy of soot and related carbonaceous materials: spectral analysis and structural information[J]. Carbon, 2005, 43(8): 1731-1742. |
| 29 | Li Y M, Mu L Q, Hu Y S, et al. Pitch-derived amorphous carbon as high performance anode for sodium-ion batteries[J]. Energy Storage Materials, 2016, 2: 139-145. |
| 30 | Cao Y L, Xiao L F, Sushko M L, et al. Sodium ion insertion in hollow carbon nanowires for battery applications[J]. Nano Letters, 2012, 12(7): 3783-3787. |
| 31 | Qi Y R, Lu Y X, Ding F X, et al. Slope-dominated carbon anode with high specific capacity and superior rate capability for high safety Na-ion batteries[J]. Angewandte Chemie International Edition, 2019, 58(13): 4361-4365. |
| 32 | Alvin S, Yoon D, Chandra C, et al. Revealing sodium ion storage mechanism in hard carbon[J]. Carbon, 2019, 145: 67-81. |
| 33 | Jiang B, Zhang Y, Yan D, et al. Ultrafast sodium storage through capacitive behaviors in carbon nanosheets with enhanced ion transport[J]. ChemElectroChem, 2019, 6(12): 3043-3048. |
| 34 | Xia J L, Yan D, Guo L P, et al. Hard carbon nanosheets with uniform ultramicropores and accessible functional groups showing high realistic capacity and superior rate performance for sodium-ion storage[J]. Advanced Materials, 2020, 32(21): 2000447. |
| [1] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [2] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [3] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [4] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [5] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [6] | 吴文涛, 褚良永, 张玲洁, 谭伟民, 沈丽明, 暴宁钟. 腰果酚生物基自愈合微胶囊的高效制备工艺研究[J]. 化工学报, 2023, 74(7): 3103-3115. |
| [7] | 王志龙, 杨烨, 赵真真, 田涛, 赵桐, 崔亚辉. 搅拌时间和混合顺序对锂离子电池正极浆料分散特性的影响[J]. 化工学报, 2023, 74(7): 3127-3138. |
| [8] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [9] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [10] | 张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| [11] | 杨峥豪, 何臻, 常玉龙, 靳紫恒, 江霞. 生物质快速热解下行式流化床反应器研究进展[J]. 化工学报, 2023, 74(6): 2249-2263. |
| [12] | 朱风, 陈凯琳, 黄小凤, 鲍银珠, 李文斌, 刘嘉鑫, 吴玮强, 高王伟. KOH改性电石渣脱除羰基硫的性能研究[J]. 化工学报, 2023, 74(6): 2668-2679. |
| [13] | 李靖, 沈聪浩, 郭大亮, 李静, 沙力争, 童欣. 木质素基碳纤维复合材料在储能元件中的应用研究进展[J]. 化工学报, 2023, 74(6): 2322-2334. |
| [14] | 李瑞康, 何盈盈, 卢维鹏, 王园园, 丁皓东, 骆勇名. 电化学强化钴基阴极活化过一硫酸盐的研究[J]. 化工学报, 2023, 74(5): 2207-2216. |
| [15] | 王承泽, 顾凯丽, 张晋华, 石建轩, 刘艺娓, 李锦祥. 硫化协同老化零价铁增效去除水中Cr(Ⅵ)的作用机制[J]. 化工学报, 2023, 74(5): 2197-2206. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号