化工学报 ›› 2021, Vol. 72 ›› Issue (12): 6086-6092.DOI: 10.11949/0438-1157.20211409
收稿日期:2021-10-08
修回日期:2021-11-19
出版日期:2021-12-05
发布日期:2021-12-22
通讯作者:
戈钧,刘铮
作者简介:夏欢(1989—),男,博士,基金资助:
Huan XIA1( ),Diannan LU2,Jun GE1,2(
),Diannan LU2,Jun GE1,2( ),Jianzhong WU3,Zheng LIU2(
),Jianzhong WU3,Zheng LIU2( )
)
Received:2021-10-08
Revised:2021-11-19
Online:2021-12-05
Published:2021-12-22
Contact:
Jun GE,Zheng LIU
摘要:
将酶负载于载体上可以提高工业应用中酶的稳定性并便于重复使用。纳米材料的发展为固定化酶提供了新的契机,利用纳米材料固定化酶有望进一步提高酶在工业环境中的催化性能、拓展固定化酶的应用范围。本文主要关注近年来在纳米结构酶催化剂方面的研究进展,重点介绍本研究组以无机晶体、金属有机骨架材料、石墨烯和功能高分子等为载体进行酶固定化以及酶催化过程多尺度分子模拟方法的研究进展,讨论了纳米结构酶催化剂的发展前景。
中图分类号:
夏欢, 卢滇楠, 戈钧, 吴建中, 刘铮. 纳米结构酶催化剂研究进展[J]. 化工学报, 2021, 72(12): 6086-6092.
Huan XIA, Diannan LU, Jun GE, Jianzhong WU, Zheng LIU. Advances in nanostructured enzyme catalysts[J]. CIESC Journal, 2021, 72(12): 6086-6092.
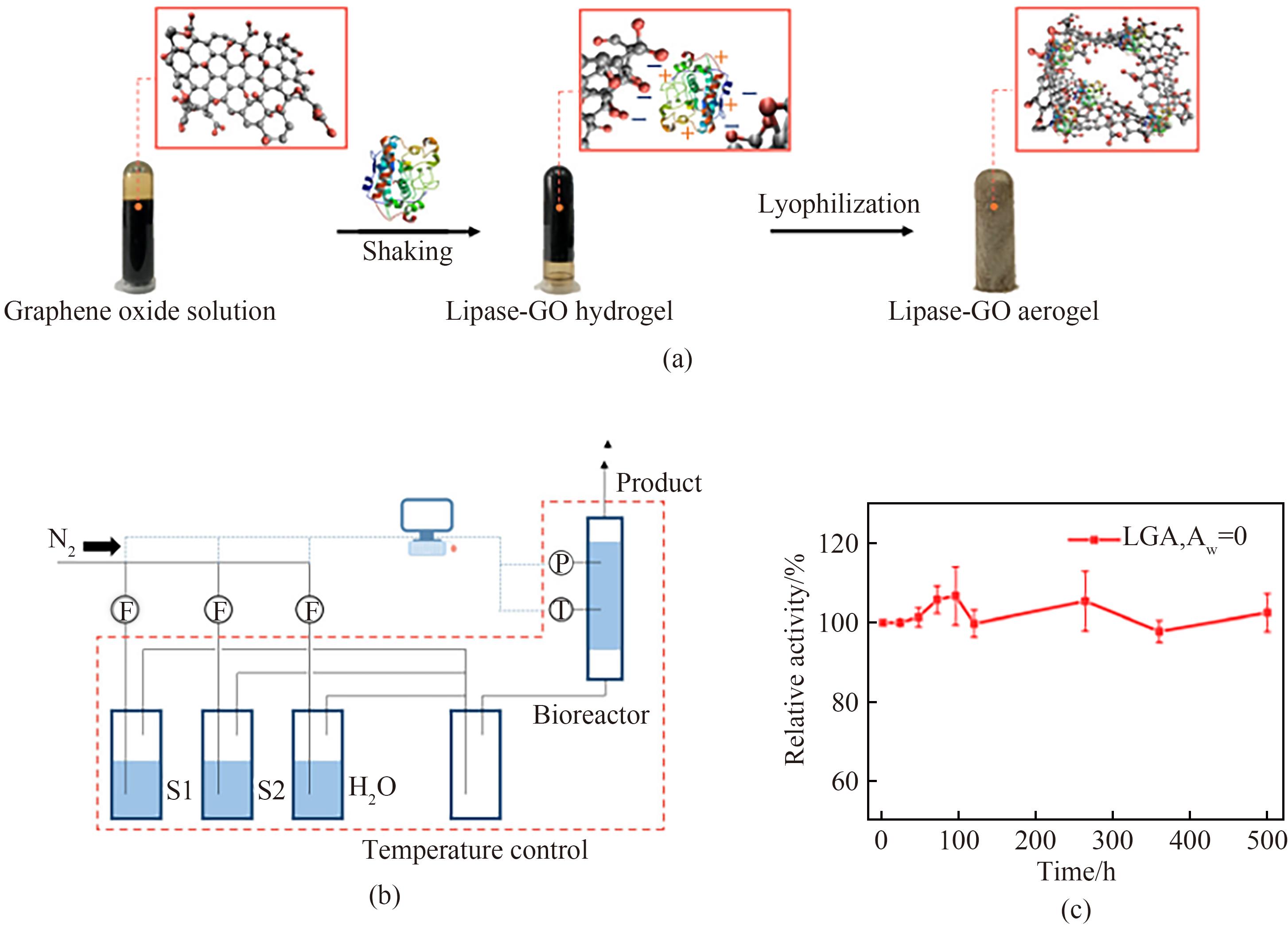
图3 脂肪酶-氧化石墨烯气凝胶复合物的制备过程(a),气态酶催化实验装置示意图(b),连续操作下脂肪酶-氧化石墨烯气凝胶的活性变化图(c)[50]
Fig.3 Preparation of procedures of lipase-GO aerogel (a), schematic diagram of experimental setup for gaseous enzymatic catalysis (b), the activity of lipase-GO aerogel during a continuous operation for 500 h (c)[50]
| 1 | Schmid A, Dordick J S, Hauer B, et al. Industrial biocatalysis today and tomorrow[J]. Nature, 2001, 409(6817): 258-268. |
| 2 | Hanefeld U, Cao L, Magner E. Enzyme immobilisation: fundamentals and application[J]. Chemical Society Reviews, 2013, 42(15): 6211-6212. |
| 3 | Luan P Q, Liu Y T, Li Y X, et al. Aqueous chemoenzymatic one-pot enantioselective synthesis of tertiary α-aryl cycloketones via Pd-catalyzed C―C formation and enzymatic C̿ C asymmetric hydrogenation[J]. Green Chemistry, 2021, 23(5): 1960-1964. |
| 4 | Cao Y F, Li X Y, Ge J. Enzyme catalyst engineering toward the integration of biocatalysis and chemocatalysis[J]. Trends in Biotechnology, 2021, 39(11): 1173-1183. |
| 5 | Hyun K H, Han S W, Koh W G, et al. Fabrication of biofuel cell containing enzyme catalyst immobilized by layer-by-layer method[J]. Journal of Power Sources, 2015, 286: 197-203. |
| 6 | Prodromidis M, Karayannis M. Enzyme based amperometric biosensors for food analysis[J]. Electroanalysis, 2002, 14(4): 241-261. |
| 7 | Ianniello R M, Yacynych A M. Immobilized enzyme chemically modified electrode as an amperometric sensor[J]. Analytical Chemistry, 1981, 53(13): 2090-2095. |
| 8 | Shoda S, Uyama H, Kadokawa J, et al. Enzymes as green catalysts for precision macromolecular synthesis[J]. Chemical Reviews, 2016, 116(4): 2307-2413. |
| 9 | Liu Q, Xun G H, Feng Y. The state-of-the-art strategies of protein engineering for enzyme stabilization[J]. Biotechnology Advances, 2019, 37(4): 530-537. |
| 10 | Singh R K, Tiwari M K, Singh R, et al. From protein engineering to immobilization: promising strategies for the upgrade of industrial enzymes[J]. International Journal of Molecular Sciences, 2013, 14(1): 1232-1277. |
| 11 | Santos J C S D, Barbosa O, Ortiz C, et al. Importance of the support properties for immobilization or purification of enzymes[J]. ChemCatChem, 2015, 7(16): 2413-2432. |
| 12 | Rodrigues R C, Berenguer-Murcia Á, Fernandez-Lafuente R. Coupling chemical modification and immobilization to improve the catalytic performance of enzymes[J]. Advanced Synthesis & Catalysis, 2011, 353(13): 2216-2238. |
| 13 | Rueda N, dos Santos J C S, Ortiz C, et al. Chemical modification in the design of immobilized enzyme biocatalysts: drawbacks and opportunities[J]. The Chemical Record, 2016, 16(3): 1436-1455. |
| 14 | Mamun A A, Bledzki A K. Micro fibre reinforced PLA and PP composites: enzyme modification, mechanical and thermal properties[J]. Composites Science and Technology, 2013, 78: 10-17. |
| 15 | Sartore L, Caliceti P, Schiavon O, et al. Enzyme modification by MPEG with an amino acid or peptide as spacer arms[J]. Applied Biochemistry and Biotechnology, 1991, 27(1): 45-54. |
| 16 | Goldstein L. Kinetic behavior of immobilized enzyme systems[J]. Methods in Enzymology, 1976, 44: 397-443. |
| 17 | Boudrant J, Woodley J M, Fernandez-Lafuente R. Parameters necessary to define an immobilized enzyme preparation[J]. Process Biochemistry, 2020, 90: 66-80. |
| 18 | Nisha S, Karthick A, Gobi N. A review on methods, application and properties of immobilized enzyme[J]. Chemical Science Review and Letters, 2012, 1: 148-155. |
| 60 | Lian X, Fang Y, Joseph E, et al. Enzyme-MOF (metal-organic framework) composites[J]. Chemical Society Reviews, 2017, 46(11): 3386-3401. |
| 61 | Xia H, Li Z X, Zhong X, et al. HKUST-1 catalyzed efficient in situ regeneration of NAD+ for dehydrogenase mediated oxidation[J]. Chemical Engineering Science, 2019, 203: 43-53. |
| 62 | Liang K, Coghlan C J, Bell S G, et al. Enzyme encapsulation in zeolitic imidazolate frameworks: a comparison between controlled co-precipitation and biomimetic mineralisation[J]. Chemical Communications, 2016, 52(3): 473-476. |
| 63 | Tocco D, Carucci C, Todde D, et al. Enzyme immobilization on metal organic frameworks: laccase from Aspergillus sp. is better adapted to ZIF-zni rather than Fe-BTC[J]. Colloids and Surfaces B: Biointerfaces, 2021, 208: 112147. |
| 19 | Liang S, Wu X L, Xiong J, et al. Metal-organic frameworks as novel matrices for efficient enzyme immobilization: an update review[J]. Coordination Chemistry Reviews, 2020, 406: 213149. |
| 20 | Wang L B, Wang Y C, He R, et al. A new nanobiocatalytic system based on allosteric effect with dramatically enhanced enzymatic performance[J]. Journal of the American Chemical Society, 2013, 135(4): 1272-1275. |
| 21 | Borzouee F, Varshosaz J, Cohan R A, et al. A comparative analysis of different enzyme immobilization nanomaterials: progress, constraints and recent trends[J]. Current Medicinal Chemistry, 2021, 28(20): 3980-4003. |
| 22 | Sharifi M, Sohrabi M J, Hosseinali S H, et al. Enzyme immobilization onto the nanomaterials: application in enzyme stability and prodrug-activated cancer therapy[J]. International Journal of Biological Macromolecules, 2020, 143: 665-676. |
| 23 | Liu W S, Wang L, Jiang R R. Specific enzyme immobilization approaches and their application with nanomaterials[J]. Topics in Catalysis, 2012, 55: 1146-1156. |
| 64 | Tuninetti J S, Serrano M P, Thomas A H, et al. Shelter for biologically relevant molecules: photoprotection and enhanced thermal stability of folic acid loaded in a ZIF-8 MOF porous host[J]. Industrial & Engineering Chemistry Research, 2020, 59: 22155-22162. |
| 65 | Badoei-dalfard A, Khankari S, Karami Z. One-pot synthesis and biochemical characterization of protease metal organic framework (protease@MOF) and its application on the hydrolysis of fish protein-waste[J]. Colloids and Surfaces B: Biointerfaces, 2020, 196: 111318. |
| 66 | Hu C, Bai Y, Hou M, et al. Defect-induced activity enhancement of enzyme-encapsulated metal-organic frameworks revealed in microfluidic gradient mixing synthesis[J]. Science Advances, 2020, 6(5): 5785. |
| 67 | Xia H, Li N, Huang W Q, et al. Enzymatic cascade reactions mediated by highly efficient biomimetic quasi metal-organic frameworks[J]. ACS Applied Materials & Interfaces, 2021, 13(19): 22240-22253. |
| 68 | Lykourinou V, Chen Y, Wang X S, et al. Immobilization of MP-11 into a mesoporous metal–organic framework, MP-11@mesoMOF: a new platform for enzymatic catalysis[J]. Journal of the American Chemical Society, 2011, 133(27): 10382-10385. |
| 69 | Li P, Moon S Y, Guelta M A, et al. Nanosizing a metal–organic framework enzyme carrier for accelerating nerve agent hydrolysis[J]. ACS Nano, 2016, 10(10): 9174-9182. |
| 70 | Deng H X, Grunder S, Cordova K E, et al. Large-pore apertures in a series of metal-organic frameworks[J]. Science, 2012, 336(6084): 1018-1023. |
| 24 | Husain Q. Nanomaterials as novel supports for the immobilization of amylolytic enzymes and their applications: a review[J]. Biocatalysis, 2017, 3(1): 37-53. |
| 25 | Hong T T, Liu W F, Li M, et al. Recent advances in the fabrication and application of nanomaterial-based enzymatic microsystems in chemical and biological sciences[J]. Analytica Chimica Acta, 2019, 1067: 31-47. |
| 26 | Rani M, Shanker U, Chaurasia A K. Catalytic potential of laccase immobilized on transition metal oxides nanomaterials: degradation of alizarin red S dye[J]. Journal of Environmental Chemical Engineering, 2017, 5(3): 2730-2739. |
| 71 | Kimura K, Suzuki A, Inokuchi H, et al. Hydrogenase activity in the dry state: isotope exchange and reversible oxidoreduction of cytochrome c3[J]. Biochimica et Biophysica Acta, 1979, 567(1): 96-105. |
| 72 | Fu Z, Xu W, Chen G, et al. Molecular dynamics simulations reveal how graphene oxide stabilizes and activates lipase in an anhydrous gas[J]. Physical Chemistry Chemical Physics, 2019, 21(45): 25425-25430. |
| 27 | Barbosa C G, Caseli L, Péres L O. Conjugated polymers nanostructured as smart interfaces for controlling the catalytic properties of enzymes[J]. Journal of Colloid and Interface Science, 2016, 476: 206-213. |
| 28 | Xue Q N, Li Z Y, Wang Q K, et al. Nanostrip flexible microwave enzymatic biosensor for noninvasive epidermal glucose sensing[J]. Nanoscale Horizons, 2020, 5(6): 934-943. |
| 29 | Wu X L, Ou G, Yang C, et al. Enhanced enzymatic reactions by solar-to-thermal conversion nanoparticles[J]. Chemical Communications, 2017, 53(36): 5048-5051. |
| 30 | Ji Y, Wang Y, Zeng W, et al. A heparin derivatives library constructed by chemical modification and enzymatic depolymerization for exploitation of non-anticoagulant functions[J]. Carbohydrate Polymers, 2020, 249: 116824. |
| 31 | Yong Y, Su R, Liu X R, et al. Lectin corona enhances enzymatic catalysis on the surface of magnetic nanoparticles[J]. Biochemical Engineering Journal, 2018, 129: 26-32. |
| 32 | Yan M, Ge J, Liu Z, et al. Encapsulation of single enzyme in nanogel with enhanced biocatalytic activity and stability[J]. Journal of the American Chemical Society, 2006, 128(34): 11008-11009. |
| 33 | Ge J, Lu D N, Wang J, et al. Molecular fundamentals of enzyme nanogels[J]. Journal of Physical Chemistry B, 2008, 112(45): 14319-14324. |
| 34 | Zhang Y, Chen Q, Ge J, et al. Controlled display of enzyme activity with a stretchable hydrogel[J]. Chemical Communications, 2013, 49(84): 9815-9817. |
| 35 | Wang R, Zhang Y F, Ge J, et al. Activation of enzyme nanogel in organic solvents by PEG–substrate joint imprinting[J]. RSC Advances, 2014, 4(76): 40301. |
| 36 | Ge J, Lu D, Wang J, et al. Lipase nanogel catalyzed transesterification in anhydrous dimethyl sulfoxide[J]. Biomacromolecules, 2009, 10(6): 1612-1618. |
| 37 | Zhu J Y, Zhang Y F, Lu D N, et al. Temperature-responsive enzyme–polymer nanoconjugates with enhanced catalytic activities in organic media[J]. Chemical Communications, 2013, 49(54): 6090. |
| 38 | Ge J, Lu D N, Yang C, et al. A lipase-responsive vehicle using amphipathic polymer synthesized with the lipase as catalyst[J]. Macromolecular Rapid Communications, 2011, 32(6): 546-550. |
| 39 | Zhang Y F, Dai Y, Hou M, et al. Chemo-enzymatic synthesis of valrubicin using Pluronic conjugated lipase with temperature responsiveness in organic media[J]. RSC Advances, 2013, 3(45): 22963. |
| 40 | Wu X L, Ge J, Zhu J Y, et al. A general method for synthesizing enzyme–polymer conjugates in reverse emulsions using Pluronic as a reactive surfactant[J]. Chemical Communications, 2015, 51(47): 9674-9677. |
| 41 | Hou M, Wang R, Wu X L, et al. Synthesis of lutein esters by using a reusable lipase-pluronic conjugate as the catalyst[J]. Catalysis Letters, 2015, 145(10): 1825-1829. |
| 42 | Ge J, Lei J D, Zare R N. Protein–inorganic hybrid nanoflowers[J]. Nature Nanotechnology, 2012, 7(7): 428-432. |
| 43 | Zhu L, Gong L, Zhang Y F, et al. Rapid detection of phenol using a membrane containing laccase nanoflowers[J]. Chemistry - an Asian Journal, 2013, 8(10): 2358-2360. |
| 44 | Li Z, Ding Y, Li S, et al. Highly active, stable and self-antimicrobial enzyme catalysts prepared by biomimetic mineralization of copper hydroxysulfate[J]. Nanoscale, 2016, 8(40): 17440-17445. |
| 45 | Li Z X, Zhang Y F, Su Y C, et al. Spatial co-localization of multi-enzymes by inorganic nanocrystal–protein complexes[J]. Chemical Communications, 2014, 50(83): 12465-12468. |
| 46 | Lyu F J, Zhang Y F, Zare R N, et al. One-pot synthesis of protein-embedded metal-organic frameworks with enhanced biological activities[J]. Nano Letters, 2014, 14(10): 5761-5765. |
| 47 | Wu X L, Ge J, Yang C, et al. Facile synthesis of multiple enzyme-containing metal–organic frameworks in a biomolecule-friendly environment[J]. Chemical Communications, 2015, 51(69): 13408-13411. |
| 48 | Zhang C, Wang X R, Hou M, et al. Immobilization on metal–organic framework engenders high sensitivity for enzymatic electrochemical detection[J]. ACS Applied Materials & Interfaces, 2017, 9(16): 13831-13836. |
| 49 | Wu X L, Yue H, Zhang Y, et al. Packaging and delivering enzymes by amorphous metal-organic frameworks[J]. Nature Communications, 2019, 10: 5165. |
| 50 | Xu W N, Fu Z W, Chen G, et al. Graphene oxide enabled long-term enzymatic transesterification in an anhydrous gas flux[J]. Nature Communications, 2019, 10: 2684. |
| 51 | López-Gallego F, Yate L. Selective biomineralization of Co3(PO4)2-sponges triggered by His-tagged proteins: efficient heterogeneous biocatalysts for redox processes[J]. Chemical Communications, 2015, 51(42): 8753-8756. |
| 52 | Silva-Torres O, Bojorquez-Vazquez L, Simakov A, et al. Enhanced laccase activity of biocatalytic hybrid copper hydroxide nanocages[J]. Enzyme and Microbial Technology, 2019, 128: 59-66. |
| 53 | Zhang B L, Li P T, Zhang H P, et al. Papain/Zn3(PO4)2 hybrid nanoflower: preparation, characterization and its enhanced catalytic activity as an immobilized enzyme[J]. RSC Advances, 2016, 6(52): 46702-46710. |
| 54 | Salvi H M, Yadav G D. Organic-inorganic epoxide hydrolase hybrid nanoflowers with enhanced catalytic activity: hydrolysis of styrene oxide to 1-phenyl-1,2-ethanediol[J]. Journal of Biotechnology, 2021, 341: 113-120. |
| 73 | Wu X L, Wang R, Zhang Y F, et al. Enantioselective ammonolysis of phenylglycine methyl ester with lipase-pluronic nanoconjugate in tertiary butanol[J]. Catalysis Letters, 2014, 144(8): 1407-1410. |
| 74 | Yuki O, Zhang Y F, Ge J, et al. Epoxidation of fatty acids by pluronic-conjugated lipase in organic media[J]. Catalysis Letters, 2016, 146(6): 1073-1078. |
| 75 | Cheng H, Zhao Y L, Luo X J, et al. Cross-linked enzyme-polymer conjugates with excellent stability and detergent-enhanced activity for efficient organophosphate degradation[J]. Bioresources and Bioprocessing, 2018, 5(1): 1-9. |
| 76 | Cao Y, Li X, Xiong J, et al. Investigating the origin of high efficiency in confined multienzyme catalysis[J]. Nanoscale, 2019, 11(45): 22108-22117. |
| 55 | Gul O T, Ocsoy I. Co-enzymes based nanoflowers incorporated-magnetic carbon nanotubes: a new generation nanocatalyst for superior removal of cationic and anionic dyes with great repeated use[J]. Environmental Technology & Innovation, 2021, 24: 101992. |
| 56 | Dube S, Rawtani D. Understanding intricacies of bioinspired organic-inorganic hybrid nanoflowers: a quest to achieve enhanced biomolecules immobilization for biocatalytic, biosensing and bioremediation applications[J]. Advances in Colloid and Interface Science, 2021, 295: 102484. |
| 57 | Gkaniatsou E, Sicard C, Ricoux R, et al. Metal–organic frameworks: a novel host platform for enzymatic catalysis and detection[J]. Materials Horizons, 2017, 4(1): 55-63. |
| 58 | Doonan C, Riccò R, Liang K, et al. Metal-organic frameworks at the biointerface: synthetic strategies and applications[J]. Accounts of Chemical Research, 2017, 50(6): 1423-1432. |
| 59 | Xia H, Zhong X, Li Z X, et al. Palladium-mediated hybrid biocatalysts with enhanced enzymatic catalytic performance via allosteric effects[J]. Journal of Colloid and Interface Science, 2019, 533: 1-8. |
| 77 | Chen G, Kong X, Lu D N, et al. Kinetics of CO2 diffusion in human carbonic anhydrase: a study using molecular dynamics simulations and the Markov-state model[J]. Physical Chemistry Chemical Physics, 2017, 19(18): 11690-11697. |
| 78 | Chen G, Lu D N, Wu J Z, et al. Detachment of HCO3- from the active site of carbonic anhydrase: molecular dynamics simulation and machine learning[J]. The Journal of Physical Chemistry C, 2018, 122(35): 20539-20549. |
| [1] | 刘远超, 关斌, 钟建斌, 徐一帆, 蒋旭浩, 李耑. 单层XSe2(X=Zr/Hf)的热电输运特性研究[J]. 化工学报, 2023, 74(9): 3968-3978. |
| [2] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [3] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| [4] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [5] | 陈雅鑫, 袁航, 刘冠章, 毛磊, 杨纯, 张瑞芳, 张光亚. 蛋白质纳米笼介导的酶自固定化研究进展[J]. 化工学报, 2023, 74(7): 2773-2782. |
| [6] | 邢美波, 张中天, 景栋梁, 张洪发. 磁调控水基碳纳米管协同多孔材料强化相变储/释能特性[J]. 化工学报, 2023, 74(7): 3093-3102. |
| [7] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [8] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [9] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [10] | 董茂林, 陈李栋, 黄六莲, 吴伟兵, 戴红旗, 卞辉洋. 酸性助水溶剂制备木质纳米纤维素及功能应用研究进展[J]. 化工学报, 2023, 74(6): 2281-2295. |
| [11] | 杨琴, 秦传鉴, 李明梓, 杨文晶, 赵卫杰, 刘虎. 用于柔性传感的双形状记忆MXene基水凝胶的制备及性能研究[J]. 化工学报, 2023, 74(6): 2699-2707. |
| [12] | 刘远超, 蒋旭浩, 邵钶, 徐一帆, 钟建斌, 李耑. 几何尺寸及缺陷对石墨炔纳米带热输运特性的影响[J]. 化工学报, 2023, 74(6): 2708-2716. |
| [13] | 胡阳, 孙彦. 酶分子的自驱动及其介导的微纳马达[J]. 化工学报, 2023, 74(1): 116-132. |
| [14] | 张浩, 王子悦, 程钰洁, 何晓辉, 纪红兵. 单原子催化剂规模化制备的研究进展[J]. 化工学报, 2023, 74(1): 276-289. |
| [15] | 张静, 刘涛, 张伟, 储震宇, 金万勤. 一种新型分离传感膜的制备及其血糖的动态监测[J]. 化工学报, 2023, 74(1): 459-468. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号