化工学报 ›› 2023, Vol. 74 ›› Issue (1): 116-132.DOI: 10.11949/0438-1157.20221053
收稿日期:2022-07-26
修回日期:2022-09-16
出版日期:2023-01-05
发布日期:2023-03-20
通讯作者:
孙彦
作者简介:胡阳(1992—),男,博士,讲师,huyang@ouc.edu.cn
基金资助:Received:2022-07-26
Revised:2022-09-16
Online:2023-01-05
Published:2023-03-20
Contact:
Yan SUN
摘要:
在生命活动中扮演重要角色的生物催化剂酶,被发现在催化底物转化的过程中能表现分子水平的扩散增强行为。这种自驱动的扩散增强现象提供了一个研究酶的新角度:酶分子马达(EMM)。受到天然生物分子马达的启发,EMM被用作“引擎”开发出了一系列的酶驱动微纳马达和微泵,将催化过程中的化学能转化为动能,驱动微纳尺度的运动。通过巧妙的设计,酶驱动微纳设备可以实现功能化、完成各种任务,引起了广泛的关注。然而,EMM和酶驱动微纳设备的运动机理尚处于争论之中,酶驱动设备尺寸、结构、酶的性质对运动性能的影响也尚未明晰,制约着EMM和微纳设备的研究和应用。因此,本文综述EMM的自驱动运动以及作为“引擎”驱动的微纳马达和微泵。首先,简述低Reynolds数环境中实现微观自驱动运动的条件,阐述酶分子的自驱动和趋化行为,归类EMM运动机理;其次,归纳酶驱动微纳马达和微泵,重点评述酶分子作为“引擎”驱动人工合成微纳马达的实现途径及其潜在应用;最后,总结酶分子自驱动及其驱动微纳尺度运动存在的主要挑战,并提出进一步研究的重点方向。
中图分类号:
胡阳, 孙彦. 酶分子的自驱动及其介导的微纳马达[J]. 化工学报, 2023, 74(1): 116-132.
Yang HU, Yan SUN. Self-propulsion of enzyme and enzyme-induced micro-/nanomotor[J]. CIESC Journal, 2023, 74(1): 116-132.

图1 低Reynolds数环境的运动(a) 三联杆有效运动机理[15]; (b) 酶分子马达和酶驱动微纳设备的运动
Fig.1 The movement at a low Reynolds number(a) the motion mechanism of a theoretical 3-link swimmer[15]; (b) the movement of EMM and enzyme-propelled micro/nanodevice
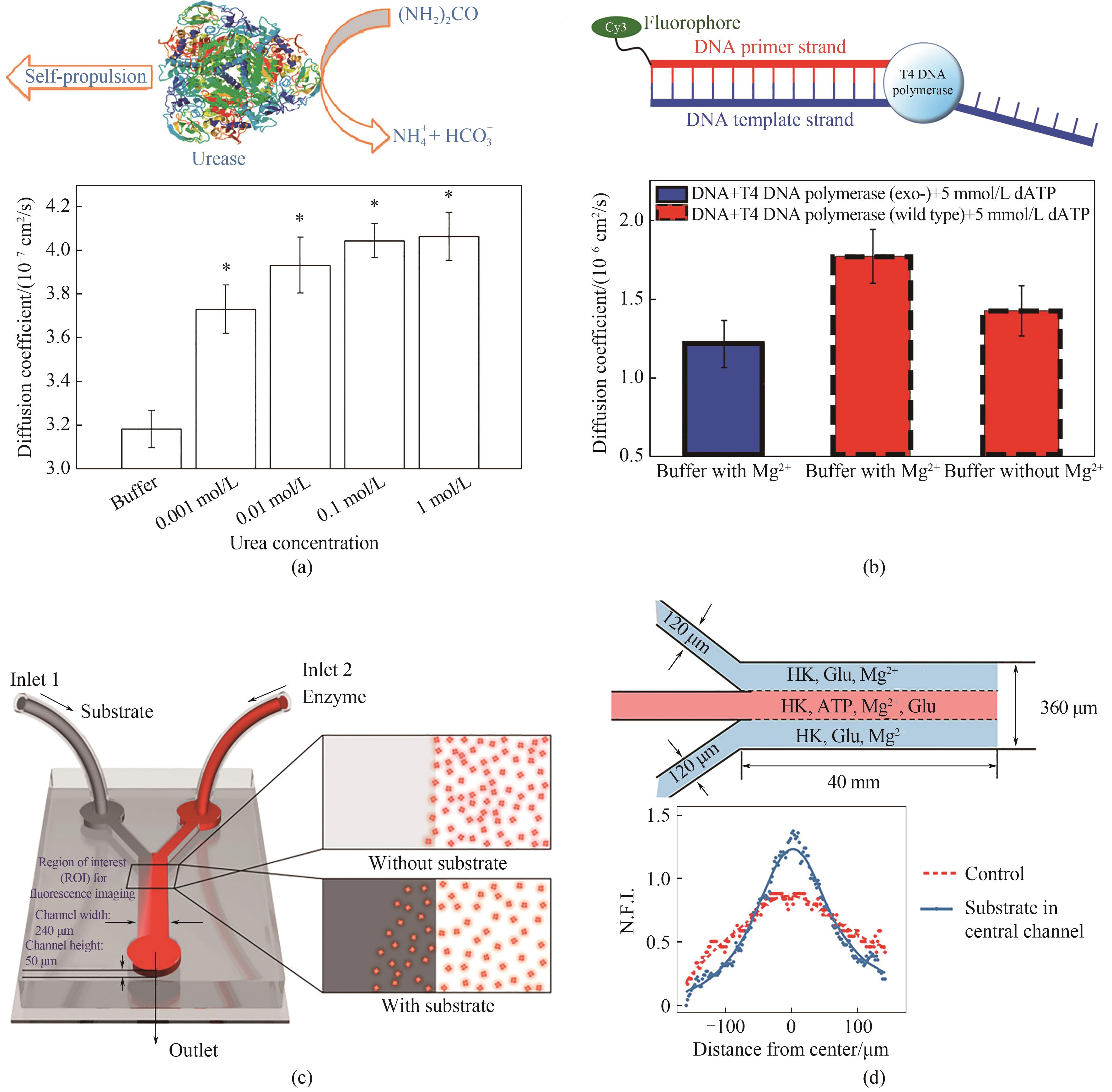
图2 酶分子马达及其趋化行为(a) 脲酶分子马达底物依赖性的扩散增强行为[5]; (b) DNA聚合酶在底物和辅酶存在时的扩散增强行为[23]; (c) 观测酶分子马达趋化行为微流控设备示意图[24]; (d) 催化级联反应酶分子马达的趋化行为[31]
Fig.2 Enzyme molecular motor and its chemotaxis(a) schematic and experiment result illustrating the substrate-dependent diffusion enhancement of urease[5]; (b) schematic and experiment result illustrating the enhanced diffusion of DNA polymerase[23]; (c) schematic illustration of the microfluidics for the observation of EMM chemotaxis[24]; (d) the chemotaxis behavior of the enzyme catalyzing a cascade reaction[31]

图3 酶驱动人工合成微纳马达(a) 过氧化氢酶驱动管状微米马达[10]; (b) 脲酶驱动微米马达及其运动控制示意图[50]; (c) 酯酶驱动微米马达[52]; (d) 级联酶催化驱动微米马达的设计及其催化网络[58]
Fig.3 Artificial enzyme-powered micro-/nanomotor(a) schematic illustrating the micromotor propelled by catalase[10]; (b) illustration of the propulsion and movement control of urease-based micromotor[50]; (c) lipase-powered micromotor[52]; (d) design and catalytic network of micromotor powered by enzymatic cascade reactions[58]

图5 酶驱动微纳马达的运动控制(a) 过氧化氢酶驱动马达在不同底物浓度下的扩散系数[64]; (b) 不同酶驱动纳米马达的运动速度[72]; (c) 不同尺寸过氧化氢酶驱动微米马达运动行为示意图[77]; (d) 光热效应调控酶驱动纳米马达运动速度示意图[80]
Fig.5 Motion control of EMNM(a) diffusion coefficient of nanomotor powered by catalase at different substrate concentrations[64]; (b) speed of nanomotor propelled by different enzymes[72]; (c) schematic illustrating the movement of micromotors with different sizes[77]; (d) illustration showing the movement control of enzyme-powered nanomotor through photothermal effect[80]
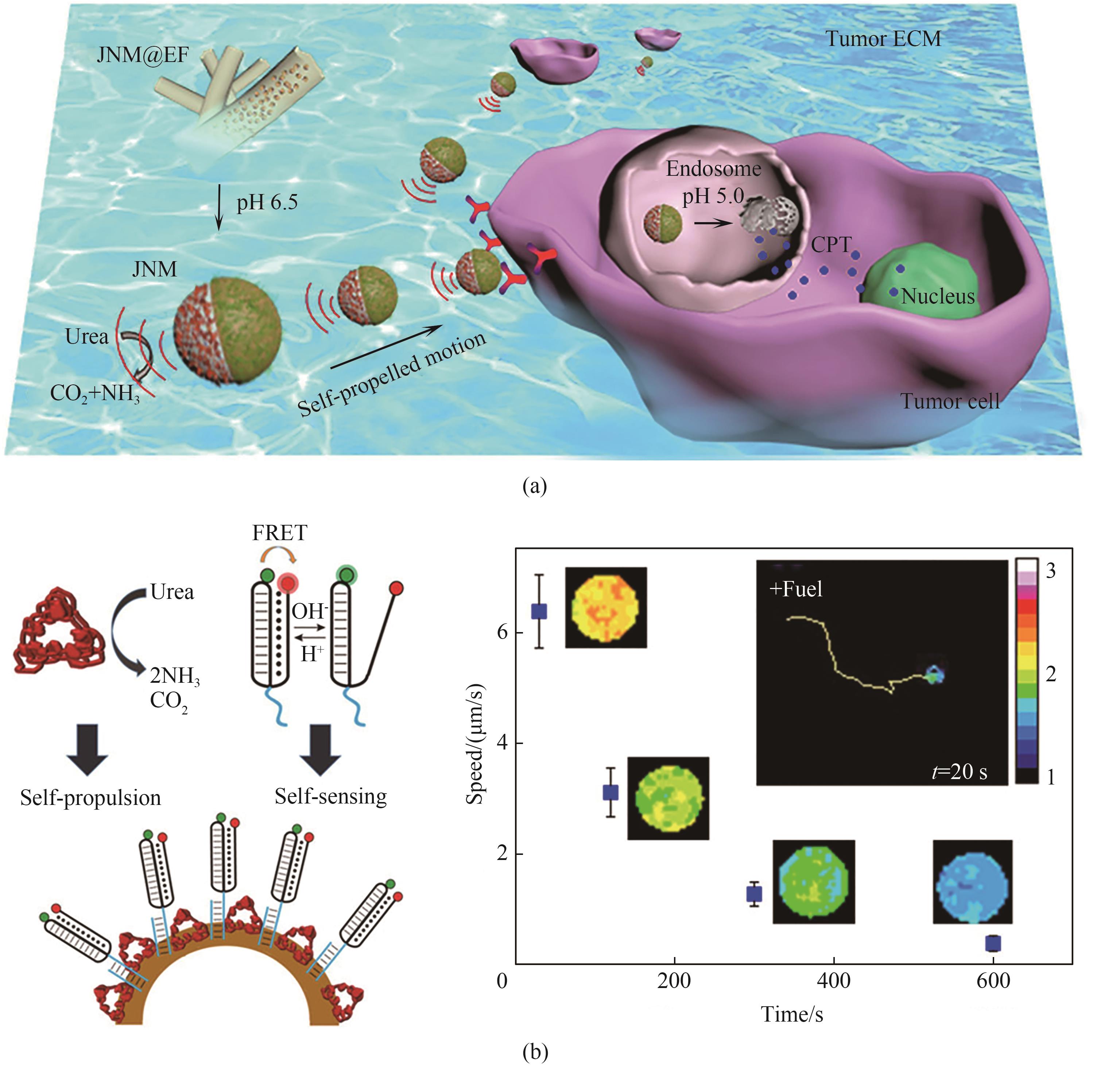
图6 酶驱动微纳马达的应用(a) 脲酶驱动纳米马达用于药物递送示意图[85]; (b) 脲酶驱动纳米马达用于检测局部pH[91]
Fig.6 Application of EMNM(a) schematic illustrating the urease-powered nanomotor for drug delivery[85]; (b) urease-powered nanomotor used to detect local pH[91]
| 1 | Feringa B L. The art of building small: from molecular switches to motors (Nobel lecture)[J]. Angewandte Chemie International Edition, 2017, 56(37): 11060-11078. |
| 2 | Stoddart J F. Mechanically interlocked molecules (MIMs)—molecular shuttles, switches, and machines (Nobel lecture)[J]. Angewandte Chemie International Edition, 2017, 56(37): 11094-11125. |
| 3 | Sauvage J P. From chemical topology to molecular machines (Nobel lecture)[J]. Angewandte Chemie International Edition, 2017, 56(37): 11080-11093. |
| 4 | Guix M, Mayorga-Martinez C C, Merkoçi A. Nano/micromotors in (bio)chemical science applications[J]. Chemical Reviews, 2014, 114(12): 6285-6322. |
| 5 | Muddana H S, Sengupta S, Mallouk T E, et al. Substrate catalysis enhances single-enzyme diffusion[J]. Journal of the American Chemical Society, 2010, 132(7): 2110-2111. |
| 6 | Butler P J, Dey K K, Sen A. Impulsive enzymes: a new force in mechanobiology[J]. Cellular and Molecular Bioengineering, 2015, 8(1): 106-118. |
| 7 | Zhao X, Gentile K, Mohajerani F, et al. Powering motion with enzymes[J]. Accounts of Chemical Research, 2018, 51(10): 2373-2381. |
| 8 | Mathesh M, Sun J W, Wilson D A. Enzyme catalysis powered micro/nanomotors for biomedical applications[J]. Journal of Materials Chemistry B, 2020, 8(33): 7319-7334. |
| 9 | Ghosh S, Somasundar A, Sen A. Enzymes as active matter[J]. Annual Review of Condensed Matter Physics, 2021, 12: 177-200. |
| 10 | Snchez S, Solovev A A, Mei Y F, et al. Dynamics of biocatalytic microengines mediated by variable friction control[J]. Journal of the American Chemical Society, 2010, 132(38): 13144-13145. |
| 11 | Feng M D, Gilson M K. Enhanced diffusion and chemotaxis of enzymes[J]. Annual Review of Biophysics, 2020, 49: 87-105. |
| 12 | Purcell E M. Life at low Reynolds number[J]. American Journal of Physics, 1977, 45(1): 3-11. |
| 13 | Wang W, Duan W T, Ahmed S, et al. Small power: autonomous nano- and micromotors propelled by self-generated gradients[J]. Nano Today, 2013, 8(5): 531-554. |
| 14 | 金东东, 俞江帆, 黄天云, 等. 磁性微纳米尺度游动机器人: 现状与应用前景[J]. 科学通报, 2017, 62(Z1): 136-151. |
| Jin D D, Yu J F, Huang T Y, et al. Magnetic micro-/nanoscale swimmers: current status and potential applications[J]. Chinese Science Bulletin, 2017, 62(Z1): 136-151. | |
| 15 | Lauga E. Life around the scallop theorem[J]. Soft Matter, 2011, 7(7): 3060-3065. |
| 16 | Pelz B, Žoldák G, Zeller F, et al. Subnanometre enzyme mechanics probed by single-molecule force spectroscopy[J]. Nature Communications, 2016, 7: 10848. |
| 17 | Patiño T, Arqué X, Mestre R, et al. Fundamental aspects of enzyme-powered micro- and nanoswimmers[J]. Accounts of Chemical Research, 2018, 51(11): 2662-2671. |
| 18 | Agudo-Canalejo J, Adeleke-Larodo T, Illien P, et al. Enhanced diffusion and chemotaxis at the nanoscale[J]. Accounts of Chemical Research, 2018, 51(10): 2365-2372. |
| 19 | Mirkovic T, Zacharia N S, Scholes G D, et al. Nanolocomotion—catalytic nanomotors and nanorotors[J]. Small, 2010, 6(2): 159-167. |
| 20 | Hassan P A, Rana S M, Verma G. Making sense of Brownian motion: colloid characterization by dynamic light scattering[J]. Langmuir, 2015, 31(1): 3-12. |
| 21 | Ries J, Schwille P. Fluorescence correlation spectroscopy[J]. BioEssays, 2012, 34(5): 361-368. |
| 22 | Ma X, Hortelão A C, Patiño T, et al. Enzyme catalysis to power micro/nanomachines[J]. ACS Nano, 2016, 10(10): 9111-9122. |
| 23 | Sengupta S, Spiering M M, Dey K K, et al. DNA polymerase as a molecular motor and pump[J]. ACS Nano, 2014, 8(3): 2410-2418. |
| 24 | Sengupta S, Dey K K, Muddana H S, et al. Enzyme molecules as nanomotors[J]. Journal of the American Chemical Society, 2013, 135(4): 1406-1414. |
| 25 | Ghosh S, Mohajerani F, Son S, et al. Motility of enzyme-powered vesicles[J]. Nano Letters, 2019, 19(9): 6019-6026. |
| 26 | Jee A Y, Dutta S, Cho Y K, et al. Enzyme leaps fuel antichemotaxis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(1): 14-18. |
| 27 | Riedel C, Gabizon R, Wilson C A M, et al. The heat released during catalytic turnover enhances the diffusion of an enzyme[J]. Nature, 2015, 517(7533): 227-230. |
| 28 | Yu H, Jo K, Kounovsky K L, et al. Molecular propulsion: chemical sensing and chemotaxis of DNA driven by RNA polymerase[J]. Journal of the American Chemical Society, 2009, 131(16): 5722-5723. |
| 29 | Mohajerani F, Zhao X, Somasundar A, et al. A theory of enzyme chemotaxis: from experiments to modeling[J]. Biochemistry, 2018, 57(43): 6256-6263. |
| 30 | Wu F, Pelster L N, Minteer S D. Krebs cycle metabolon formation: metabolite concentration gradient enhanced compartmentation of sequential enzymes[J]. Chemical Communications, 2015, 51(7): 1244-1247. |
| 31 | Zhao X, Palacci H, Yadav V, et al. Substrate-driven chemotactic assembly in an enzyme cascade[J]. Nature Chemistry, 2018, 10(3): 311-317. |
| 32 | An S, Kumar R, Sheets E D, et al. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells[J]. Science, 2008, 320(5872): 103-106. |
| 33 | Dennison M, Kapral R, Stark H. Diffusion in systems crowded by active force-dipole molecules[J]. Soft Matter, 2017, 13(20): 3741-3749. |
| 34 | Slochower D R, Gilson M K. Motor-like properties of nonmotor enzymes[J]. Biophysical Journal, 2018, 114(9): 2174-2179. |
| 35 | Lauga E. Enhanced diffusion by reciprocal swimming[J]. Physical Review Letters, 2011, 106(17): 178101. |
| 36 | Illien P, Zhao X, Dey K K, et al. Exothermicity is not a necessary condition for enhanced diffusion of enzymes[J]. Nano Letters, 2017, 17(7): 4415-4420. |
| 37 | Golestanian R. Enhanced diffusion of enzymes that catalyze exothermic reactions[J]. Physical Review Letters, 2015, 115(10): 108102. |
| 38 | Dey K K, Zhao X, Tansi B M, et al. Micromotors powered by enzyme catalysis[J]. Nano Letters, 2015, 15(12): 8311-8315. |
| 39 | Golestanian R. Anomalous diffusion of symmetric and asymmetric active colloids[J]. Physical Review Letters, 2009, 102(18): 188305. |
| 40 | Zhang Y F, Hess H. Enhanced diffusion of catalytically active enzymes[J]. ACS Central Science, 2019, 5(6): 939-948. |
| 41 | Zhao X, Dey K K, Jeganathan S, et al. Enhanced diffusion of passive tracers in active enzyme solutions[J]. Nano Letters, 2017, 17(8): 4807-4812. |
| 42 | Sitt A, Soukupova J, Miller D, et al. Microscale rockets and picoliter containers engineered from electrospun polymeric microtubes[J]. Small, 2016, 12(11): 1432-1439. |
| 43 | Dey K K, Wong F, Altemose A, et al. Catalytic motors—quo vadimus?[J]. Current Opinion in Colloid & Interface Science, 2016, 21: 4-13. |
| 44 | Pavel I A, Bunea A I, David S, et al. Nanorods with biocatalytically induced self-electrophoresis[J]. ChemCatChem, 2014, 6(3): 866-872. |
| 45 | Gao C Y, Zhou C, Lin Z H, et al. Surface wettability-directed propulsion of glucose-powered nanoflask motors[J]. ACS Nano, 2019, 13(11): 12758-12766. |
| 46 | Li H A, Sun Z Y, Jiang S Q, et al. Tadpole-like unimolecular nanomotor with sub-100 nm size swims in a tumor microenvironment model[J]. Nano Letters, 2019, 19(12): 8749-8757. |
| 47 | Wang Z, Yan Y, Li C, et al. Fluidity-guided assembly of Au@Pt on liposomes as a catalase-powered nanomotor for effective cell uptake in cancer cells and plant leaves[J]. ACS Nano, 2022, 16(6): 9019-9030. |
| 48 | Keller S, Teora S P, Hu G X, et al. High-throughput design of biocompatible enzyme-based hydrogel microparticles with autonomous movement[J]. Angewandte Chemie International Edition, 2018, 57(31): 9814-9817. |
| 49 | Ma X, Hortelao A C, Miguel-López A, et al. Bubble-free propulsion of ultrasmall tubular nanojets powered by biocatalytic reactions[J]. Journal of the American Chemical Society, 2016, 138(42): 13782-13785. |
| 50 | Ma X, Wang X, Hahn K, et al. Motion control of urea-powered biocompatible hollow microcapsules[J]. ACS Nano, 2016, 10(3): 3597-3605. |
| 51 | Patiño T, Feiner-Gracia N, Arqué X, et al. Influence of enzyme quantity and distribution on the self-propulsion of non-Janus urease-powered micromotors[J]. Journal of the American Chemical Society, 2018, 140(25): 7896-7903. |
| 52 | Hu Y, Sun Y. Autonomous motion of immobilized enzyme on Janus particles significantly facilitates enzymatic reactions[J]. Biochemical Engineering Journal, 2019, 149: 107242. |
| 53 | Schattling P S, Ramos-Docampo M A, Salgueiriño V, et al. Double-fueled Janus swimmers with magnetotactic behavior[J]. ACS Nano, 2017, 11(4): 3973-3983. |
| 54 | Ramos-Docampo M A, Fernández-Medina M, Taipaleenmäki E, et al. Microswimmers with heat delivery capacity for 3D cell spheroid penetration[J]. ACS Nano, 2019, 13(10): 12192-12205. |
| 55 | Ji Y X, Lin X K, Wu Z G, et al. Macroscale chemotaxis from a swarm of bacteria-mimicking nanoswimmers[J]. Angewandte Chemie International Edition, 2019, 58(35): 12200-12205. |
| 56 | Abdelmohsen L K E A, Nijemeisland M, Pawar G M, et al. Dynamic loading and unloading of proteins in polymeric stomatocytes: formation of an enzyme-loaded supramolecular nanomotor[J]. ACS Nano, 2016, 10(2): 2652-2660. |
| 57 | Schattling P, Thingholm B, Städler B. Enhanced diffusion of glucose-fueled Janus particles[J]. Chemistry of Materials, 2015, 27(21): 7412-7418. |
| 58 | Nijemeisland M, Abdelmohsen L K E A, Huck W T S, et al. A compartmentalized out-of-equilibrium enzymatic reaction network for sustained autonomous movement[J]. ACS Central Science, 2016, 2(11): 843-849. |
| 59 | Laskar A, Shklyaev O E, Balazs A C. Collaboration and competition between active sheets for self-propelled particles[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(19): 9257-9262. |
| 60 | Sengupta S, Patra D, Ortiz-Rivera I, et al. Self-powered enzyme micropumps[J]. Nature Chemistry, 2014, 6(5): 415-422. |
| 61 | Ortiz-Rivera I, Shum H, Agrawal A, et al. Convective flow reversal in self-powered enzyme micropumps[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(10): 2585-2590. |
| 62 | Shao J X, Cao S P, Che H L, et al. Twin-engine Janus supramolecular nanomotors with counterbalanced motion[J]. Journal of the American Chemical Society, 2022, 144(25): 11246-11252. |
| 63 | Hortelão A C, Patiño T, Perez-Jiménez A, et al. Enzyme-powered nanobots enhance anticancer drug delivery[J]. Advanced Functional Materials, 2018, 28(25): 1705086. |
| 64 | Ma X, Snchez S. Bio-catalytic mesoporous Janus nano-motors powered by catalase enzyme[J]. Tetrahedron, 2017, 73(33): 4883-4886. |
| 65 | Ma X, Jannasch A, Albrecht U R, et al. Enzyme-powered hollow mesoporous Janus nanomotors[J]. Nano Letters, 2015, 15(10): 7043-7050. |
| 66 | Zhang C Y, Dong X Y, Guo Z, et al. Remarkably enhanced activity and substrate affinity of lipase covalently bonded on zwitterionic polymer-grafted silica nanoparticles[J]. Journal of Colloid and Interface Science, 2018, 519: 145-153. |
| 67 | Alarcón-Correa M, Günther J P, Troll J, et al. Self-assembled phage-based colloids for high localized enzymatic activity[J]. ACS Nano, 2019, 13(5): 5810-5815. |
| 68 | Ye Y C, Tong F, Wang S H, et al. Apoptotic tumor DNA activated nanomotor chemotaxis[J]. Nano Letters, 2021, 21(19): 8086-8094. |
| 69 | Jang W S, Kim H J, Gao C, et al. Enzymatically powered surface-associated self-motile protocells[J]. Small, 2018, 14(36): 1801715. |
| 70 | Joseph A, Contini C, Cecchin D, et al. Chemotactic synthetic vesicles: design and applications in blood-brain barrier crossing[J]. Science Advances, 2017, 3(8): e1700362. |
| 71 | Somasundar A, Ghosh S, Mohajerani F, et al. Positive and negative chemotaxis of enzyme-coated liposome motors[J]. Nature Nanotechnology, 2019, 14(12): 1129-1134. |
| 72 | Arqué X, Romero-Rivera A, Feixas F, et al. Intrinsic enzymatic properties modulate the self-propulsion of micromotors[J]. Nature Communications, 2019, 10: 2826. |
| 73 | Luo M, Li S, Wan J, et al. Enhanced propulsion of urease-powered micromotors by multilayered assembly of ureases on Janus magnetic microparticles[J]. Langmuir, 2020, 36(25): 7005-7013. |
| 74 | Tang S S, Zhang F Y, Gong H, et al. Enzyme-powered Janus platelet cell robots for active and targeted drug delivery[J]. Science Robotics, 2020, 5(43): eaba6137. |
| 75 | Wang D, Chen C, Sun J, et al. Refillable fuel-loading microshell motors for persistent motion in a fuel-free environment[J]. ACS Applied Materials & Interfaces, 2022, 14(23): 27074-27082. |
| 76 | Zhang X Q, Chen C T, Wu J, et al. Bubble-propelled jellyfish-like micromotors for DNA sensing[J]. ACS Applied Materials & Interfaces, 2019, 11(14): 13581-13588. |
| 77 | Chen C T, He Z Q, Wu J, et al. Motion of enzyme-powered microshell motors[J]. Chemistry—An Asian Journal, 2019, 14(14): 2491-2496. |
| 78 | Tu Y F, Peng F, Sui X F, et al. Self-propelled supramolecular nanomotors with temperature-responsive speed regulation[J]. Nature Chemistry, 2017, 9(5): 480-486. |
| 79 | Che H L, Buddingh’ B C, van Hest J C M. Self-regulated and temporal control of a “breathing” microgel mediated by enzymatic reaction[J]. Angewandte Chemie International Edition, 2017, 56(41): 12581-12585. |
| 80 | Wu M Y, Liu S P, Liu Z C, et al. Photothermal interference urease-powered polydopamine nanomotor for enhanced propulsion and synergistic therapy[J]. Colloids and Surfaces B: Biointerfaces, 2022, 212: 112353. |
| 81 | Wang D, Zhao G, Chen C H, et al. One-step fabrication of dual optically/magnetically modulated walnut-like micromotor[J]. Langmuir, 2019, 35(7): 2801-2807. |
| 82 | Hu Y, Li Z X, Sun Y. Ultrasmall enzyme/light-powered nanomotor facilitates cholesterol detection[J]. Journal of Colloid and Interface Science, 2022, 621: 341-351. |
| 83 | Venugopalan P L, de Ávila B E F, Pal M, et al. Fantastic voyage of nanomotors into the cell[J]. ACS Nano, 2020, 14(8): 9423-9439. |
| 84 | Gao C Y, Wang Y, Ye Z H, et al. Biomedical micro-/nanomotors: from overcoming biological barriers to in vivo imaging[J]. Advanced Materials, 2021, 33(6): 2000512. |
| 85 | Chen Z J, Xia T, Zhang Z L, et al. Enzyme-powered Janus nanomotors launched from intratumoral depots to address drug delivery barriers[J]. Chemical Engineering Journal, 2019, 375: 122109. |
| 86 | Yuan H, Liu X X, Wang L Y, et al. Fundamentals and applications of enzyme powered micro/nano-motors[J]. Bioactive Materials, 2021, 6(6): 1727-1749. |
| 87 | Wu Z G, Lin X K, Zou X, et al. Biodegradable protein-based rockets for drug transportation and light-triggered release[J]. ACS Applied Materials & Interfaces, 2015, 7(1): 250-255. |
| 88 | Llopis-Lorente A, García-Fernández A, Murillo-Cremaes N, et al. Enzyme-powered gated mesoporous silica nanomotors for on-command intracellular payload delivery[J]. ACS Nano, 2019, 13(10): 12171-12183. |
| 89 | Llopis-Lorente A, García-Fernández A, Lucena-Sánchez E, et al. Stimulus-responsive nanomotors based on gated enzyme-powered Janus Au-mesoporous silica nanoparticles for enhanced cargo delivery[J]. Chemical Communications, 2019, 55(87): 13164-13167. |
| 90 | Hu Y, Liu W, Sun Y. Self-propelled micro-/nanomotors as “on-the-move” platforms: cleaners, sensors, and reactors[J]. Advanced Functional Materials, 2022, 32(10): 2109181. |
| 91 | Patiño T, Porchetta A, Jannasch A, et al. Self-sensing enzyme-powered micromotors equipped with pH-responsive DNA nanoswitches[J]. Nano Letters, 2019, 19(6): 3440-3447. |
| 92 | Bunea A I, Pavel I A, David S, et al. Sensing based on the motion of enzyme-modified nanorods[J]. Biosensors and Bioelectronics, 2015, 67: 42-48. |
| 93 | Singh V V, Kaufmann K, de Ávila B E F, et al. Nanomotors responsive to nerve-agent vapor plumes[J]. Chemical Communications, 2016, 52(16): 3360-3363. |
| 94 | Ortiz-Rivera I, Courtney T M, Sen A. Enzyme micropump-based inhibitor assays[J]. Advanced Functional Materials, 2016, 26(13): 2135-2142. |
| 95 | Xie Y Z, Fu S Z, Wu J, et al. Motor-based microprobe powered by bio-assembled catalase for motion detection of DNA[J]. Biosensors and Bioelectronics, 2017, 87: 31-37. |
| 96 | Fu S Z, Zhang X Q, Xie Y Z, et al. An efficient enzyme-powered micromotor device fabricated by cyclic alternate hybridization assembly for DNA detection[J]. Nanoscale, 2017, 9(26): 9026-9033. |
| 97 | Simmchen J, Baeza A, Ruiz D, et al. Asymmetric hybrid silica nanomotors for capture and cargo transport: towards a novel motion-based DNA sensor[J]. Small, 2012, 8(13): 2053-2059. |
| 98 | Zhang Y, Gregory D A, Zhang Y, et al. Reactive inkjet printing of functional silk stirrers for enhanced mixing and sensing[J]. Small, 2019, 15(1): 1804213. |
| 99 | Karshalev E, de Ávila B E F, Wang J. Micromotors for “chemistry-on-the-fly”[J]. Journal of the American Chemical Society, 2018, 140(11): 3810-3820. |
| 100 | Sattayasamitsathit S, Kaufmann K, Galarnyk M, et al. Dual-enzyme natural motors incorporating decontamination and propulsion capabilities[J]. RSC Advances, 2014, 4(52): 27565-27570. |
| 101 | Wang L, Hortelão A C, Huang X, et al. Lipase-powered mesoporous silica nanomotors for triglyceride degradation[J]. Angewandte Chemie International Edition, 2019, 58(24): 7992-7996. |
| 102 | Valdez L, Shum H, Ortiz-Rivera I, et al. Solutal and thermal buoyancy effects in self-powered phosphatase micropumps[J]. Soft Matter, 2017, 13(15): 2800-2807. |
| 103 | Das S, Shklyaev O E, Altemose A, et al. Harnessing catalytic pumps for directional delivery of microparticles in microchambers[J]. Nature Communications, 2017, 8(1): 14384. |
| [1] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [2] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [3] | 陈雅鑫, 袁航, 刘冠章, 毛磊, 杨纯, 张瑞芳, 张光亚. 蛋白质纳米笼介导的酶自固定化研究进展[J]. 化工学报, 2023, 74(7): 2773-2782. |
| [4] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [5] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [6] | 张兰河, 赖青燚, 王铁铮, 关潇卓, 张明爽, 程欣, 徐小惠, 贾艳萍. H2O2对SBR脱氮效率和污泥性能的影响[J]. 化工学报, 2023, 74(5): 2186-2196. |
| [7] | 刘瑞琪, 周栖桐, 张悦, 贺莹, 高静, 马丽. 基于金纳米颗粒修饰二氧化硅纳米花的生物传感器构建及应用[J]. 化工学报, 2023, 74(3): 1247-1259. |
| [8] | 贾露凡, 王艺颖, 董钰漫, 李沁园, 谢鑫, 苑昊, 孟涛. 微流控双水相贴壁液滴流动强化酶促反应研究[J]. 化工学报, 2023, 74(3): 1239-1246. |
| [9] | 苏伟怡, 丁佳慧, 李春利, 王洪海, 姜艳军. 酶促反应结晶研究进展[J]. 化工学报, 2023, 74(2): 617-629. |
| [10] | 刘昕, 戈钧, 李春. 光驱动微生物杂合系统提高生物制造水平[J]. 化工学报, 2023, 74(1): 330-341. |
| [11] | 谭卓涛, 齐思雨, 许梦蛟, 戴杰, 朱晨杰, 应汉杰. 辅酶自循环的氧化还原级联体系在生物催化过程中的应用:机遇与挑战[J]. 化工学报, 2023, 74(1): 45-59. |
| [12] | 安绍杰, 许洪峰, 李思, 许远航, 李佳锡. 利用分子机器的组装与分解构建pH敏感性谷胱甘肽过氧化物人工酶[J]. 化工学报, 2022, 73(8): 3669-3678. |
| [13] | 张劢, 田瑶, 郭之旗, 王叶, 窦广进, 宋浩. 光催化-生物杂合系统设计优化用于燃料和化学品绿色合成[J]. 化工学报, 2022, 73(7): 2774-2789. |
| [14] | 黄丽菁, 黄继娇, 李鹏辉, 刘芷诺, 蒋康杰, 吴文娟. 木质素羟丙基磺甲基化改性及其对纤维素酶水解的影响[J]. 化工学报, 2022, 73(7): 3232-3239. |
| [15] | 张昕哲, 孙文涛, 吕波, 李春. 植物天然产物氧化与微生物制造[J]. 化工学报, 2022, 73(7): 2790-2805. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号
